Abstract
The vitamin D receptor (VDR) normally functions as a ligand-dependent transcriptional activator. Here we show that, in the presence of Ets-1, VDR stimulates the prolactin promoter in a ligand-independent manner, behaving as a constitutive activator. Mutations in the AF2 domain abolish vitamin D-dependent transactivation but do not affect constitutive activation by Ets-1. Therefore, in contrast with the actions of vitamin D, activation by Ets-1 is independent of the AF2 domain. Ets-1 also conferred a ligand-independent activation to the estrogen receptor and to peroxisome proliferator-activated receptor α. In addition, Ets-1 cooperated with the unliganded receptors to stimulate the activity of reporter constructs containing consensus response elements fused to the thymidine kinase promoter. There is a direct interaction of the receptors with Ets-1 which requires the DNA binding domains of both proteins. Interaction with Ets-1 induces a conformational change in VDR which can be detected by an increased resistance to proteolytic digestion. Furthermore, a retinoid X receptor-VDR heterodimer in which both receptors lack the core C-terminal AF2 domain can recruit coactivators in the presence, but not in the absence, of Ets-1. This suggests that Ets-1 induces a conformational change in the receptor which creates an active interaction surface with coactivators even in the AF2-defective mutants. These results demonstrate the existence of a novel mechanism, alternative to ligand binding, which can convert an unliganded receptor from an inactive state into a competent transcriptional activator.
Nuclear receptors normally act as ligand-inducible transcriptional factors by binding as homodimers or as heterodimers with the retinoid X receptor (RXR) to hormone response elements (HREs) in target genes (15). Transcriptional regulation by nuclear receptors is achieved through autonomous activation functions (AFs): a constitutive N-terminal AF1 and a C-terminal ligand-dependent AF2. Ligand binding causes a conformational change in the receptors that allows recruitment of CREB binding protein (CBP)- and p160-related coactivator proteins with histone acetylase activity (26). The multisubunit coactivator complex TRAC-DRIP also binds the nuclear receptors in a ligand- and AF2-dependent manner and may interact directly with the basic transcriptional machinery (9, 21). However, recent evidence has shown that several receptors may also be activated in a ligand-independent manner. A variety of agents including growth factors and cyclic AMP activate the receptors, presumably by stimulation of cellular protein kinases which cause receptor phosphorylation (3, 12, 22, 24, 31). This activation appears to involve a ligand- and AF2-independent recruitment of p160 coactivators (8, 27). Furthermore, the estrogen receptor (ER) can be also stimulated in a ligand-independent manner by association with cyclin D1 (35). Cyclin D1 interacts with p160 coactivators (36) and also with the acetylase p/CAF (CBP-associated factor) (18) and can recruit the coactivators to ER in the absence of estrogens.
The vitamin D receptor (VDR), ERα, and peroxisome proliferator-activated receptor α (PPARα) stimulate prolactin gene expression (5, 16, 25). In the context of the prolactin gene, the nuclear receptors require the presence of the pituitary-specific transcription factor GHF-1 (or Pit-1) to activate the promoter, and a direct protein-to-protein interaction between the receptors and GHF-1 appears to be involved in this regulation. The prolactin promoter contains several binding sites for Ets factors. The Ets family of transcription factors, a target of the Ras–mitogen-activated protein kinase signaling pathway, plays an important role in cell growth and development (29). The Ets family is defined by a conserved DNA binding domain (DBD), also known as the ETS domain. Ets factors bind DNA as monomers and recognize a consensus sequence that contains a core 5′-GGA(A/T)-3′ motif. Ets-1 acts in conjunction with GHF-1 to fully reconstitute prolactin promoter activity in nonpituitary cells. This functional interaction also involves a physical association between both transcription factors. It has been shown that Ets-1 physically associates with GHF-1 and that both factors synergistically activate the prolactin promoter (2, 4). Additionally, CBP also interacts with Ets-1 (33) and GHF-1 (25, 32, 34) and plays a coactivator role in transactivation by these factors. Therefore, a multicomponent activating complex appears to be responsible for prolactin gene transcription.
In this study we have analyzed the effect of Ets factors on transcriptional regulation by the receptors. We have found that, in the presence of Ets-1, VDR stimulates the prolactin promoter in a ligand-independent manner, behaving as a constitutive activator. There is a direct interaction of the VDR with Ets-1 which induces a conformational change in VDR and renders an active receptor in the absence of vitamin D. This activation is AF2 independent, since Ets-1 also conferred activation to AF2-defective VDR mutants. Furthermore, receptors lacking the AF2 domain can recruit the p160 coactivator ACTR in the presence but not in the absence of Ets-1. Ets-1 also conferred ligand-independent activation to other nuclear receptors such as ERα and PPARα. These observations demonstrate the existence of a novel mechanism of activation of nuclear receptors, different from ligand binding, which could have important effects on transcriptional regulation.
MATERIALS AND METHODS
Expression vectors and transfections.
Reporter plasmids containing different fragments of the rat prolactin promoter fused to the chloramphenicol acetyltransferase (CAT) gene have been previously described (4). The consensus vitamin D response element (VDRE), peroxisome proliferator response element (PPRE), and estrogen response element (ERE) were cloned upstream of the thymidine kinase (TK) promoter of pBL-CAT8 (5, 10). Expression vectors for VDR, VDR mutants, ERα, PPARα, RXR, GHF-1, SRC-1, CBP, Ets-1 (p54), and dominant-negative Ets have been also described (4, 5, 10, 11). HeLa cells were transfected by calcium phosphate with 5 μg of reporter plasmids and the amounts of expression vectors indicated in the figure legends. Unless otherwise stated cells were incubated in the presence and absence of vitamin D (100 nM), estradiol (1 μM), Wy14,643 (100 μM), or 9-cis-retinoic acid (1 μM), for 48 h in Dulbecco's modified Eagle medium supplemented with 10% AG1-X8 resin and charcoal-stripped newborn calf serum. All data shown are means ± standard deviations obtained from at least four independent transfections, and the experiments were repeated at least twice with similar relative differences in regulated expression.
Immunoprecipitation and GST pull-down assays.
Coding sequences for VDR, PPARα, and Ets-1 were fused in frame with that for glutathione S-transferase (GST) in the pGEX 2TK-P vector. GST-ACTR (6), GST-SMRT (7), and GST-CBP (11) were also used. Recombinant proteins were synthetized, purified on glutathione-Sepharose resin, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). GST alone and GST–Ets-1 (1 μg) were exposed to 900 μg of whole-cell extract (WCE) from HeLa cells transfected with 15 μg of VDR 24 h before. Proteins were eluted from the resin and resolved by SDS-PAGE. VDR was detected by Western blotting with a VDR antibody [VDR (C-20); Santa Cruz Biotechnology] and visualized with ECL (Amersham). 35S-labeled Ets-1, VDR, ER, PPAR, RXR, and SRC-1 were generated with TNT T7 Quick coupled in vitro transcription and translation and used in pull-down assays with 1 μg of GST or GST-fused proteins as described previously (5). 35S-labeled p68 Ets-1 deletion mutants in pSG5 (2) were also used in the assays. The p54 Ets-1 isoform lacks the extra N-terminal domain. For immunoprecipitation, HeLa cells were transfected with 15 μg of expression vectors for Ets-1 and/or VDR. The cells were harvested in 200 μl of lysis buffer, and 700 μg of WCE was incubated with anti-Ets antibody [Ets-1/Ets-2 (C-275); Santa Cruz Biotechnology] and protein A-Sepharose at 4°C overnight. WCEs (5 mg) of untransfected pituitary GH4C1 cells were also used for immunoprecipitation with the Ets antibody. The cells were either untreated or treated with 100 nM vitamin D for 24 h. The immunocomplexes were resolved by SDS-PAGE and analyzed by Western blotting with the VDR antibody.
Gel retardation assays.
Mobility shift assays were performed with 1 μl of in vitro-translated VDR and/or RXR in the presence and absence of recombinant Ets-1 (300 ng) and ACTR (600 ng) as previously described (10, 11). The VDRE oligonucleotide used was 5′-AGCTCAGGTCAAGGAGGTCAG-3′.
Limited proteolytic digestion.
In vitro-translated 35S-VDR (8 μl) or 35S-VDR(112–427) (8 μl) was incubated in the presence of 400 ng of GST–Ets-1, the same amount of GST alone, or 100 nM vitamin D for 20 min at room temperature. The receptors were then incubated for 2 min with increasing concentrations of trypsin, and the proteolytic fragments were separated and identified by autoradiography as described previously (11).
RESULTS
VDR shows a constitutive activity in the presence of Ets-1.
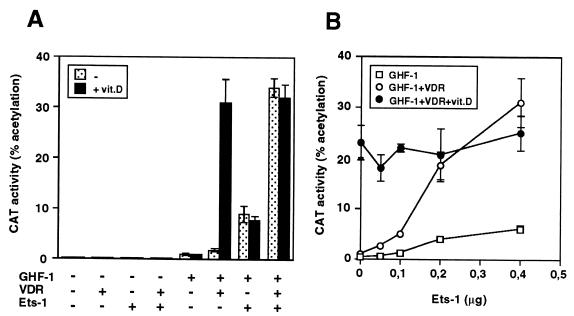
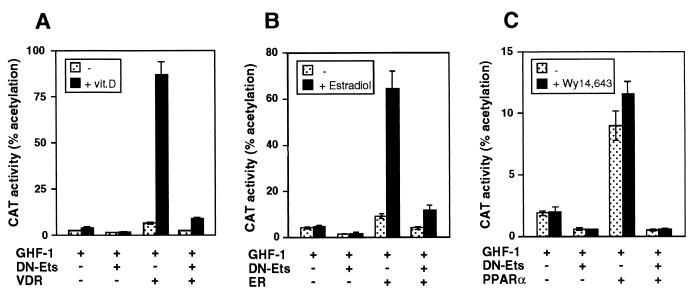
In order to analyze the influence of Ets factors on the transcriptional response to VDR, a plasmid containing the 5′-flanking region of the rat prolactin promoter was transiently transfected into HeLa cells with expression vectors encoding Ets-1, VDR, and/or GHF-1. Figure 1A shows that, in agreement with our previous observations (5), activation of the prolactin promoter construct −3000Prl-CAT by vitamin D requires the presence of GHF-1. Ets-1, which by itself caused little stimulation, had a synergistic effect with GHF-1, and this response was further induced upon expression of VDR. Remarkably, in the presence of Ets-1, VDR stimulated the promoter in a ligand-independent manner, behaving as a constitutive activator. Under these conditions, incubation with vitamin D did not cause a further increase. Figure 1B shows that activation by unliganded VDR was dependent on the amount of transfected Ets-1 being markedly enhanced as the concentration of Ets-1 increased. In the absence of Ets-1 there was a strong vitamin D-dependent stimulation. At intermediate concentrations of Ets-1, VDR stimulated the promoter in a ligand-independent manner and incubation with vitamin D was able to induce a further increase in transactivation. However, high levels of Ets-1 caused a strong ligand-independent activation, and a ligand-dependent response was not observed.
FIG. 1.
Ligand-independent activation of nuclear receptors by Ets-1. (A) HeLa cells were cotransfected with −3000Prl-CAT (5 μg) and vectors for GHF-1 (0.4 μg), VDR (2.5 μg), and/or Ets-1 (0.5 μg). (B) The reporter plasmid was cotransfected with GHF-1 and VDR in the presence of the indicated amounts of Ets-1. CAT activity was determined in untreated cells and cells treated with vitamin D for 48 h.
Ets-1 conferred ligand-independent activation to ERα and PPARα.
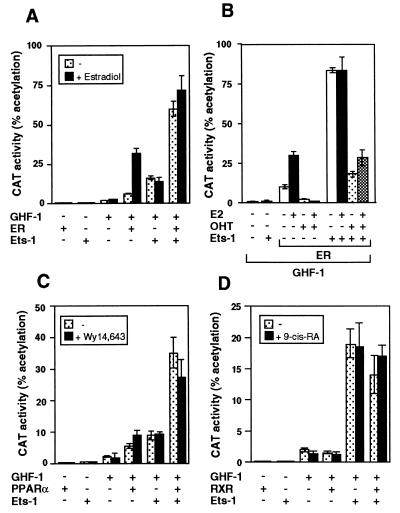
Figure 2 shows that Ets-1 also caused a constitutive activation of ERα and PPARα. Expression of ERα increased −3000Prl-CAT activity, and incubation with estradiol caused a further increase. On the other hand, expression of Ets-1 caused a strong transactivation by the unliganded ERα that was not further induced upon estradiol incubation (Fig. 2A). To analyze whether Ets-1 was able to induce ERα activity also when the receptor was bound to an antagonist, prolactin promoter activity was also determined in cells transfected with ERα and incubated with estradiol and/or 4-hydroxytamoxifen (OHT). As shown in Fig. 2B, OHT treatment markedly reduced basal CAT activity as well as estradiol-induced stimulation in the absence of Ets-1. After expression of Ets-1, the unliganded ERα stimulated the promoter even in the presence of OHT. Although promoter activity was lower in cells incubated with the antagonist, stimulation of ERα transcriptional activity by Ets-1 in cells incubated with OHT was similar to that in cells incubated with estradiol when activity was expressed as fold induction. Furthermore, identical results were obtained with the pure antiestrogen ICI182.780, which blocks not only AF2 but also AF1 functions (not illustrated).
FIG. 2.
Ets-1 causes constitutive activation of ERα and PPARα. (A) Cells were transfected with −3000Prl-CAT (5 μg) and vectors for GHF-1 (0.4 μg) and Ets-1 (0.5 μg) alone or in combination with 2.5 μg of ERα. CAT activity was determined in cells treated in the presence and absence of estradiol (1 μM) for 48 h. (B) cells were transfected with the same vectors and treated with 10 nM estradiol (E2) and/or 1 μM antagonist OHT. (C and D) The reporter plasmid was cotransfected with the same amounts of GHF-1 and Ets-1 together with 5 μg of PPARα (C) or 1 μg RXR (D) vectors. CAT activity was determined in untreated cells and in cells treated with Wy14,643 or 9-cis-retinoic acid.
As shown in Fig. 2C, PPARα also activated the prolactin promoter, and this response was only slightly increased by Wy14,643, a PPAR activator. This may reflect the presence of endogenous PPAR ligands in the cells (25). Ets-1 also had a strong synergistic effect with PPARα, which was not induced further in the presence of Wy14,643. The influence of Ets was also examined in cells transfected with RXR. As shown in Fig. 2D, RXR did not affect prolactin promoter activity and cooperation with Ets-1 was not observed.
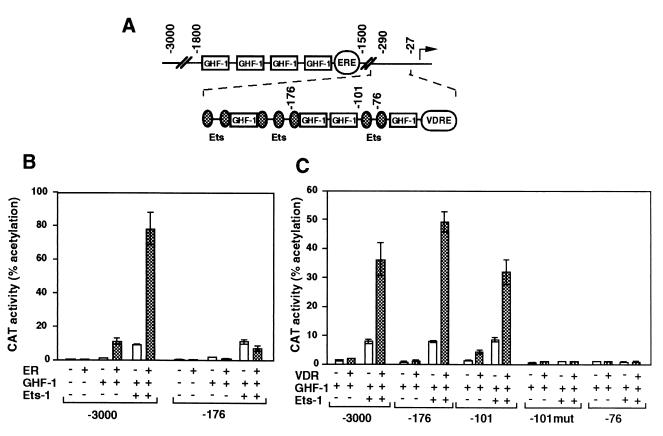
Stimulation of prolactin gene transcription by ER is mediated by an ERE located in a distal enhancer between nucleotides −1592 and −1580 (16), whereas the proximal promoter contains the sequences responsible for VDR responsiveness (5) (Fig. 3A). Accordingly, a promoter construct extending to bp −3000 exhibited ligand-independent activation upon cotransfection with expression vectors for Ets-1 and either ER (Fig. 3B) or VDR (Fig. 3C). However, the response to ER was lost in cells transfected with the −176Prl-CAT plasmid, which contains three Ets-binding sites but which does not contain the ERE. In contrast, the response of VDR to Ets-1 was maintained with the −176Prl-CAT and −101Prl-CAT constructs but was lost in a construct with a deletion to bp −76 which contains a unique Ets binding site. This construct was not stimulated by GHF-1 either. Mutation of the Ets sites in the plasmid extending to bp −101 (−101mut Prl-CAT) also abolished regulation.
FIG. 3.
Influence of Ets-1 on different prolactin promoter fragments. (A) Schematic representation of the rat prolactin 5′-flanking region showing the structure of the distal enhancer (bp −1500 to −1800) and the proximal promoter region. Binding sites for GHF-1 and Ets factors, as well as ERE and VDRE are depicted. (B and C) Cells were transfected with 5 μg of reporter CAT constructs and 0.5 μg of Ets-1 alone or in combination with unliganded ER (B) or VDR (C). The reporter plasmids have progressive deletions of the prolactin promoter (from bp −3000 to −76), and in the −101mut construct the Ets binding sites have been mutated (4). CAT activities were determined 48 h after transfection.
Ets-1 cooperates with unliganded receptors to stimulate other HRE-containing promoters.
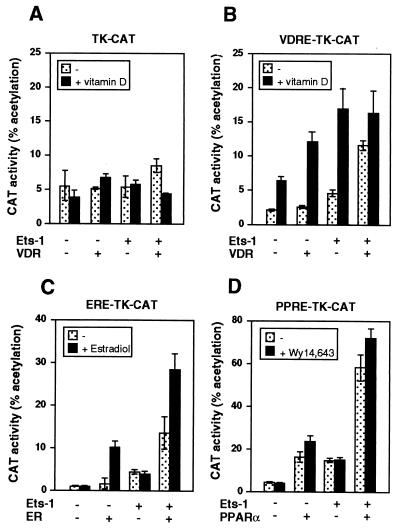
In order to analyze whether activation by the unoccupied receptors in the presence of Ets-1 is independent of the promoter context, reporter constructs containing consensus response elements for VDR, ER, or PPAR ligated to the TK promoter were transfected into HeLa cells. A TK-CAT construct that does not contain a response element was also used as a control. The TK promoter was not significantly stimulated by VDR either in the absence or presence of ligand or Ets-1 (Fig. 4A). In contrast, the activity of the same plasmid containing a VDRE was stimulated by vitamin D, and this response increased after expression of either VDR or Ets-1. Similar to the results obtained with the prolactin promoter, the unliganded receptor caused a significant increase in promoter activity only in cells expressing Ets-1. In addition, vitamin D-dependent stimulation was less marked in cells expressing both the VDR and Ets-1 (Fig. 4B). ERα also cooperated with Ets-1 to stimulate in a ligand-independent manner the ERα-containing TK-CAT plasmid, although some ligand-dependent activity was observed upon incubation with estradiol (Fig. 4C). PPARα increased constitutively the activity of the PPRE-TK-CAT construct, and expression of Ets-1 also significantly induced ligand-independent activity (Fig. 4D).
FIG. 4.
Ets increases activity of unliganded receptors in other promoter constructs. HeLa cells were transfected with 5 μg of a reporter CAT plasmid under control of the TK promoter (TK-CAT) (A) or with the same plasmid containing a consensus VDRE, PPRE, or ERE. These plasmids were cotransfected with 0.5 μg of Ets-1 and 2.5 μg of expression vectors for VDR (A and B), ERα (C), or PPARα (D). CAT activity was determined in cells incubated with or without vitamin D, estradiol, or Wy14,643, respectively.
Influence of a dominant-negative Ets vector on transactivation of the prolactin promoter by nuclear receptors.
Above results demonstrate that exogenous expression of Ets factors plays an important role in activation of the prolactin promoter. To examine the role of endogenous Ets factors on the regulation of basal and receptor-mediated stimulation of the prolactin promoter, a dominant-negative Ets construct which lacks the transactivation domain was employed in cotransfection assays (Fig. 5). For this purpose, HeLa cells were transfected with −3000Prl-CAT and an expression vector encoding the ETS domain of Ets-2. The DBD is highly conserved among the members of the Ets family, and therefore overexpression of this domain interferes with the action of the different Ets factors. Expression of dominant-negative Ets had little effect on basal promoter activity, which was essentially undetectable in the absence of GHF-1 (data not shown), and reduced significantly GHF-1-mediated activation. In addition, the dominant-negative vector interfered strongly with VDR and vitamin D-dependent stimulation (Fig. 5A). Similar results were obtained in cells transfected with ER (Fig. 5B), in which expression of this vector abolished estrogen regulation. The dominant-negative Ets also neutralized the responses to PPARα, which again were similar in the presence and absence of Wy14,643 (Fig. 5C). Therefore, endogenous Ets factors play a crucial role not only in basal activity but also in activation of the prolactin promoter by the nuclear receptors.
FIG. 5.
A dominant-negative Ets inhibits receptor-mediated stimulation. Cells were transfected with −3000Prl-CAT (5 μg) and GHF-1 (0.4 μg), alone or in combination with VDR (A), ERα (B), or PPARα (C). Five micrograms of a vector encoding a dominant-negative Ets vector (DN-Ets) was cotransfected as indicated. Activity was determined in untreated cells and cells treated with vitamin D, estradiol, or Wy14,643.
Ets-1 confers constitutive activity to AF2-defective receptor mutants.
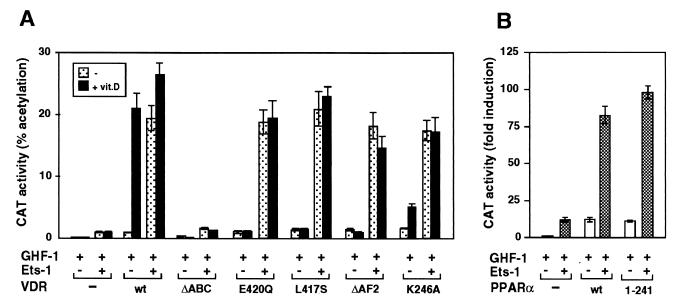
We have previously shown that coactivators which bind to the AF2 domain of VDR play an important role in vitamin D-dependent stimulation of the prolactin promoter (5). To evaluate the role of the AF2 domain in activation by Ets-1, we used the AF2-defective mutants VDR-ΔAF2, L417S, E420Q, and K246A. These mutants are unable to recruit coactivators in a vitamin D-dependent manner (5, 11). Figure 6A shows the effect of cotransfection of VDR expression plasmids with Ets-1 on the induction of −3000Prl-CAT by vitamin D. In the absence of Ets-1, vitamin D caused a strong stimulation in cells transfected with wild-type VDR, whereas the AF2 mutants exhibited no vitamin D-dependent activation. In contrast, in the presence of Ets-1, the AF2-defective VDR mutants were able to activate the prolactin promoter in a ligand-independent manner with the same potency as that of the wild-type receptor. These results show that, in contrast with the actions of vitamin D, activation by Ets-1 is independent of the AF2 domain. An N-terminally truncated VDR (the ΔABC mutant) which lacks 111 N-terminal amino acids displayed neither vitamin D-dependent activation nor constitutive activity in the presence of Ets-1. That the nuclear AF2 receptor domain is not required for stimulation by Ets-1 is also shown by the results obtained with a PPARα truncated in the hinge region (Fig. 6B). PPARα(1–241), which totally lacks the ligand binding domain (LBD), stimulated as efficiently as the native receptor the activity of −3000Prl-CAT in the presence of Ets-1.
FIG. 6.
Ets-1 confers activation to AF2-defective VDR mutants. (A) −3000Prl-CAT was transfected into HeLa cells together with vectors encoding GHF-1 (0.4 μg), Ets-1 (0.5 μg), and wild-type VDR (wt) or the VDR mutants indicated (2.5 μg each). VDR-ΔAF2 lacks the C-terminal helix 12 of the LBD, and ΔABC lacks the 110 N-terminal VDR residues. Two different point mutations in helix 12 (L417S and E420Q), as well as mutation K246A in helix 3, were also used. CAT activity was determined in untreated cells and in cells incubated with vitamin D. (B) The Prl-CAT plasmid was cotransfected with an expression vector for the native PPARα (wt) or for PPARα(1–241), which lacks the LBD. CAT activities were determined 48 h later.
Influence of the coactivators SRC-1 and CBP on ligand-dependent and constitutive activation.
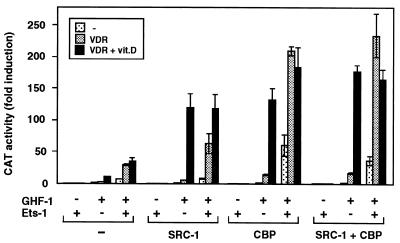
To analyze whether coactivators could also modulate Ets-1-mediated stimulation, the cells were transfected with expression vectors for VDR, GHF-1, and the coactivators SRC-1 and CBP in the presence and absence of Ets-1. Figure 7 shows the functional effects of these factors on prolactin promoter stimulation. Basal promoter activity was essentially undetectable in cells expressing Ets-1 alone or in combination with the coactivators unless GHF-1 was expressed. However, in the presence of the pituitary factor and in the absence of transfected Ets-1, SRC-1 acted as a potent ligand-dependent coactivator for VDR, enhancing very significantly the response to vitamin D. Ets-1 increased constitutive activation by VDR, and expression of the coactivators potentiated Ets-mediated constitutive activity of VDR, but vitamin D was not able to stimulate reporter activity above the levels found in the absence of Ets-1. For CBP, a strong synergistic response with Ets-1 was found even in the absence of VDR. This result agrees with the idea that CBP is also a coactivator for Ets-1 (33) and GHF-1 (25, 32, 34). This response was further induced in cells expressing unliganded VDR. However, again the activity of VDR was constitutive, and CBP, which potentiates very significantly vitamin D-dependent transactivation in the absence of Ets-1, was not able to elicit a ligand-dependent response when Ets was present. The results obtained with the combination of SRC-1 and CBP were similar to those found with CBP alone.
FIG. 7.
Expression of coactivators potentiates Ets-mediated constitutive activity of VDR. The prolactin reporter plasmid −3000Prl-CAT and expression vectors for GHF-1 (0.4 μg), VDR (2.5 μg), and Ets-1 (0.5 μg) were transfected alone or in combination with 2 μg of vectors for the coactivators SRC-1 and CBP, as indicated. CAT activity was determined in cells treated for 48 h in the presence and absence of vitamin D.
Ets-1 interacts with the receptors.
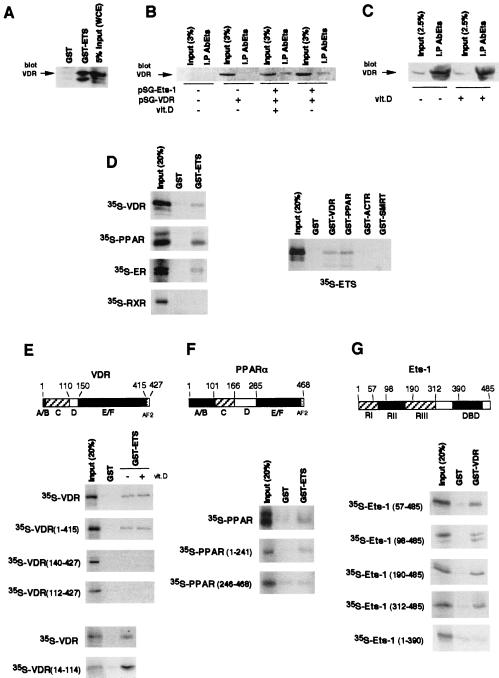
The finding that Ets-1 stimulates the transcriptional activity of VDR is compatible with the existence of an interaction between them. To test this interaction, extract from HeLa cells transfected with VDR was incubated with GST–Ets-1. This fusion protein, but not GST alone, interacted with VDR (Fig. 8A). To prove that Ets and VDR can associate in vivo, cells were transfected either with an empty vector or with vectors encoding Ets-1 and/or VDR. Extracts were immunoprecipitated with an anti-Ets antibody, and the amount of VDR in the precipitates was determined by Western blotting. As shown in Fig. 8B, VDR was undetectable in the immunoprecipitates from cells transfected with the empty vector (lane 2) or with VDR alone (lane 4). In contrast, a band corresponding to VDR was readily detected in cells both untreated and treated with vitamin D and coexpressing Ets-1 and VDR (lanes 6 and 8).
FIG. 8.
Interaction of Ets-1 with nuclear receptors. (A) GST or GST-ETS was incubated with 900 μg of WCE, and the VDR bound was analyzed by Western blotting. The input represents 5% of proteins used. (B) HeLa cells were transfected with an empty vector or with vectors encoding Ets-1 and/or VDR. Immunoprecipitates with the anti-Ets antibody (AbEts) from cells treated in the presence and absence of vitamin D were subjected to Western analysis with the VDR antibody together with 3% of the WCE used (input). (C) VDR was detected by Western analysis in immunoprecipitates from untreated and vitamin D-treated GH4C1 cells. Input represents 2.5% (125 μg) of the WCE used. (D, left) Pull-down assays were performed with GST alone or GST-ETS and different in vitro-translated 35S-labeled receptors. (Right) In vitro-translated Ets-1 was used in pull-down experiments with GST-fused VDR and PPARα as well as with the receptor-interacting domains of the receptor coactivator ACTR and the corepressor SMRT. The inputs represent 20% of the proteins used. (E and F) Representation of VDR and PPARα, showing the different functional domains. The indicated 35S-VDR and PPARα deletion mutants were used in pull-down assays with GST and GST-ETS. The pull-down assays with labeled VDR were performed in the presence and absence of vitamin D (1 μM). (G) Schematic representation of the p68 Ets-1 protein. RI and RIII, transcriptional activation domains; RII, regulatory domain; DBD, DNA binding ETS domain. The 35S-Ets-1 fragments indicated were used in pull-down assays with GST or GST-VDR.
The lack of a detectable interaction between VDR and Ets-1 in untransfected HeLa cells is most likely due to the low levels of expression of these proteins in this cell type (2). However, the association between VDR and Ets-1 in transfected HeLa cells could represent a nonspecific association between the overexpressed proteins. To demonstrate that this interaction could also occur in vivo in nontransfected cells, coimmunoprecipitation experiments were also performed in prolactin-producing pituitary GH4C1 cells which express high levels of Ets-1 (2) and VDR (10). As shown in Fig. 8C, a strong association between both endogenous factors was found, as demonstrated by the presence of an intense VDR band in the Ets-1 immunoprecipitates. Therefore, association between VDR and Ets-1 can be also detected in vivo in pituitary cells under physiological conditions.
A direct interaction between Ets-1 and the receptors was demonstrated by pull-down studies using GST–Ets-1 and 35S-labeled receptors (Fig. 8D). 35S-VDR, 35S-PPARα, and 35S-ERα bound specifically to GST–Ets-1, whereas 35S-RXR did not bind to this factor. Interaction of 35S-Ets-1 with GST-VDR and GST-PPARα confirmed the association. The nuclear receptor-interacting domains of the coactivator ACTR and the corepressor SMRT, used as controls, did not interact with Ets-1 in the assay.
A series of 35S-VDR mutants was used to delineate the domains involved in the association with Ets-1. Figure 8E shows that VDR bound GST–Ets-1 with similar strengths in the presence and absence of vitamin D. Deletion of helix 12 of the LBD (residues 415 to 427), which contains the core AF2, did not affect interaction with Ets-1. In contrast, deletion of the 140 N-terminal residues abolished this interaction. Similar results were obtained with a truncated VDR lacking the first 111 residues. This truncation eliminates the A/B domain, which is an atypical region in VDR since it contains only 20 amino acids, and the C domain, which contains the DBD. To dismiss the possibility that the short A/B region could participate in binding to Ets-1, a pull-down study with GST–Ets-1 and the 35S-labeled DBD of VDR (amino acids 14 to 114) was also carried out. The results obtained demonstrated that Ets-1 interacted even more strongly with the DBD alone than with the complete receptor. The interaction between PPARα and Ets-1 also mapped to the N terminus of the receptor. Whereas 35S-Ets-1 interacted specifically with PPRα(1–241), no specific interaction between this transcription factor and PPARα(246–468), which contains the LBD, was found (Fig. 8F).
The domain of Ets-1 involved in association with VDR was also mapped by using 35S-Ets-1 deletion mutants (Fig. 8G). Deletion of 98, 190, or 312 N-terminal residues did not affect interaction with GST-VDR. This shows that the transcription activation domains, as well as the regulatory domain of Ets-1, are dispensable for association with the receptor. In contrast, truncation of the 95 C-terminal amino acids, which deletes the DBD, abolishes interaction.
Ets-1 causes conformational changes in VDR.
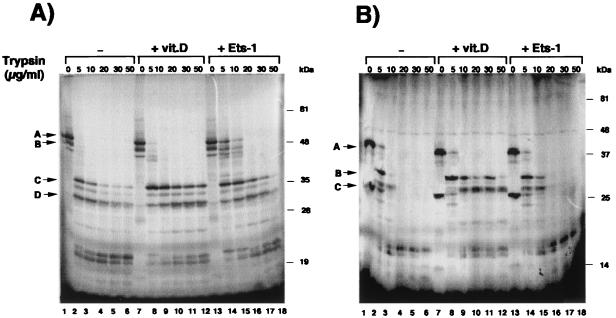
The association of Ets-1 with VDR could induce a conformational change in the receptor. Previous studies have shown that differences in the conformations of unoccupied and ligand-occupied VDR can be detected by an increased resistance to limited proteolytic digestion (11). We therefore tested whether interaction with Ets-1 might also induce differences in protease sensitivity. The influence of incubation with vitamin D or Ets-1 on tryptic digestion patterns of 35S-VDR is shown in Fig. 9A. The unoccupied VDR is highly sensitive to proteolysis (lanes 1 to 6). When the receptor is occupied with vitamin D, the proteolysis of several resistant fragments with molecular masses between 30 and 38 kDa is inhibited (lanes 7 to 12). Incubation with Ets-1 also strongly increased resistance to tryptic digestion. While in the absence of Ets-1 no undigested receptor remained upon incubation of VDR with 5 and 10 μg of trypsin/ml, a significant fraction of the receptor was undigested in the presence of Ets-1 (lanes 14 and 15). With higher trypsin concentrations stabilization of the smaller-size fragments was also noticed. As a control that a VDR protein that does not interact with Ets-1 does not alter the pattern of proteolysis, a similar experiment was performed with the N-terminally truncated VDR(112–427). This receptor lacks the DBD and, as shown in Fig. 8E, does not interact with Ets-1. As illustrated in Fig. 9B, whereas incubation with vitamin D increased the resistance of the truncated VDR to trypsin digestion, Ets-1 did not alter the proteolytic pattern.
FIG. 9.
Ets-1 induces a conformational change in VDR. (A) 35S-VDR preincubated with GST (lanes 1 to 6), GST–Ets-1 (lanes 13 to 18), or vitamin D (lanes 7 to 12) was digested with increasing concentrations of trypsin and analyzed by SDS-PAGE and autoradiography. Arrows A and B, undigested VDR; arrows C and D, resistant protein fragments. (B) The 35S-VDR fragment spanning residues 112 to 427 was used in a similar experiment. Arrow A, mobility of the undigested VDR fragment; arrows B and C, main fragments resistant to proteolysis.
Ets-1 causes AF2-independent recruitment of coactivators.
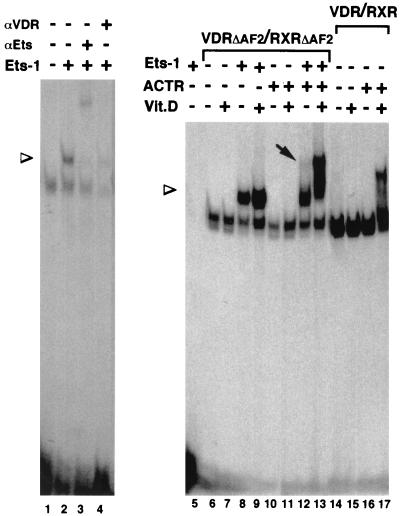
The conformational change in VDR caused by association with Ets-1 could promote an AF2-independent coactivator recruitment. This possibility was explored by mobility shift assays performed with the VDRE in the presence of a RXRΔAF2-VDRΔAF2 heterodimer (in which both receptors lack helix 12), Ets-1, and the coactivator ACTR (Fig. 10). Ets-1 does not bind to the VDRE (lane 5), and association with Ets-1 does not allow binding of VDR alone or in combination with ACTR, as no binding to the element was found unless RXR was present (data not shown). However, the RXRΔAF2-VDRΔAF2 heterodimer bound readily to the VDRE, and the presence of Ets-1 caused the appearance of a superretarded band with a slower mobility (lane 2). The mobility of the supershifted complex was further retarded by an anti-Ets antibody (lane 3), and formation of the complex was reversed by an anti-VDR antibody (lane 4), demonstrating that this band represents a ternary complex containing Ets-1 and the heterodimer. This result demonstrates again the existence of a direct interaction between Ets-1 and the receptors. The coactivator ACTR was not recruited by the defective receptors in the absence of Ets-1 (lane 10). However, in the presence of Ets-1, the RXRΔAF2-VDRΔAF2 heterodimer was able to cause a ligand-independent recruitment of ACTR detectable as a weak supershifted complex (lane 12). As expected, ACTR did not bind the AF2-defective receptors upon incubation with vitamin D (lane 11), whereas a ligand-dependent recruitment of ACTR to a native RXR-VDR heterodimer which contains the AF2 domains was readily observed (lanes 14 to 17). The binding of AF2-defective heterodimers to the coactivator in the presence of Ets-1 was further increased in the presence of vitamin D, resulting in the formation of a strong complex with a low mobility (lane 13). These results suggest that, indeed, association with Ets-1 can cause an AF2-independent recruitment of coactivators that could account for the receptor activation.
FIG. 10.
Association with Ets-1 allows an AF2-independent recruitment of coactivators. Shown are gel retardation assays with the VDRE oligonucleotide and in vitro-translated VDR and/or RXR. Receptors VDR(1–415) (VDRΔAF2) and RXR(1–445) (RXRΔAF2), lacking helix 12, as well as wild-type receptors (lanes 14 to 17) were used. The assays were performed in the presence and absence of recombinant Ets-1 (300 ng) and/or the p160 coactivator ACTR (600 ng). When indicated, vitamin D (100 nM) was present in the binding reaction mixtures. The presence of VDR and Ets-1 in the complexes was analyzed by incubation with 1 μl of specific antibodies (αVDR and αEts, respectively). Arrowheads, mobilities of the supershifted complexes containing the receptor heterodimer and Ets-1; arrow, appearance of a retarded complex with the RXRΔAF2-VDRΔAF2 heterodimer in the presence of ACTR.
DISCUSSION
Activation of nuclear receptors is normally dependent on ligand binding. In the present work, using the stimulation of the prolactin promoter as a model, we demonstrate the existence of a novel mechanism of ligand-independent activation of VDR that involves interaction with Ets transcription factors. We show that VDR transactivation by Ets-1 is associated with a direct physical interaction between both proteins which maps to the receptor DBD and the C terminus of Ets-1, which also contains the DBD. Ets-1 also causes a constitutive activation of other nuclear receptors, such as ERα or PPARα, and interacts in vitro with these receptors. However, the action of Ets-1 does not extend to all nuclear receptors, as RXR does not associate with Ets. Stimulation of ERα by Ets-1 appears to require the prolactin distal enhancer that contains the ERE (16), whereas stimulation of VDR maps to proximal promoter sequences in which a VDRE has been identified (5). The Ets binding sites in the promoter also appear to be important for stimulation, as mutation of these sites abolishes stimulation by either the receptors, Ets-1, or the pituitary transcription factor GHF-1.
The particular structure of the prolactin promoter, which contains several Ets binding sites as well as binding elements for VDR and GHF-1, appears to facilitate constitutive receptor activation by Ets-1. It is therefore likely that cooperativity between Ets-1 and VDR can be influenced by the spacing of their respective binding sites. However, we have found that the influence of Ets-1 on the receptor is not restricted to the prolactin promoter, as it also provokes a ligand-independent activation of reporter genes in which response elements for the receptors are fused to an heterologous promoter. The existence of Ets binding sites in the TK promoter has not been documented, and no significant stimulation by Ets-1 of this promoter in the absence of an HRE was found. However, some stimulation by Ets-1 was observed when such an element was ligated to the promoter, suggesting that endogenous receptors could synergize with this transcription factor. Stimulation of these constructs indicates that Ets-1 could be involved in stimulation of other HRE-containing genes and that therefore interaction with this factor could represent a more general mechanism of receptor activation. However, there were some differences between the activation of constructs containing the consensus response elements and the activation of the prolactin promoter. Although Ets-1 clearly increased constitutive activity of the unoccupied receptors in the VDRE- or ERE-containing plasmids, incubation with vitamin D or estradiol caused a further transcriptional stimulation. This was not the case with the prolactin promoter, in which constitutive stimulation was very strong and did not increase upon ligand binding. It is possible that this promoter context-specific response could reflect a differential sensitivity to Ets-1, since stimulation of the prolactin promoter was also partially ligand dependent when the receptors were transfected with smaller amounts of Ets-1.
We have previously shown that truncation of VDR helix 12 abolishes stimulation of the prolactin promoter by vitamin D and that expression of the coactivators SRC-1 and CBP very significantly enhances the stimulatory effect of vitamin D mediated by the wild-type VDR but not by the AF2 mutant receptor (5). This suggests that AF2-dependent recruitment of coactivators indeed mediates ligand-dependent stimulation. This conclusion is further supported by the results obtained in the present study with different VDR point mutants. Thus, mutation of conserved residues both in helix 12 and in helix 3 which are required for the recruitment of coactivators and AF2 activity of VDR (11) severely compromised vitamin D-dependent transactivation. In contrast, these mutations did not affect activation of VDR by Ets-1. The finding that Ets-1 causes stimulation of transcription in the AF2-defective mutants demonstrates that vitamin D and Ets-1 require different regions of the receptor to achieve their effects upon transcription and that, in contrast with the actions of vitamin D, activation by Ets-1 appears to be independent of the AF2 domain.
Previous studies have shown that receptors activated by ligand binding as well as constitutively active mutant receptors show a structural condensation of the LBD that is manifested as an enhanced resistance to proteolytic digestion (11, 14, 28). Our data show that, when associated with Ets-1, VDR exhibited a strongly increased resistance to tryptic digestion in the absence of vitamin D. These data suggest a model in which interactions between VDR and Ets-1 trigger receptor activation by means of a conformational change.
The current view of transcriptional regulation by nuclear receptors is that the conformational changes elicited by ligand binding allow the recruitment of multicomponent coactivator complexes (17, 26). Ligand-independent activation by Ets-1 presumably requires similar changes. This notion is supported by our observation that, as assessed in vitro by gel retardation assays, interaction of Ets-1 with a receptor which lacks the C-terminal AF2 domain promotes some recruitment of the p160 coactivator ACTR in a vitamin D-independent manner. This is a most striking finding, even though, in contrast with the results obtained in vivo with the prolactin promoter, in which a maximal ligand-independent stimulation can be obtained in the presence of Ets-1, in vitro binding of the coactivator to the AF2-defective receptor still increased significantly in the presence of vitamin D. It is conceivable that this apparent discrepancy may simply reflect a lack of optimal folding of the recombinant proteins interacting with DNA or the need of additional factors required for optimal vitamin D-independent, Ets-dependent conformational transitions. On the other hand, these in vitro data correlate better with the results obtained with the HRE-containing heterologous promoter (Fig. 4) or with the prolactin promoter in cells expressing low concentrations of Ets-1 (Fig. 1B), where, besides an increase in ligand-independent stimulation, we also observe a ligand-dependent enhancement of transcription in the presence of Ets-1. This suggests again that the promoter architecture is important in determining the response to Ets-1. In the context of the prolactin promoter it is likely that interaction of the receptors not only with Ets factors but also with GHF-1 could favor the formation of complexes containing p160 coactivators and CBP, which may be stabilized by protein-protein interactions. This would lead to the formation of transcriptionally competent multicomponent complexes and to constitutive prolactin promoter stimulation.
In any case, our data suggest that Ets-1 induces a conformational change in the receptors which creates an active interaction surface with coactivators even in the AF2-defective mutants. In agreement with our results, recent observations have shown an AF2-independent recruitment of coactivators by nuclear receptors. For instance, phosphorylation of ERβ and SF-1 causes direct coactivator recruitment by the ligand-independent AF1 domain (8, 27), and it has also been demonstrated that members of the p160 family of coactivators interact weakly with the N-terminal regions of several receptors (13, 20, 30). However, this is observed with receptors having a strong constitutive activation function in the N terminus and is not the case with VDR, which has an extremely short A/B domain with no known AF1 activity (23). On the other hand, cyclin D1 can associate with ER and stimulate its transcriptional functions in the absence of estrogen. By acting as a bridging factor between ER and coactivators, cyclin D1 can also recruit p160 coactivators to ER in the absence of ligand (36). However, it should be noted that we have not observed an interaction between Ets-1 and ACTR and that therefore the mechanisms of receptor activation by cyclin D1 and Ets-1 appear to be different.
Taken together, the data presented in this work reveal the existence of a novel mechanism of receptor activation which involves interaction with Ets-1 and which can be independent of the classical ligand activation pathway and of coactivator recruitment by the AF2 domain. Since Ets transcription factors are targets of the Ras/mitogen-activated protein kinase signaling pathway and play an important role in the control of growth, development, and tumorigenesis, the functional interaction described here reveals the existence of a novel mode of cross talk between the nuclear receptors and other signaling pathways elicited by different extracellular stimuli which could have important physiological consequences.
ACKNOWLEDGMENTS
We thank R. Evans and M. Parker for plasmids used in this study. The Ets-1 fragments were a kind gift from A. Gutierrez-Hartmann.
This work was supported by grant PM97-0135 from the D.G.E.S., by grants 08.1/0032 and 08.6/0010.1/1999 from the Comunidad de Madrid, and by the Fundación Salud 2000 (Serono).
R.M.T. and A.I.C. contributed equally to this work.
REFERENCES
- 1.Bevan C L, Hoare S, Claessens F, Heery D M, Parker M G. The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC-1. Mol Cell Biol. 1999;19:8383–8392. doi: 10.1128/mcb.19.12.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford A P, Wasylyk C, Wasylyk B, Gutierrez-Hartmann A. Interaction of Ets-1 and the POU-homeodomain protein GHF-1/Pit-1 reconstitutes pituitary-specific gene expression. Mol Cell Biol. 1997;17:1065–1074. doi: 10.1128/mcb.17.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunone G, Briand P-A, Miksicek R J, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAPK pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo A I, Tolon R M, Aranda A. Insulin-like growth factor-1 stimulates rat prolactin gene expression by a Ras, ETS and phosphatidylinositol 3-kinase dependent mechanism. Oncogene. 1998;16:1981–1991. doi: 10.1038/sj.onc.1200204. [DOI] [PubMed] [Google Scholar]
- 5.Castillo A I, Jiménez-Lara A M, Tolon R M, Aranda A. Synergistic activation of the prolactin promoter by vitamin D and GHF-1: role of the coactivators CBP and SRC-1. Mol Endocrinol. 1999;13:1141–1154. doi: 10.1210/mend.13.7.0320. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalski M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a histone acetyltransferase and forms a multimeric activation complex with p/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 7.Chen J D, Evans R M. A transcriptional corepressor that interacts with nuclear hormone receptors. Nature. 1995;377:455–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 8.Hammer G D, Krylova I, Zhang Y, Darimont B D, Simpson K, Weigel N L, Ingraham H A. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Yuan C X, Malik S, Gu W, Fondell J D, Yamamura S, Fu Z Y, Zhang X, Qin J, Roeder R G. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol Cell. 1999;3:361–370. doi: 10.1016/s1097-2765(00)80463-3. [DOI] [PubMed] [Google Scholar]
- 10.Jiménez-Lara A M, Aranda A. The vitamin D receptor binds in a transcriptionally inactive form and without a defined polarity on a retinoic acid response element. FASEB J. 1999;13:1073–1081. doi: 10.1096/fasebj.13.9.1073. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez-Lara A M, Aranda A. Lysine-246 of the vitamin D receptor is crucial for ligand-dependent interaction with coactivators and transcriptional activity. J Biol Chem. 1999;274:13503–13510. doi: 10.1074/jbc.274.19.13503. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 13.Lavinsky R M, Jepsen K, Heinzel T, Torchia J, Mullen T M, Schiff R, Del-Rio A L, Ricote M, Ngo S, Gemsch J, Hilsenbeck S G, Osborne C K, Glass C K, Rosenfeld M G, Rose D W. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA. 1998;95:2920–2925. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazennec G, Ediger T R, Petz L N, Nardulli A M, Katzenellenbogen B S. Mechanistic aspects of estrogen receptor activation probed with constitutively active estrogen receptors: correlations with DNA and coregulator interactions and receptor conformational changes. Mol Endocrinol. 1997;11:1375–1386. doi: 10.1210/mend.11.9.9983. [DOI] [PubMed] [Google Scholar]
- 15.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer R A, Notides A C. Identification of an estrogen-responsive element from the 5′-flanking region of the rat prolactin gene. Mol Cell Biol. 1987;7:4247–4254. doi: 10.1128/mcb.7.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenna N J, Lanz R B, O'Malley B W. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 18.McMahon C, Suthiphongchai T, DiRenzo J, Ewen M E. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc Natl Acad Sci USA. 1999;96:5382–5387. doi: 10.1073/pnas.96.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberste-Berghaus C, Zanger K, Hashimoto K, Cohen R N, Hollenberg A N, Wondisdorf F E. Thyroid hormone-independent interaction between the thyroid hormone receptor beta2 amino terminus and coactivators. J Biol Chem. 2000;275:1787–1792. doi: 10.1074/jbc.275.3.1787. [DOI] [PubMed] [Google Scholar]
- 20.Oñate S A, Boonyaratanakorkit V, Spencer T E, Tsai S Y, Tsai M-J, Edwards D P, O'Malley B W. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptor. J Biol Chem. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- 21.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Naar A M, Erdjument-Bromage H, Tempst P, Freedman L P. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 22.Rochette-Egly C, Adam S, Rossignol M, Egly J-M, Chambon P. Stimulation of RARα activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell. 1997;90:97–107. doi: 10.1016/s0092-8674(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 23.Sone T, Kerner S, Pike J W. Vitamin D receptor interaction with specific DNA: association as a 1,24-hydroxyvitamin D3-modulated heterodimer. J Biol Chem. 1991;266:23296–23305. [PubMed] [Google Scholar]
- 24.Taneja R, Rochette-Egly C, Plassat J L, Penna L, Gaub M P, Chambon P. Phosphorylation of activation functions AF-1 and AF-2 of RARα and RARγ is indispensable for differentiation of F9 cells upon retinoic acid and cAMP treatment. EMBO J. 1997;16:6452–6455. doi: 10.1093/emboj/16.21.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolon R M, Castillo A I, Aranda A. Activation of the prolactin gene by peroxisome proliferator activated receptor-α appears to be DNA binding-independent. J Biol Chem. 1998;273:26652–26661. doi: 10.1074/jbc.273.41.26652. [DOI] [PubMed] [Google Scholar]
- 26.Torchia J, Glass C K, Rosenfeld M G. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 27.Tremblay A, Tremblay G B, Labrie F, Guiguère V. Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 28.Vivat V, Zechel C, Wurtz J M, Bourguet W, Kagechika H, Umemiya H, Shudo K, Moras D, Gronemeyer H, Chambon P. A mutation mimicking ligand-induced conformational change yields a constitutive RXR that senses allosteric effects in heterodimers. EMBO J. 1997;15:5697–5709. doi: 10.1093/emboj/16.18.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasylyk B, Hagman J, Gutiérrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 30.Webb P, Neguyen P, Sinshako J, Anderson C, Feng W, Nguyen M P, Chen D, Huang S-M, Subramanian S, McKinerney E, Katzenellenbogen B, Stallcup M R, Kushner P J. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- 31.Weigel N L, Zhang Y. Ligand-independent activation of steroid hormone receptors. J Mol Med. 1998;76:469–479. doi: 10.1007/s001090050241. [DOI] [PubMed] [Google Scholar]
- 32.Xu L, Lavinsky R M, Dasen J S, Flyn S E, McInerney E M, Mullen T-M, Heinzel T, Szeto D, Korzus E, Kurokawal R, Aggarwal A K, Rose D W, Glass C K, Rosenfeld M G. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- 33.Yang C, Shapiro L H, Ribera M, Kumar A, Brindle P K. A role for CREB binding protein and p300 transcriptional coactivators in Ets-1 transactivation functions. Mol Cell Biol. 1998;18:2218–2229. doi: 10.1128/mcb.18.4.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanger K, Cohen L E, Hashimoto K, Radovick S, Wondisdorf F E. A novel mechanism for cyclic adenosine 3′,5′-monophosphate regulation of gene expression by CREB-binding protein. Mol Endocrinol. 1999;13:268–275. doi: 10.1210/mend.13.2.0245. [DOI] [PubMed] [Google Scholar]
- 35.Zwijsen R M L, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides R. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 36.Zwijsen R M L, Buckle R S, Hijmans E M, Loomans C J M, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]