Vaccine-related severe allergic reactions are of growing public concern given reports of anaphylaxis after messenger RNA (mRNA)–based vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),1 the virus that causes coronavirus disease 2019 (COVID-19) and may contribute to vaccine hesitancy and incomplete vaccination.2 Little is known on previous trends in health care utilization for vaccine-related severe allergic reactions among the US adult population.3 Understanding these trends over time, especially before the COVID-19 pandemic, may be helpful to contextualize COVID-19 vaccine-related allergic reactions and inform public understanding of the overall risk of vaccine-related severe allergic reactions.
Our objectives were to characterize the trends in rates of emergency department (ED) visits for vaccine-related severe allergic reactions among the US adult population from 2006 to 2018 and to evaluate factors associated with these severe allergic reactions (including anaphylaxis). We performed cross-sectional analyses of (1) ED visits for vaccine-related severe allergic reactions among US adults (≥18 years) using a nationally representative sample of US ED visits, the Nationwide Emergency Department Sample (NEDS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality,4 and (2) severe allergic events requiring ED care from a national passive reporting system, the Vaccine Adverse Event Reporting System (VAERS).5 The NEDS is the largest all-payer US ED database that provides nationally representative data from approximately 145 million ED visits each year using discharge data from 990 hospitals located in 36 States and the District of Columbia.
Vaccine-related severe allergic reactions were determined in NEDS using the following International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9 or 10-CM) diagnostic codes for anaphylaxis, guided by previous studies3: ICD-9-CM code 999.4 (anaphylactic shock-serum) for years 2006 to 2010; ICD-9-CM codes 999.4 (anaphylactic shock-serum), 999.42 (anaphylactic reaction owing to vaccination), and 999.52 and 995.0 (other serum reaction owing to vaccination and other anaphylactic reaction) for years 2011 to 2015 quarter 3; ICD-10-CM codes T80.52 (anaphylactic reaction owing to vaccination), T80.62 and T78.2 (other serum reaction owing to vaccination and anaphylactic shock, unspecified), T88.1 and T78.2 (other complications after immunization, not elsewhere classified, and anaphylactic shock, unspecified), T50.Z95 and T78.2 (adverse effect of other vaccines and biological substances and anaphylactic shock, unspecified), T50.B95 and T78.2 (adverse effect of other viral vaccines and anaphylactic shock, unspecified), and Z88.7 and T78.2 (allergy status to serum and vaccine and anaphylactic shock, unspecified) for years 2015 quarter 4 to 2018.
For identification of severe allergic events in VAERS,5 , 6 cases occurring within 0 to 1 day of vaccination were included if the following terms of the Medical Dictionary for Regulatory Activities were documented: anaphylactic reaction, anaphylactic shock, and anaphylactoid reaction. In addition, cases occurring within 0 to 1 day of vaccination were included if major or minor skin, respiratory, or cardiovascular symptoms or minor gastrointestinal symptoms were documented in combination based on the Brighton Collaboration criteria (levels 1 to 3 of diagnostic certainty).7
We used US Census population estimates for the respective years to determine population rates.8 Chronic conditions were identified using the Healthcare Cost and Utilization Project Chronic Condition Indicator. Documented epinephrine use for the visits was determined by the following codes: Healthcare Common Procedure Coding System Current Procedural Terminology code J0170 for years 2006 to 2010 and code J0171 for years 2011 to 2018. Focusing on NEDS, we constructed a multivariable logistic regression model to identify factors associated with severe allergic reactions, defined as hospitalization, cardiac arrest, intubation, or death.
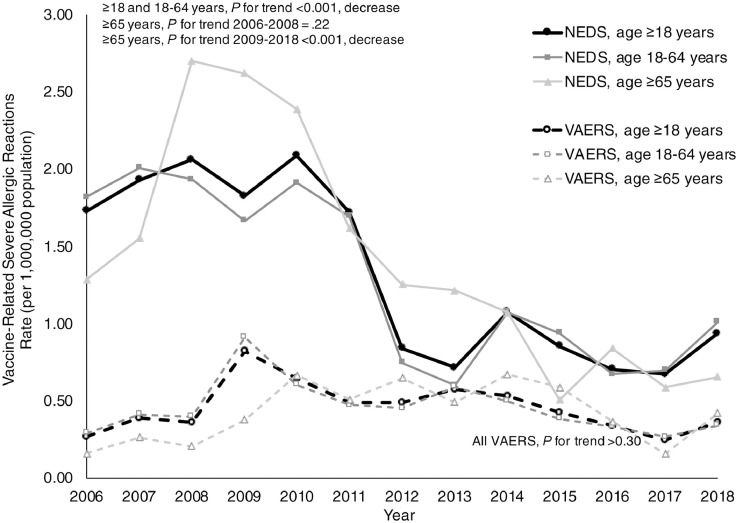
From 2006 to 2018, US adults experienced approximately 4027 (95% confidence interval [CI], 3654-4400) vaccine-related severe allergic reactions resulting in ED visits. The rate of these severe allergic reactions resulting in ED visits decreased over time, from 1.73 to 0.94 visits per million population per year (P for trend < .001) (Fig 1 ). The VAERS captured less vaccine-related severe allergic reactions resulting in ED events (n = 1412) and a lower rate of severe allergic reactions resulting in ED events (0.27-0.36 events per million population per year; P for trend > .30), with events most often associated with influenza vaccines. Most vaccine-related severe allergic reactions resulting in ED visits involved patients who were of female sex (n = 2571; 64%), had private insurance (n = 2193; 54%), visited urban hospitals (n = 3124; 78%), or had a chronic condition (n = 2257; 56%). Few ED visits had documented epinephrine use (n = 267; 7%).
Figure 1.
ED visit rates for vaccine-related severe allergic reactions per million population by age group and data source, 2006 to 2018. ED, emergency department; NEDS, Nationwide Emergency Department Sample; VAERS, Vaccine Adverse Event Reporting System.
Approximately one-third of these ED visits (weighted n = 1364; 34%) were considered severe, defined as resulting in hospitalization (weighted n = 1335; 34%), cardiac arrest or intubation (weighted n = 271; 7%), or death (n < 10, 1%). After controlling for age, sex, primary payer, geographic region, urban vs rural hospital, and presence of chronic conditions, factors associated with vaccine-related severe allergic reactions included increasing age (odds ratio [OR], 2.24; 95% CI, 1.04-4.86 for ≥65 years compared with ages 18-24 years), male sex (OR, 1.56; 95% CI, 1.08-2.24 compared with female sex), public insurance (OR, 1.84; 95% CI, 1.18-2.82 compared with private insurance), or having any chronic condition (OR, 14.36; 95% CI, 8.88-23.21).
Using nationally representative data, we report that vaccine-related severe allergic reactions resulting in ED visits among adults were rare and decreased considerably from 2006 to 2018, before the COVID-19 pandemic. Vaccine-related anaphylaxis had previously been estimated to be 1.3 cases per million vaccine doses given from 2009 to 2011.3 Slightly higher rates of anaphylaxis have been reported after administration of mRNA COVID-19 vaccinations, with recent estimates of 7.9 cases per million vaccinations.1 Previous reports of vaccine-related anaphylaxis may not be comparable to our findings given that we report population-based rates. Similar to studies on mRNA COVID-19 vaccines, we found that passive reporting of vaccine-related events through VAERS underestimated the rates.9
Reassuringly, death from vaccine-related severe allergic reactions was exceedingly rare. Older adults, especially more than or equal to 65 years, were more likely to experience vaccine-related severe allergic reactions compared with younger adults, which may be secondary to preexisting comorbidities. Older adults have been noted to have increased risk of fatal anaphylaxis, especially secondary to drug-related anaphylaxis.10 Documented epinephrine use was noted to be low perhaps secondary to prehospital use or inadequate documentation.
Limitations include potential coding errors and minimal clinical information, including lack of allergist-performed diagnostic testing to confirm vaccine-related severe allergic reactions such as anaphylaxis. Nevertheless, we have used national data from the following 2 different sources to capture health care utilization for vaccine-related severe allergic reactions: (1) voluntarily reported data (VAERS) and (2) a population-based sample of ED discharges based on physician diagnosis (NEDS). Together, these data may provide a more comprehensive picture of trends in vaccine-related severe allergic reactions.
Vaccine-related severe allergic reactions are rare and health care utilization for these severe allergic reactions decreased from 2006 to 2018 (before the COVID-19 pandemic). Reassuringly, fatal vaccine-related allergic reactions were exceedingly rare. Recognition, diagnosis, and appropriate treatment of vaccine-related severe allergic reactions should be considered important components of public health vaccination efforts.
Footnotes
2006 to 2018
Disclosures: Dr Blumenthal reports receiving grants from the Massachusetts General Hospital, outside of the submitted work. Dr Camargo, Dr Blumenthal, and Dr Arroyo report receiving grants from the National Institutes of Health (NIH), outside of the submitted work. Dr Robinson reports receiving honoraria for speaking from Viatris. Dr Cash has no conflicts of interest to disclose.
Funding: Dr Arroyo was supported by the NIH (grant number R25 AI147369) and Dr Blumenthal by the NIH (grant number K01 AI125631) and the Massachusetts General Hospital Department of Medicine Transformative Scholar Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH nor the Massachusetts General Hospital.
References
- 1.Greenhawt M, Abrams EM, Shaker M, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson LB, Landman AB, Shenoy ES, et al. Allergic symptoms after mRNA COVID-19 vaccination and risk of incomplete vaccination. J Allergy Clin Immunol Pract. 2021;9(8):3200–3202. doi: 10.1016/j.jaip.2021.05.031. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agency for Healthcare Research and Quality. NEDS overview: Healthcare Cost and Utilization Project (HCUP). 2021. Available at: https://www.hcup-us.ahrq.gov/nedsoverview.jsp. Accessed February 26, 2021.
- 5.Centers for Disease Control and Prevention. Vaccine adverse reporting system. Available at: https://vaers.hhs.gov. Accessed March 11, 2021.
- 6.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2015;33(36):4398–4405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rüggeberg JU, Gold MS, Bayas JM, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 8.United States Census Bureau. Annual estimates of the resident population for selected age groups by sex for the United States: April 1, 2010 to July 1, 2019; table 1. Intercensal estimates of the resident population by sex and age for the United States: April 1, 2000 to July 1, 2010. Available at: https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html; https://www.census.gov/data/tables/time-series/demo/popest/intercensal-2000-2010-national.html. Accessed March 3, 2021.
- 9.Blumenthal KG, Robinson LB, Camargo CA, Jr, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo AC, Robinson LB, Cash RE, Faridi MK, Hasegawa K, Camargo CA., Jr. Trends in emergency department visits and hospitalizations for acute allergic reactions and anaphylaxis among US older adults: 2006-2014. J Allergy Clin Immunol Pract. 2021;9(7):2831–2843. doi: 10.1016/j.jaip.2021.03.032. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]