TO THE EDITOR:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is of special concern to patients with chronic lymphocytic leukemia (CLL).1,2 Over time, individuals with CLL experience impaired B-cell function and antibody production, leaving them at an increased risk for severe infection or death. Patients with CLL suffer immune dysregulation from the disease, which is further disrupted by the effects of CLL-specific treatments. There are 3 vaccines for SARS-CoV-2 approved in the United States,3 with high immunogenicity in immunocompetent subjects.4-6
The postimmunization dynamics in patients with CLL are different from those observed in healthy subjects. Attenuated humoral responses to vaccination have been documented.7-10 Patients with CLL have among the lowest immune responses, which are influenced by disease status, immunoglobulin levels, and active or recent therapies.11-14 In particular, treatment with anti-CD20 monoclonal antibodies (mAbs) or Bruton tyrosine kinase inhibitors (BTKi’s) is associated with poor vaccine response.10,15
In this longitudinal cohort study, we interrogated the cellular and humoral immune response to novel vaccine antigen BNT162b2 (Pfizer-BioNTech) or messenger RNA (mRNA)-1273 (Moderna), as well as the humoral recall response to measles, in 16 subjects with CLL. In response to vaccination, immunocompetent individuals generate an antigen-specific response that results in cellular and humoral memory that persists long after vaccination,16 including CD4+ T cells, CD8+ T cells, and 2 distinct long-lived populations of B cells: long-lived plasma cells (LLPCs), and memory B cells (MBCs). LLPCs traffic to the bone marrow and continuously secrete the antibodies that make up polyclonal immune serum, whereas MBCs, which do not secrete antibodies, circulate in peripheral blood surveying for invading pathogens. MBCs are especially important in the face of waning antibody titers or the emergence of new variants that might escape neutralization by serum antibodies.17
We enrolled subjects who were ≥18 years of age and without a known history of COVID-19 infection, prior to receiving the Moderna or Pfizer-BioNTech 2-dose SARS-CoV-2 mRNA vaccine series. This study reports the presence and magnitude of humoral and cellular immune responses, including quantitative receptor-binding domain (RBD)-specific antibody titers, RBD-specific MBC frequency following in vitro stimulation, and functional tumor necrosis factor-α and interferon-γ–secreting spike (S) peptide-specific CD4+ and CD8 T cells at baseline (prior to vaccination) and ∼1-month (24-103 days) following the 2-dose mRNA vaccination series.
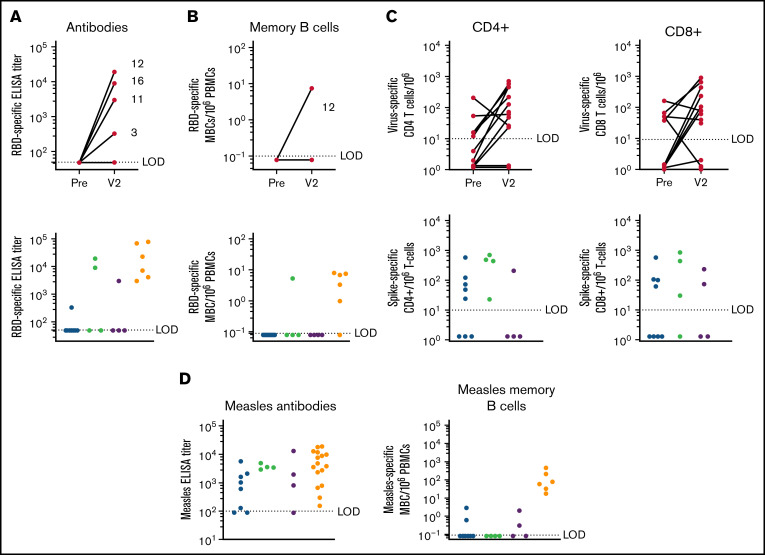
We observed a 25% seroconversion rate. Four patients with vaccine-mediated antibody responses were diverse: 1 was treatment naive, 1 was receiving treatment with bcl-2 inhibitor, and 2 were under observation. Of the patients under observation, 1 was in remission, whereas the other had relapsed disease. When stratified by treatment, 50% of subjects currently under observation following treatment seroconverted compared with 12.5% of subjects currently receiving active treatment (Figure 1A; Table 1). Of the responders (4/16), 1 had never received anti-CD20 mAb treatment, and 3 had received treatment >12 months earlier, consistent with previous studies.18 In an attempt to identify potential predictors of response, we evaluated a number of clinical factors, as well as immune profiling. Although no significant differences were appreciated, responders had overall higher immunoglobulin G (IgG) serum levels and lower absolute lymphocyte count (ALC), mean B-cell percentage, class-switched MBCs, and B1 B cells compared with nonresponders (supplemental Tables 3 and 4). Interestingly, only 1 subject (subject 12), who was in disease remission, with bcl-2 inhibitor treatment occurring >6 months prior to vaccination in combination with an anti-CD20 mAb treatment given >12 months prior to vaccination, exhibited an RBD-specific memory B-cell response. The observation that 3 of 4 patients with an RBD-specific antibody response did not have detectable RBD-specific MBCs is notable (Figure 1B). All subjects who had an RBD-specific antibody response also had an S-specific CD4+ T-cell response, indicating that a population of T helper cells was available for B-cell priming.
Figure 1.
Immune response to vaccination. (A) Antibodies: RBD-specific end point enzyme-linked immunosorbent assay (ELISA) titer following COVID-19 mRNA vaccination: prior to vaccination and V2 following 2-dose vaccination series (24-103 days) (upper panel) is shown. Individual subject numbers are shown (3, 11, 12, and 16) for responders. RBD-specific ELISA titer stratified by treatment group; geometric mean titer (GMT) of responders is shown above the graph (lower panel). The limit of detection (LOD) is set at 50; samples below the LOD were given an arbitrary value of 49. Healthy subject samples were taken (13-28 days) following the 2-dose vaccination series (lower left panel). (B) RBD-specific memory B-cell frequency per 106 peripheral blood mononuclear cells (PBMCs) following COVID-19 mRNA vaccination: prior to vaccination and V2 (24-103 days) following the 2-dose vaccination series (upper panel). Only subject 12 developed an MBC response. RBD-specific MBC frequency stratified by treatment group (lower panel). Geometric mean titer of responders is shown above the graph. Healthy subject samples (247-264) post 2-dose vaccine series are included (lower right panel). LOD = 0.1; an arbitrary number (0.08) was assigned to samples below the LOD. (C) S-specific CD4 (left upper panel) and CD8 (right upper panel) T-cell frequency per 106 T cells following COVID-19 mRNA vaccination: prior to vaccination and V2 following 2-dose vaccination series (24-103 days) (lowerpanel). S-specific CD4+ and CD8+ response to vaccination: the increase in T-cell expansion from baseline, stratified by treatment group (lower panel). Geometric mean titer of responders is shown above the graph. LOD = 10; for subjects without a vaccine-specific response, an arbitrary value between 1.1 and 1.5 was assigned. (D) Humoral immune recall response to a childhood antigen (measles) in subjects with CLL and age/sex-matched healthy controls. Antibodies: measles-specific end point ELISA titer stratified by treatment group (left panel). LOD = 100; samples below the LOD were assigned an arbitrary value of 80. Geometric mean titer of responders is shown above the graph for each group. Memory B cells: measles-specific MBC frequency stratified by treatment group (right panel). Geometric mean frequency of responders is shown above the graph. LOD = 0.1; an arbitrary number between .05 and .1 was assigned to those samples. Red, active treatment; blue, observation after treatment; green, treatment naive; yellow, healthy age/sex-matched controls.
Table 1.
Summary of subject immune response to mRNA COVID-19 vaccination and recall response to measles antigen
| Subject ID | Age, y/sex | Vaccine | Response to mRNA COVID-19 vaccine | Response to measles | Treatment | CD20 Ab | ALC 1-4.8* |
IgG 700-1600 mg/dL* |
CD19+ 4-17%* |
IgD−CD27+ 5-21%* |
CD4 30-60%* |
CD8 10-30%* |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab | CD4 | CD8 | MBC | Ab | MBC | |||||||||||
| 1 | 62/Male | P | — | — | — | — | — | — | N | No | 48.00 | 85 | 96.00 | 3.60 | 2.00 | 0.70 |
| 2 | 63/Female | P | — | — | + | — | + | + | N | No | 21.00 | NA | 76.00 | 59.00 | 9.30 | 10.00 |
| 3 | 48/Male | ? | + | + | — | — | — | — | C | Yes (≤12) | 1.00 | NA | 2.10 | 17.00 | 39.00 | 33.00 |
| 4 | 77/Female | M | — | - | — | — | + | — | N | No | 87.00 | 573 | NA | NA | NA | NA |
| 5 | 81/Female | P | — | - | + | — | + | + | C | Yes (>12) | 5.80 | NA | NA | NA | 19.00 | 3.80 |
| 6 | 67/Male | M | — | + | + | — | + | + | C | No | 2.90 | 918 | 30.00 | 2.30 | 41.00 | 19.00 |
| 7 | 60/Female | P | — | + | + | — | + | — | O (6) | Yes (>12) | 1.60 | NA | 0.00 | 0.00 | 86.00 | 8.00 |
| 8 | 66/Male | P | — | - | — | — | + | — | C | Yes (>12) | 0.43 | 526 | 3.00 | 22.00 | 35.00 | 47.00 |
| 9 | 65/Female | ? | — | + | + | — | + | — | C | Yes (≤12) | 1.30 | NA | 0.07 | 0.00 | 62.00 | 21.00 |
| 10 | 62/Female | P | — | + | - | — | — | — | C | Yes (>12) | 30.00 | 262 | 65.00 | 2.40 | 23.00 | 8.00 |
| 11 | 63/Male | P | + | + | + | — | + | + | N | No | 17.00 | 780 | 83.00 | 0.21 | 13.00 | 2.10 |
| 12 | 61/Male | P | + | + | + | — | + | — | O (6-12) | Yes (>12) | 0.75 | 593 | 8.00 | 3.80 | 42.00 | 15.00 |
| 13 | 70/Male | P | — | + | — | — | + | — | C | Yes (>12) | 5.80 | 101 | 45.00 | 1.50 | 20.00 | 20.00 |
| 14 | 65/Male | P | — | + | — | — | + | — | O (>12) | Yes (>12) | 0.33 | 405 | NA | NA | 77.00 | 9.30 |
| 15 | 64/Male | M | — | — | + | — | + | — | C | Yes (>12) | 1.70 | 100 | 0.41 | 0.94 | 26.00 | 42.00 |
| 16 | 75/Male | P | + | + | + | — | + | — | O (>12) | Yes (>12) | 0.29 | 547 | 0.10 | 0.00 | 22.00 | 19.00 |
For T-cell–specific responses, “+” indicates an increase in S-specific T cells compared with baseline and “—“ indicates no change (or a decrease) in S-specific T cells following vaccination. Current treatment status, CD20 Ab treatment, and clinical values were recorded at baseline (time of enrollment) when available.
Ab, antibody; ALC, absolute lymphocyte count; C, currently on treatment; M, Moderna; N, treatment naive; NA, baseline values were not available; O (6), observation, last treatment within 6 months; O (6-12), observation, 6 to 12 months since last treatment; O (>12), observation, >12 months since last treatment; P, Pfizer-BioNTech; ?, unknown; +, response above the LOD; —, response below the LOD.
Normal range.
SARS-CoV-2 S-reactive T cells were present at baseline in some of the subjects (Figure 1C). Subjects 5 and 8 and subjects 3 and 13 did not exhibit any expansion of S-responsive CD4+ T cells and or and S-reactive CD8+ T cells, respectively, following vaccination. This is consistent with previous reports of S-reactive T cells in naive individuals without prior antigen exposure.19 The cellular immune response seemed to be fairly robust compared with the humoral immune response in these subjects with CLL, consistent with previous studies.20,21 We observed a 62.5% CD4+ T-cell response and a 56% CD8+ T-cell response. Four subjects had an S-specific CD4+ response alone, 3 subjects had an S-specific CD8+ response alone, and 6 subjects had CD4+ and CD8+ responses. Four of the 10 CD4+ responders seroconverted, providing supporting evidence for the importance of CD4+ T-cell help in generating a B-cell response.
Active treatment with BTKi’s has a significant impact on B-cell survival, differentiation, and the development of an antigen-specific antibody response to novel antigen exposure. B cells are dependent on Bruton tyrosine kinase signaling for differentiation and proliferation signals, and immune response to novel antigens, either by natural infection or vaccination, is severely limited in these subjects15; however, recall to previously encountered antigens remains largely intact (Figure 1D). Seventy-one percent of subjects on BTKi’s had a cellular immune response with CD4+ and/or CD8+ T cells. Whether this finding translates to an effective T-cell response associated with a clinical benefit is of interest. Because BTKi’s are administered daily, further studies that evaluate the timing of vaccines or interruption of ongoing BTKi therapy in an attempt to enhance vaccine response are warranted. This approach showed success in patients with rheumatologic disease on immunosuppressive therapies.22 Bcl-2 is a protein regulator of apoptosis, and preclinical data suggest that bcl-2 inhibition affects T-cell function.23 The impact of ongoing bcl-2 inhibition with venetoclax remains an unanswered question that is worthy of additional study.
A recent study15 reported an impaired vaccine response to novel antigens in patients with CLL, resulting in seroconversion in 28.1% of treatment-naive subjects and only 3.8% of patients on BTKi’s. Compared with the humoral response to previously vaccinated antigens, the response was 41.5% in subjects on BTKi’s and 59.1% for treatment-naive subjects, indicating that BTKi’s disrupt the generation of novel immune responses but do not necessarily interfere with recall. We explored the recall response to measles and observed that 81% of subjects were seropositive for measles serum antibodies; subjects 3 and 10 were on active treatment with bcl-2 inhibitor and BTKi’s, respectively, and 1 subject was treatment naive. This is a slightly higher response rate than was observed in a recent cross-sectional study of 959 patients24 that detected a 63% measles seropositivity rate in subjects with hematological malignancies. The antibody response to measles seems to be largely unaffected in subjects with CLL, indicating that LLPCs responsible for maintaining circulating serum antibodies remain stable throughout CLL immune dysfunction and treatment. However, the MBC recall response to measles was highly disrupted in these subjects. Only 25% retained a detectable population of measles-specific MBCs: of these 4 subjects, 2 were on active treatment (subjects 5 and 6), and 2 were treatment naive. Although a population of measles-specific MBCs was detected in these subjects, the frequencies were lower than those observed in age/sex-matched healthy controls (geometric mean frequency, 78.4).
In summary, the results of this study provide a thorough evaluation of the humoral and cellular immune response to the initial 2-dose mRNA COVID-19 vaccine series in patients with CLL. Our results highlight the limitations of serology studies alone in defining vaccine-mediated immune responses, particularly in this immune-dysregulated patient population. Larger longitudinal studies incorporating clinical outcomes in vaccinated patients with CLL, as well as the impact of a third booster or heterologous vaccine, are needed.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
Acknowledgments: The authors thank the subjects for participating in this research study.
This work was supported in part by National Institutes of Health National Institute of Allergy and Infectious Diseases grant 1R01AI145835 (W.B.M.), by National Institutes of Health grant P51 OD011092 (M.K.S.), and endowed funds from the Knight Cancer Institute's Scholar Award for Leukemia and Lymphoma Research (S.E.S.).
The funders had no involvement in study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Contribution: Z.L.L.: investigation, visualization, writing-original draft, and editing and reviewing; M.S.K.: investigation, project administration, resources, visualization, and writing-original draft; D.X.L.: investigation and formal analysis; H.-P.R.: formal analysis, investigation, and writing—review & editing; V.R.: investigation, project administration, resources; J.G. and D.R.: project administration and resources; A.E.B.: formal analysis and resources; M.E.C.: resources, writing—review & editing; M.K.S. acquired funding, supervised the study, and reviewed and edited the manuscript; W.B.M.: conceptualization, funding acquisition, supervision, and writing—review & editing; and S.E.S.: conceptualization, funding acquisition, project administration, supervision, writing—original draft, and writing—review & editing.
Conflict-of-interest disclosure: S.E.S. has acted as a consultant for Velos Bio, Karyopharm, Genentech, Janssen, and Pharmacyclics and his institution has received research funding from Acerta Pharma, Astra Zeneca, BeiGene, Bristol Myers Squibb, Genentech, Gilead Sciences, Ionis, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Stephen E. Spurgeon, Knight Cancer Institute OHSU, 3303 S. Bond Ave, Portland, OR 97239; e-mail: spurgeous@ohsu.edu.
References
- 1.Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roeker LE, Eyre TA, Thompson MC, et al. COVID-19 in patients with CLL: improved survival outcomes and update on management strategies. Blood. 2021;138(18):1768-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Different COVID-19 vaccines. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html. Accessed 30 August 2021.
- 4.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadoff J, Gray G, Vandebosch A, et al. ; ENSEMBLE Study Group . Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauro FR, Giannarelli D, Galluzzo CM, et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia. 2021;35(3): 737-746. [DOI] [PubMed] [Google Scholar]
- 8.Hartkamp A, Mulder AH, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19(13-14):1671-1677. [DOI] [PubMed] [Google Scholar]
- 9.van der Velden AM, Mulder AH, Hartkamp A, Diepersloot RJ, van Velzen-Blad H, Biesma DH. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med. 2001;12(5):420-424. [DOI] [PubMed] [Google Scholar]
- 10.Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9):2703-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv. 2021;2021.04.06.21254949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903-1915. [DOI] [PubMed] [Google Scholar]
- 17.Lyski ZL, et al. SARS-CoV-2 specific memory B-cells from individuals with diverse disease severities recognize SARS-CoV-2 variants of concern. medRxiv. 2021;Jun 3:2021.05.28.21258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchesi F, Pimpinelli F, Giannarelli D, et al. Impact of anti-CD20 monoclonal antibodies on serologic response to BNT162b2 vaccine in B-cell non-Hodgkin’s lymphomas. Leukemia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malard F, Gaugler B, Gozlan J, et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021;11(8):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilich T, Nelde A, Heitmann JS, et al. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci Transl Med. 2021;13(590):eabf7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76(9):1559-1565. [DOI] [PubMed] [Google Scholar]
- 23.Farsaci B, Sabzevari H, Higgins JP, et al. Effect of a small molecule BCL-2 inhibitor on immune function and use with a recombinant vaccine. Int J Cancer. 2010;127(7):1603-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquis SR, Logue JK, Chu HY, et al. Seroprevalence of measles and mumps antibodies among individuals with cancer. JAMA Netw Open. 2021;4(7):e2118508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.