To the editor:
Solid organ transplant recipients have a weaker humoral response to coronavirus disease 2019 (COVID-19) vaccination because of several factors, including lymphopenia associated with immunosuppressive therapies (particularly belatacept, antiproliferative drugs, and steroids).1 Because of the high probability of severe COVID-19 symptoms in this at-risk population,2 a third vaccine dose has been proposed for immunocompromised patients by the French National Authority for Health to improve humoral responses and vaccine efficiency.3 Despite this improved vaccination schedule, >30% of kidney transplant recipients (KTRs) do not develop a humoral response and remain at risk of severe COVID-19 infection.
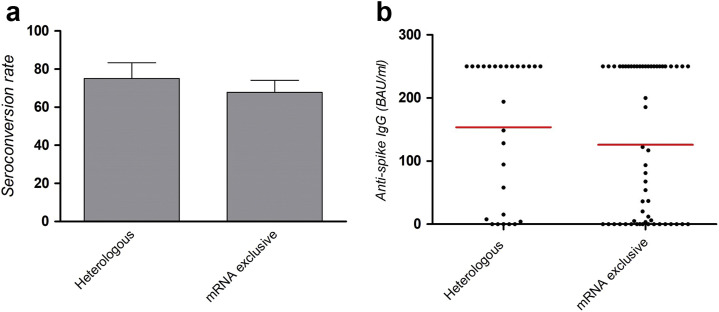
ChAdOx1-nCov vaccine (i.e., AstraZeneca) has been sparingly used by transplant centers, because of the low representation of patients with vulnerability in the initial trial4 but also its rare but serious thrombotic complications.5 Recently, emerging data reported that heterologous vaccination using an mRNA booster after ChAdOx1-nCov primed vaccination induced a good—and in some cases an even better—humoral response than exclusive mRNA vaccination.6 There are currently no data that assess the benefit of heterologous vaccination in solid organ transplant recipients, or whether this can improve the humoral response. A total of 373 KTRs from our institution had a serologic assessment 1 month after the third vaccine injection (screening and binding antibody unit [BAU]/ml quantification of anti-spike IgG by ECLIA Roche, Architect Abbott, or Diasorin). Among them, 28 had a heterologous vaccination schedule (ChAdOx1-nCov priming, 1 or 2 injections, followed by 1 or 2 mRNA injections), and 345 received 3 mRNA injections. On the basis of established risk factors of nonhumoral response after mRNA vaccination, we identified a matched 2:1 control cohort having received 3 mRNA vaccines (mRNA exclusive) based on age (±5 years), lymphopenia (<1500/mm3), and use of antiproliferative drugs and steroids. Conditional logistic regression was used to compare heterologous and mRNA exclusive cohorts. The average age of both cohorts was 59 years, 71% received antiproliferative drugs, 39% received steroids, and the mean lymphocyte count was 1700/mm3. There was a trend of lower allograft function (assessed by the Modification of Diet in Renal Disease) in the heterologous cohort (44.6 vs. 51.5 ml/min; P = 0.06; Table 1 ). No difference in serious adverse events was observed among patients from the 2 groups. Median times of serologic screening in the heterologous group and the mRNA exclusive group were 33 and 34 days, respectively. Seroconversion (i.e., anti-spike IgG superior to laboratory threshold) was observed in 75% of patients with heterologous vaccination and 67.8% of patients with mRNA exclusive vaccination (odds ratio, 1.72; 95% confidence interval, 0.59–4.99; P = 0.32). Mean anti-spike IgG titers were 159 BAU/ml in the heterologous group and 125 BAU/ml in the mRNA exclusive group (P = 0.36; Figure 1 ). Recent data by Behrens et al. demonstrated a higher immune response induced by a heterologous schedule, including neutralization of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta variant.7 To our knowledge, we report the first study assessing humoral responses to a heterologous vaccination schedule in immunocompromised KTRs. Seroconversion rates and antibody titers induced by heterologous vaccination were at least equal to mRNA-exclusive vaccination in immunocompromised transplant recipients; although they trended higher in the heterologous group, this did not reach statistical significance because of the small cohort size. Moreover, the lower allograft function in the heterologous cohort may have weakened the observed humoral response.1 Overall, heterologous vaccination appears to induce a robust humoral response in KTRs and may be considered to improve vaccine response in this immunocompromised population.
Table 1.
Characteristics of the kidney transplant cohorts depending on their vaccination schedule (heterologous schedule: ChAdOx1-S primed vaccination, then mRNA booster; mRNA exclusive: mRNA vaccine alone)
| Characteristics | Heterologous (n = 28) |
mRNA exclusive (n = 56) |
P value | ||||
|---|---|---|---|---|---|---|---|
| N/A | No. | % | N/A | No. | % | ||
| Positive serology after 3 doses | 0 | 28 | 75.0 | 0 | 56 | 67.8 | 0.32 |
| Male recipient | 0 | 20 | 71.4 | 0 | 39 | 69.6 | 0.99 |
| Transplant rank ≥2a | 0 | 21 | 75.0 | 0 | 46 | 82.1 | 0.56 |
| Calcineurin inhibitor treatment | 0 | 24 | 85.7 | 0 | 44 | 78.5 | 0.56 |
| mTOR inhibitor treatment | 0 | 6 | 12.4 | 0 | 5 | 8.9 | 0.16 |
| Antimetabolite treatment | 0 | 20 | 71.4 | 0 | 40 | 71.4 | 1 |
| Steroid treatment | 0 | 11 | 39.3 | 0 | 22 | 39.3 | 1 |
| N/A | Mean | SD | N/A | Mean | SD | P value | |
|---|---|---|---|---|---|---|---|
| Age, yr | 0 | 58.7 | 13.3 | 0 | 58.7 | 12.0 | 0.99 |
| Time from transplantation, yr | 0 | 8.2 | 6.2 | 0 | 8.5 | 7.4 | 0.79 |
| Lymphocyte count, /mm3 | 0 | 1750 | 750 | 0 | 1730 | 1000 | 0.52 |
| Anti-spike IgG titer, BAU/ml | 1 | 159 | 110 | 0 | 125 | 116 | 0.36 |
| Allograft function by MDRD, ml/min | 0 | 44.6 | 18.0 | 0 | 51.5 | 19.2 | 0.06 |
BAU, binding antibody unit; MDRD, Modification of Diet in Renal Disease; N/A, nonavailable data.
Transplant rank ≥2: patient having received a second or more transplant kidney.
Figure 1.
(a) Seroconversion rate after 3 injections (i.e., anti-spike IgG superior to laboratory threshold) in patients having received a heterologous schedule (ChAdOx1-S primed vaccination and mRNA booster) and a standard schedule (mRNA vaccine alone). (b) Anti-spike IgGs, expressed in binding antibody unit (BAU)/ml, and their respective mean titer 1 month after the last vaccine injection in both groups.
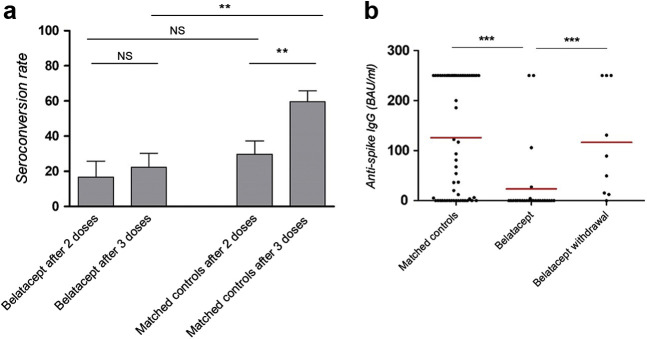
Otherwise, there are important concerns for KTRs treated with belatacept, the only costimulation blocker that has received approval for clinical use.8 Although poor humoral responses following 2 mRNA vaccine injections in KTRs treated with belatacept has been well demonstrated,9, 10, 11 whether a third dose could overcome these issues, as in patients receiving conventional therapy, remains controversial. Indeed, published rates of seroconversion vary dramatically from 6.4% (4 of 62) in the study of Chavarot et al. 12 to 41% (5 of 12) in the report by Kamar et al. 3 These discrepancies could result from differences in confounding variables, especially the association with antiproliferative drugs, usually combined with belatacept and recognized as a risk factor for poor response to mRNA vaccines.13 To avoid this pitfall, we analyzed in our institutional cohort the seroconversion rate in KTRs treated with belatacept having received 3 mRNA doses and matched them with 2 KTRs not receiving belatacept, on age (±5 years), total lymphocyte count (<1500/mm3), and use of antiproliferative and steroid drugs. Characteristics of the 27 belatacept-treated patients and 56 control patients are presented in Table 2 ; none of them had a history of COVID-19 infection. Seroconversion after the second injection was observed in 13.3% of belatacept-treated patients and 25.8% of control patients (P = 0.45). After the third mRNA injection, seroconversion was observed in 22.2% of patients exposed to belatacept and 59.7% of the matched control patients (Figure 2 a), with mean anti-spike IgG titers at 24 and 106 BAU/ml, respectively (P < 0.001; Figure 2b). The corresponding odds ratio estimated from a conditional logistic regression was 4.97 (95% confidence interval, 1.40–17.67; P = 0.01). Hence, our results confirm that belatacept severely inhibits the humoral response to a third dose of mRNA SARS-CoV-2 vaccine in an independent way.
Table 2.
Characteristics of the cohort of patients undergoing belatacept therapy and matched controls
| Characteristics | Belatacept (n = 27) |
Matched controls (n = 54) |
P value | ||||
|---|---|---|---|---|---|---|---|
| N/A | No. | % | N/A | No. | % | ||
| Positive serology after 2 doses | 12 | 2 | 13.3 | 24 | 8 | 25.8 | 0.45 |
| Positive serology after 3 doses | 0 | 6 | 22.2 | 0 | 32 | 59.7 | 0.01 |
| Male recipient | 0 | 16 | 59.2 | 0 | 38 | 70.3 | 0.33 |
| Transplant rank ≥2a | 0 | 4 | 14.8 | 0 | 15 | 38.4 | 0.26 |
| Calcineurin inhibitor treatment | 0 | 7 | 25.9 | 0 | 37 | 68.5 | <0.001 |
| mTOR inhibitor treatment | 0 | 0 | 0 | 0 | 12 | 22.2 | 0.006 |
| Antimetabolite treatment | 0 | 18 | 66.7 | 0 | 36 | 66.7 | 1 |
| Steroid treatment | 0 | 19 | 70.3 | 0 | 38 | 70.3 | 1 |
| N/A | Mean | SD | N/A | Mean | SD | P value | |
|---|---|---|---|---|---|---|---|
| Age, yr | 0 | 61.2 | 14.2 | 0 | 60.9 | 13.9 | 0.91 |
| Time from transplantation, yr | 0 | 4.4 | 3.9 | 0 | 7.4 | 7.8 | 0.27 |
| Lymphocyte count, /mm3 | 0 | 1257 | 660 | 0 | 1450 | 958 | 0.57 |
| Anti-spike IgG titer after 3 doses, BAU/ml | 0 | 24.5 | 69.6 | 0 | 106.5 | 116.4 | <0.001 |
| Allograft function by MDRD, ml/min | 0 | 34.7 | 12.5 | 0 | 45.6 | 20.6 | 0.02 |
BAU, binding antibody unit; MDRD, Modification of Diet in Renal Disease; N/A, nonavailable data.
Transplant rank ≥2: patient having received a second or more transplant kidney.
Figure 2.
Serologic assessment was performed by ECLIA Roche, Architect Abbott, or Diasorin technologies, and anti-spike IgG titers were expressed in binding antibody unit (BAU)/ml. Positivity was set as anti-spike IgG superior to laboratory threshold. Median times of serologic screening in the belatacept group and the matched control group were 42 and 36 days, respectively, after the third injection. (a) Seroconversion rate after 2 and 3 mRNA injections (i.e., anti-spike IgG level superior to laboratory threshold) in patients receiving belatacept and matched controls. (b) Anti-spike IgGs, expressed in BAU/ml, and their respective mean titer 1 month after the last vaccine injection in belatacept recipients and matched controls. Anti-spike IgGs, expressed in BAU/ml, and their respective mean titer 1 month after the last vaccine injection in belatacept recipients and patients who underwent belatacept withdrawal. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. NS, nonsignificant difference.
Given the crucial importance for KTRs to be vaccinated, it has been suggested that belatacept could be replaced with conventional maintenance therapy to improve vaccine effectiveness.13 However, whether this strategy is worthwhile deserves further investigation for several reasons: (i) recent data have shown that vaccine effectiveness is deeply impacted by preexisting cross-reactive CD4+ T cells specific for endemic human cold coronavirus14 , 15 and (ii) costimulation blockade promotes specific T-cell hyporesponsiveness and anergy.16 Consequently, vaccine responses could be impacted a long time after discontinuation of a costimulation blockade. To address these issues, we assessed the response to the mRNA SARS-CoV-2 vaccine in the KTRs of our whole cohort who had been previously exposed to belatacept for at least 1 year. In the 9 patients identified, belatacept had been intentionally withdrawn and replaced with conventional immunosuppressive drugs, mainly a mycophenolate derivative combined with a calcineurin inhibitor (see Supplementary Table S1 for details), and none had presented a rejection episode in the follow-up. The mean time between belatacept discontinuation and vaccination was 32 months. One month after the third vaccine dose, 8 of 9 patients (87.5%) had a positive serology with a mean anti-spike IgG titer at 105 BAU/ml (Figure 2b). These results are extremely encouraging with respect to withdrawing a costimulation blockade to improve vaccine effectiveness. This obviously needs to be confirmed in KTRs having stopped belatacept more recently.
In conclusion, several strategies can be considered to improve humoral responses following COVID-19 vaccination in KTRs. Heterologous vaccination, using an mRNA booster after ChAdOx1-nCov priming, induced at least as good a humoral response, if not a better response, to exclusive mRNA vaccination. Otherwise, immunosuppression modulation, notably temporary belatacept withdrawal, seems promising to improve the poor humoral response in these patients.
Footnotes
Table S1. Characteristics of the patients who underwent belatacept withdrawal.
Supplementary Material
References
- 1.Masset C., Kerleau C., Garandeau C., et al. A third injection of BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021;100:1132–1135. doi: 10.1016/j.kint.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caillard S., Anglicheau D., Matignon M., et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamar N., Abravanel F., Marion O., et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronbichler A, Anders H-J, Fernandez-Juárez GM, et al. Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases. Nephrol Dial Transplant. 2021;36:1160–1168. doi: 10.1093/ndt/gfab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A., Thiele T., Warkentin T.E., et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borobia A.M., Carcas A.J., Pérez-Olmeda M., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens G.M., Cossmann A., Stankov M.V., et al. SARS-CoV-2 delta variant neutralisation after heterologous ChAdOx1-S/BNT162b2 vaccination. Lancet. 2021;398:1041–1042. doi: 10.1016/S0140-6736(21)01891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durrbach A., Pestana J.M., Pearson T., et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT Study) Am J Transplant. 2010;10:547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 9.Chavarot N., Ovedrani A., Marion O., et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105:e94–e95. doi: 10.1097/TP.0000000000003784. [DOI] [PubMed] [Google Scholar]
- 10.Noble J., Langello A., Bouchut W., et al. Immune response post-SARS-CoV-2 mRNA vaccination in kidney-transplant recipients receiving belatacept. Transplantation. 2021;105:e259–e260. doi: 10.1097/TP.0000000000003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou M.T., Boyarsky B.J., Chiang T.P.Y., et al. Immunogenicity and reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking belatacept. Transplantation. 2021;105:2119–2123. doi: 10.1097/TP.0000000000003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavarot N., Morel A., Leruez-Ville M., et al. Weak antibody response to 3 doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am J Transplant. 2021;21:4043–4051. doi: 10.1111/ajt.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caillard S., Thaunat O. COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol. 2021;17:785–787. doi: 10.1038/s41581-021-00491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loyal L., Braun J., Henze L., et al. Cross-reactive CD4+ T cells enhance SARS-CoV-2 immune responses upon infection and vaccination. Science. 2021;374 doi: 10.1126/science.abh1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateus J., Dan J.M., Zhang Z., et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science. 2021;374 doi: 10.1126/science.abj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebille F., Vanhove B., Soulillou J.P. Mechanisms of tolerance induction: blockade of co-stimulation. Philos Trans R Soc Lond B Biol Sci. 2001;356:649–657. doi: 10.1098/rstb.2001.0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.