Abstract
Cardiovascular disease accounts for more than 17 million deaths globally every year, of which complications of hypertension account for 9.4 million deaths worldwide. Early detection and management of hypertension can prevent costly interventions, including dialysis and cardiac surgery. Non-pharmacological approaches for managing hypertension commonly involve lifestyle modification, including exercise and dietary regulations such as reducing salt and fluid intake; however, a majority of patients will eventually require antihypertensive medications. In 2020, the International Society of Hypertension published worldwide guidelines in its efforts to reduce the global prevalence of raised blood pressure (BP) in adults aged 18 years or over. Currently, several classes of medications are used to control hypertension, either as mono- or combination therapy depending on the disease severity. These drug classes include those that target the renin-angiotensin-aldosterone system (RAAS) and adrenergic receptors, calcium channel blockers, diuretics and vasodilators. While some of these classes of medications have shown significant benefits in controlling BP and reducing cardiovascular mortality, the prevalence of hypertension remains high. Significant efforts have been made in developing new classes of drugs that lower BP; these medications exert their therapeutic benefits through different pathways and mechanism of actions. With several of these emerging classes in phase III clinical trials, it is hoped that the discovery of these novel therapeutic avenues will aid in reducing the global burden of hypertension.
Key Points
| Globally, there are 1.3 billion patients with hypertension, of whom fewer than one in five have their blood pressure (BP) under control. |
| While several drug classes are available for the management of raised BP, the prevalence of hypertension continues to increase in pandemic proportions. |
| New research has identified novel pathways and mechanisms for lowering BP. |
| Despite the disruption caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in the conception, development and delivery of these novel drugs, several agents in preclinical trials are showing considerable promise in adequately controlling raised BP. |
Introduction
Hypertension is a multifactorial, complex disorder estimated to affect one in three adults in the United States (US) [1]. Globally, the prevalence of hypertension is expected to reach 1.6 billion by 2025 [2]. Several factors influencing elevated blood pressure (BP) have been identified. Modifiable risk factors affecting BP include diet, obesity, smoking and physical exercise, while non-modifiable factors include increasing age, sex and genetics. Adequately controlling high BP reduces the risk of stroke, coronary artery disease, peripheral vascular disease, congestive heart failure and end-stage renal failure. The risk of developing these complications can start at a BP level as low as 115/75 mmHg [3]. In 2017, the American College of Cardiology (ACC) and American Heart Association (AHA) released updated guidelines in which they lowered the traditional BP cut-off value for diagnosing hypertension (Table 1) [4]. Accurately diagnosing hypertension can still pose significant challenges for physicians, as BP readings can fluctuate unexpectedly, a phenomenon referred to as labile hypertension. In 2020, the International Society of Hypertension produced global guidelines to enable physicians to diagnose and manage hypertension as accurately as possible [5]. These guidelines classified hypertension based on systolic and diastolic cut-offs, taking into account the manner and environment in which the BP measurements were taken.
Table 1.
Stages of hypertension as defined by the ACC/AHA [4]
| Stage | Blood pressure (mmHg) |
|---|---|
| Normal | < 120/< 80 |
| Elevated | 120–129/<80 |
| Stage 1 | 130–139/80–89 |
| Stage 2 | ≥ 140/≥ 90 |
| Hypertensive crisis | > 180/> 120 |
ACC American College of Cardiology, AHA American Heart Association
The exact pathophysiology of hypertension remains largely unknown. It is thought that up to 5% of patients with hypertension have either renal or adrenal system impairment. In the remainder of patients, the cause is not entirely clear and these patients are labelled as having ‘essential hypertension’. The regulation of BP relies on cardiac output and systemic vascular resistance. Most patients with essential hypertension have normal cardiac output but raised peripheral resistance. Peripheral resistance is determined by the small arterioles which contain smooth muscle. A rise in intracellular calcium leads to contraction of these smooth muscles, which in turn raises peripheral resistance. The autonomic nervous system is also thought to play an important role in increasing BP. Sympathetic nervous system (SNS) stimulation can lead to an increase in cardiac output and both arteriolar constriction and dilation. The SNS has an important function in short-term changes in BP that occur in response to physical exercise, however there is a lack of evidence suggesting its involvement in the development of chronic hypertension. It has also been suggested that endothelial dysfunction can lead to essential hypertension. Currently available antihypertensive agents exert their therapeutic effect by altering these underlying pathologic processes. These drug classes include those that target the renin-angiotensin-aldosterone system (RAAS), adrenergic receptors, and calcium channel blockers (CCBs), and induce diuresis and vasodilation. The increasing global incidence and burden of hypertension and its associated complications has necessitated the development of novel drugs with greater safety and efficacy than existing treatments.
Current Pharmacological Classes of Antihypertensive Agents
Drugs Targeting the Renin-Angiotensin-Aldosterone System (RAAS)
Angiotensin-Converting Enzyme Inhibitors (ACEi)
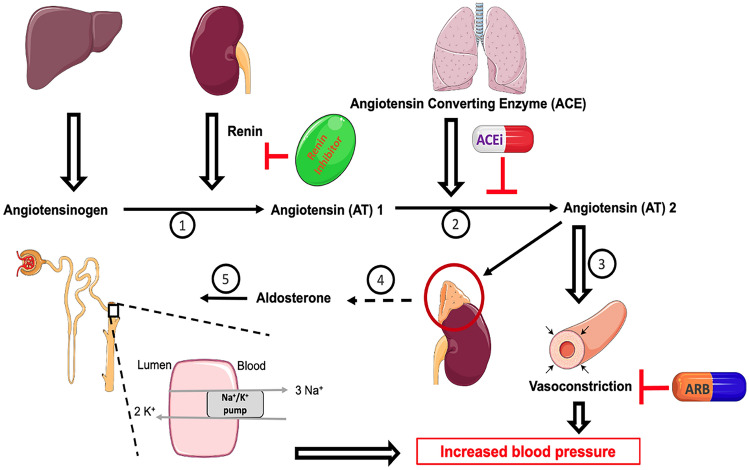
The angiotensin-converting enzyme (ACE) is an enzyme produced primarily by the pulmonary vasculature. Upon conversion of angiotensinogen to angiotensin (AT) I by renin, ACE catalyses the conversion of AT I to AT II (Fig. 1). AT II causes vasoconstriction and stimulates the release of aldosterone from the Zona Glomerulosa of the adrenal gland, which in turn leads to Na+ and water reabsorption and rise in blood volume. The observation that snake bite caused people to collapse due to a rapid decrease in BP led to the hypothesis that snake venom may induce its antihypertensive properties by inhibiting this pathway. The relationship between ACE, AT I, AT II and snake venom eventually led to the development of the first ACEi, captopril [6]. ACEi are one of the recommended first-line agents for those with uncontrolled stage 1 hypertension and non-Black patients [7]. Commonly used ACEi include benazepril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril and trandolapril. The dosage of ACEi varies by medication and population subgroups. Common adverse effects of ACEi include hypotension, therefore these drugs should not routinely be used concurrently with other drugs that suppress the RAAS, such as angiotensin receptor blockers (ARBs) or renin inhibitors. Due to their effect on decreased bradykinin breakdown, ACEi are contraindicated in patients with a history of angioedema. ACEi are known teratogens and are therefore to be avoided in pregnant women. Moreover, caution is required when using ACEi in patients with bilateral renal artery stenosis due to a risk of acute renal failure. Further common adverse effects include dry cough and hyperkalemia and there have also been reports of gynecomastia, agranulocytosis and eosinophilic pneumonitis [8].
Fig. 1.
Mechanism of action of angiotensin converting enzyme inhibitors and angiotensin receptor blocker on The renin-angiotensin-aldosterone system (RAAS). The liver secretes angiotensinogen which is then converted to angiotensin 1 via the action of renin (1), which is released by the kidneys. Angiotensin 1 is subsequently converted into its active component (2) angiotensin 2 via the action of angiotensin converting enzyme, which is produced by the pulmonary vasculature. Angiotensin 2 increases blood pressure via two mechanisms: angiotensin 2 by itself is a potent vasoconstrictor (3) which increases systemic blood pressure. Furthermore, angiotensin 2 acts on the adrenal cortex to release aldosterone from the zona glomerulosa (4). Aldosterone acts on the collecting ducts and distal convoluted tubules (DCT) to facilitate sodium ion reabsorption by acting on the sodium-potassium pump (5); water is also reabsorbed in conjunction with sodium ions during this process (not shown). The reabsorption of sodium ions and water increases intravascular volume thereby increasing blood pressure
Angiotensin Receptor Blockers (ARBs)
AT II binds to the AT1 subtype of AT II receptors, which are present predominantly in the heart, liver, kidneys, adrenal glands and brain. Activation of these receptors increase cardiac contractility, sodium reabsorption and vasoconstriction, which in turn leads to an increased blood volume and BP. Attempts have been made to develop AT II receptor antagonists to block the receptor and lower BP [9]. These studies resulted in the development of the first ARB, losartan. Other examples of commonly used ARBs include azilsartan, candesartan, eprosartan, irbesartan, olmesartan, telmisartan and valsartan. Because of their excellent tolerability profile, ARBs are one of the first-line drugs for treating hypertension. The JNC-8 guidelines suggest using ARBs as monotherapy or in combination with thiazide diuretics and dihydropyridine CCBs in non-Black populations. These guidelines also advise the use of ACEi or ARBs as a first-line option, regardless of ethnicity, for patients with chronic kidney disease (CKD) [7]. There is an estimated reduction of 8 mmHg for systolic BP (SBP) and 5 mmHg for diastolic BP (DBP), similar to ACEi [10]. The most frequent adverse effects with ARBs include dizziness, headache, and hyperkalemia, while uncommon adverse effects include first-dose hypotension, rash, diarrhoea, dyspepsia, abnormal liver function, muscle cramps, myalgias, back pain, insomnia, decreased hemoglobin, renal impairment, pharyngitis, and nasal congestion [11]. The occurrence of dry cough with ARBs is significantly lower compared with ACEi [12]. In general, withdrawal due to adverse effects is significantly less in patients taking ARBs compared with ACEi [11]. ARBs are contraindicated in patients with bilateral renal artery stenosis because they can precipitate renal failure. Elevated AT II in bilateral RAS helps maintain the glomerular filtration rate (GFR) and administration of ARBs can neutralize this beneficial effect [13]. Although there is no conclusive evidence to demonstrate the cross-reactivity of angioedema between ACEi and ARBs in patients with ACEi-induced angioedema, caution must be exercised [14].
Renin Inhibitors
Renin inhibitors bind to the active site of the renin molecule and prevent the binding of renin to angiotensinogen, which is the rate-limiting step of the RAAS cascade. This action prevents the formation of AT I and II. The first and only drug from this class to be approved for use is aliskiren, which has been approved for the treatment of mild to moderate hypertension. In experimental and clinical studies, aliskiren has been shown to reduce BP in a dose-dependent manner [15, 16]. A Cochrane review of randomized controlled trials (RCTs) conducted on aliskiren concluded that the mean reduction in BP with 300 mg was almost similar to other classes of antihypertensives [17]. Non-serious adverse effects commonly seen with this drug are headache, diarrhoea, dizziness and fatigue; hypotensive episodes were seen in volume-depleted patients. Further contraindications include aliskiren’s concurrent use with ACEi or ARBs in diabetic patients. Despite its potent antihypertensive properties, alikiren is not considered a mainstay option for pharmacological management of hypertension. Research into developing agents in this class with better bioavailability and tissue availability is ongoing.
Calcium Channel Blockers (CCBs)
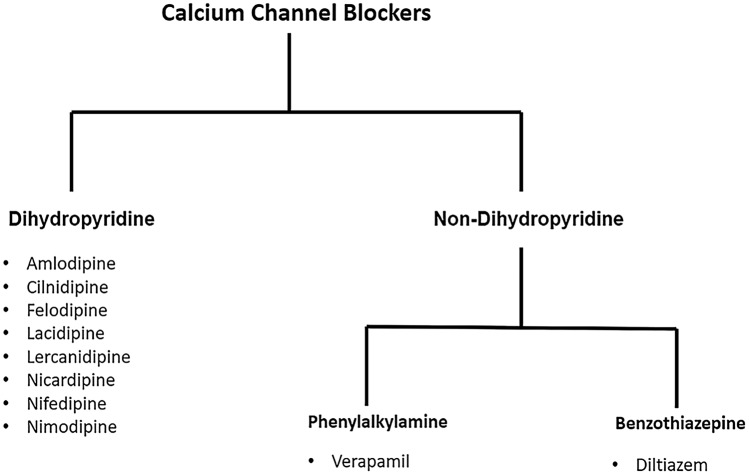
CCBs are a versatile class of drugs, which can be broadly categorized into two subtypes—dihydropyridines and non-dihydropyridines. Both dihydropyridines and non-dihydropyridines act by inhibiting the voltage-gated L-type calcium channels; the former class acts more on vascular smooth muscle and the latter is more cardioselective. By impeding the entry of extracellular Ca2+ into vascular smooth muscle, CCBs decrease excitation-contraction coupling and thus decrease peripheral vascular resistance and BP. In cardiac myocytes, these agents decrease sinoatrial nodal activity and atrioventricular conduction. Current examples of dihydropyridines that are commonly used for hypertension include amlodipine, nifedipine, felodipine, nicardipine, cilnidipine, while examples of non-dihydropyridines that are commonly used include verapamil and diltiazem (Fig. 2). The ALLHAT study reported that compared with lisinopril, amlodipine yields a 1.2 mmHg lower SBP [18]. The VALUE trial found a greater decrease in SBP and DBP, by 4.0 and 2.1 mmHg, respectively, with amlodipine than with valsartan after 1 month, and by 1.5 and 1.3 mmHg, respectively, after 1 year [19]. According to the JNC-8 criteria, along with ACEi or ARBs and thiazide diuretics, CCBs are indicated as a first-line management of high BP [7]. The ALLHAT study further found that CCBs are preferred over ACEi for the management of hypertension in patients of Afro-Caribbean origin because of the higher rates of stoke, peripheral artery disease and hospitalization due to angina among these patients in the ACEi group [18]. Common adverse effects of dihydropyridine CCBs include peripheral oedema, flushing, tachycardia, and dizziness. Non-dihydropyridine CCBs have an inhibitory effect on cardiac tissue and thus cause cardiac depression and atrioventricular block; verapamil has been known to cause constipation [20]. Rarely, both dihydropyridine CCBs and non-dihydropyridine CCBs can cause gingival hyperplasia, oesophageal dysfunction, and mild elevation of hepatic transaminases [21]. CCBs are contraindicated in patients allergic to any component of the medication and are also contraindicated in patients with severe hypotension, sick sinus syndrome, second- or third-degree heart block, and patients with an accessory bypass tract, acute myocardial infarction (MI) or pulmonary congestion [22].
Fig. 2.
Commonly used calcium channel blockers (CCBs). CCBs can be categorised into dihydropyridine, which primarily act on vascular smooth muscle and non-dihydropyridine agents. Non-dihydropyridine CCBs can be further classified into phenylalkylamines, which are more selective for the myocardium, and benzothiazepine, which have both myocardium depressant and vascular smooth muscle relaxant properties
Diuretics
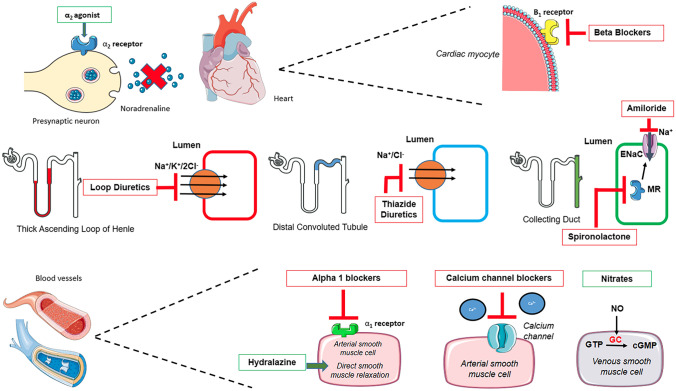
Diuretics are a vast group of drugs that can be further subdivided into carbonic anhydrase (CA) inhibitors, loop diuretics, thiazides, potassium-sparing diuretics, and osmotic diuretics. Loop diuretics, thiazides and potassium-sparing diuretics are all indicated for the management of high BP, however thiazides and thiazide-like diuretics are more commonly used. Although all diuretics induce a natriuretic action and block Na+ reabsorption, they do so at different parts of the nephron by inhibiting various transporters (Fig. 3). Loop diuretics, such as furosemide and torasemide, work at the thick ascending limb of the loop of Henle and block the Na+–K+–2Cl− transport protein. Thiazides such as hydrochlorothiazide and chlortalidone act at the distal convoluted tubule and inhibit the Na+Cl− cotransporter. Potassium-sparing diuretics, which work at the collecting duct, can be divided into two categories: teridine analogues, such as amiloride and triamterene, inhibit reabsorption of Na+ via the epithelial Na+ channel (ENaC), and the aldosterone receptor antagonists spironolactone and eplerenone are inhibitors of aldosterone at the mineralocorticoid receptor. In recent studies, it was found that thiazides reduced SBP and DBP by 9 mmHg and 6 mmHg, respectively, compared with placebo. They have further been shown to reduce pulse pressure by 4–6 mmHg, which is greater than the reduction caused by ACEi, ARBs, renin inhibitors, or β-blockers (BBs) [23]. Common adverse effects of loop diuretics include ototoxicity, hypokalemia, hypomagnesemia and metabolic alkalosis, whereas adverse effects of thiazides include hyponatremia, hyperglycemia, hyperlipidemia, hyperuricemia, and hypercalcemia. While all potassium-sparing diuretics can lead to hyperkalemia, aldosterone receptor antagonists can lead to gynecomastia and decreased libido. Diuretics are contraindicated in patients allergic to any component of the medication as well as those with hepatic and renal failure. Loop diuretics are contraindicated in gout and pregnancy, thiazides are contraindicated in gout, and potassium-sparing diuretics are contraindicated in patients with hyperkalemia and in those taking ACEi or ARBs, and in pregnancy [24].
Fig. 3.
Sites of action of current classes of antihypertensive medications (ACEi and ARB shown in Fig. 1) cGMP cyclic guanosine monophosphate, GC guanylyl cyclase, GTP guanosine triphosphate, MR Mineralocorticoid Receptor, NO nitric oxide
Drug Classes Targeting Adrenergic Receptors
β-Blockers
BBs are a class of drugs consisting of many subtypes, each with different pharmacokinetic and pharmacodynamic properties. Some of the mechanisms include a reduction in cardiac output by competitively blocking β1 receptors in the cardiac myocytes, which inhibits the signal transduction pathway of Gs protein, resulting in negative inotropic and chronotropic effects (Fig. 2). BP-lowering properties are also seen when BBs act on β1 receptors in the kidneys, resulting in inhibition of renin release from juxtaglomerular apparatus and subsequent decrease in AT II and aldosterone production, enhancing renal loss of Na+ and water and further diminishing arterial pressure. Several trials in the 1990s showed the efficacy of BBs compared with placebo in managing hypertension with a 4–27 mmHg drop in SBP [25, 26]. However, the efficacy of BB use in uncomplicated hypertension was first questioned by two large hypertension trials: the Losartan Intervention for End Point Reduction in Hypertension (LIFE) study and the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA), which found superiority of losartan and amlodipine, respectively, compared with atenolol [27, 28]. Many clinical trials and meta-analyses have demonstrated that BBs are inferior to other antihypertensive drugs on cardiovascular protection for several reasons, including their suboptimal BP-lowering efficacy [27–29], their inability to adequately lower central aortic pressure [30], their reduced effect on left ventricular hypertrophy regression [31], and their unfavourable metabolic effects [32]. A meta-analysis including all studies on cardiovascular mortality and morbidity revealed that first-line BB therapy reduced the risk of stroke, but to a lesser extent when compared with other agents, including low- and high-dose thiazide, ACEi, or CCBs [33]. Some examples of β1-selective BBs include atenolol, bisoprolol and metoprolol. The fact that more than 75% of the data in previous meta-analyses were from studies where atenolol was the main agent of interest increases the risk of selection biases and raises questions whether the lack of therapeutic benefit with BB is a class effect or is limited to atenolol [34, 35]. Due to the diversity in the location of the β receptors, BBs result in many adverse effects, including drowsiness, lethargy, sleep disturbance, visual hallucinations, depression, blurring of vision, nightmares, bronchospasm in asthmatic patients, peripheral vascular effects such as cold extremities, Raynaud’s phenomenon, erectile and orgasmic dysfunction and masking of hypoglycemic symptoms such as tachycardia [36]. Although BBs are contraindicated in patients with asthma, recommendations have supported the use of β1-selective BBs in this group of patients [4]. Other contraindications include patients with cocaine-induced coronary vasospasm, acute or chronic bradycardia and/or hypotension, long QT-syndrome, and previous Torsades de Pointes.
α-Blockers
Although the use of α-blockers in the management of primary hypertension is limited, they play a significant role in the management of secondary hypertension and benign prostatic hyperplasia (BPH). The combined treatment of hypertension and BPH has made α-blockers an attractive option. α Receptors are categorized into two subtypes–α1 and α2. α1 receptors primarily involve smooth muscle contractions causing vasoconstriction (Fig. 3) of the blood vessels, ureter, vas deferens, urothelium and several other areas. α1 is the primary receptor for the control of hypertension and BPH. Current examples of selective α1-blockers include doxazosin, prazosin, terazosin and tamsulosin; non-selective α-blockers include phentolamine and phenoxybenzamine. In a Cochrane meta-analysis comparing the BP-lowering efficacy of α-blockers with placebo, doxazosin, on average, lowered SBP by 6.42 mmHg and DBP by 3.53 mmHg, prazosin lowered SBP by 10.38 mmHg and DBP by 6.90 mmHg, and terazosin lowered SBP by 6.59 mmHg and DBP by 4.40 mmHg [37]. The primary goal of the ALLHAT trial was to find the rate of fatal coronary heart disease (CHD) and non-fatal MI, and the secondary goal was to study the rate of cardiovascular disease events. Whereas patients receiving doxazosin and chlortalidone had similar rates of fatal CHD and non-fatal MI, it was found that those taking doxazosin had a higher incidence of congestive heart failure compared with those taking chlortalidone (8.13% vs. 4.45% at 4 years; p < 0.001). Therefore, the doxazosin arm of the trial was terminated [38]. The efficacy of α-blockers on BPH was documented in the MTOPS trial [39] and the COMBAT study [40]. In the MTOPS trial, complications such as acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infections were reduced, compared with placebo, by 39% with doxazosin, 34% with finasteride, and 66% with combination therapy [39]. Phentolamine has been administered during medical management of pheochromocytoma acute hypertensive attacks [41] and phenoxybenzamine is commonly administered prior to surgery [42]. A common adverse effect of α-blockers is the ‘first dose’ effect. This phenomenon describes the severe decrease in BP, orthostatic hypotension, and syncope in patients after receiving the first dose of an α-blocker. Other common adverse effects of α-blockers are due to the drop in BP, such as dizziness, light-headedness, fatigue and headaches. Intraoperative ‘floppy iris syndrome’ (IFIS) [43] and priapism [44] are also documented adverse effects of α-blockers and therefore patients are advised to stop the medication for up to 2 weeks prior to surgery.
Combined α/β-Blockers
A number of α/β-blockers have been developed since the first, labetalol, came to clinical use in 1977. However, carvedilol is commonly used in clinical practice. Although the drugs are referred to as combined α/β-blockers, their predominant mode of action is non-selective blockade of β-adrenergic receptors, with α-adrenergic receptor blockade being a supplementary property [45]. The effects of β-blockade activate baroreceptor reflex, resulting in vasoconstriction via α-adrenergic receptor stimulation and a consequent increase in total peripheral resistance. The additional α-blocking properties of these classes of agents theoretically provides a greater BP-lowering effect [46]; however, studies comparing labetalol with the specific β-receptor blocker propranolol have failed to support this theory. Other studies have shown labetalol is able to lower BP without lowering cardiac output and heart rate, as opposed to propranolol, which causes significant reductions in both parameters [47, 48]. Systematic review of eight randomized, double-blind, placebo-controlled trials revealed α/β-blockers lowered the trough SBP and DBP by an average of 6 mmHg and 4 mmHg, respectively. [45] Labetalol is currently indicated for use in the management of hypertensive urgencies or emergencies [49], as well as the treatment of intraoperative and postoperative hypertension [50], and is also considered first-line in the management of hypertension in pregnancy [51]. The CAPRICORN [52] and COPERNICUS [53] trials demonstrated that carvedilol reduces mortality and morbidity in patients with chronic heart failure related to both ischemic and non-ischemic causes. It has been demonstrated that treatment with carvedilol is not only associated with more stabilized glycemic control and improved insulin resistance compared with metoprolol but also results in fewer new-onset diabetes and diabetic events in patients with heart failure [54, 55]. Analyses of studies comparing carvedilol with captopril, nifedipine or hydrochlorothiazide found that carvedilol achieved a reduction in BP similar to that achieved by its comparators [56]. α/β-Blockers are generally well tolerated. Adverse effects of these agents can occur as a result of antagonism of either α or β receptors. β‐blocking adverse effects include dyspnoea, bronchospasm, bradycardia, malaise, and asthenia, while adverse effects due to α‐blockade include dizziness, light-headedness, fatigue and headaches. Labetolol use has also been associated with an adverse effect of a tingling scalp. Long-term use of α/β-blockers can cause increased sensitivity to catecholamines due to upregulation of adrenergic receptors, and thus their abrupt withdrawal may precipitate tachyarrhythmias, acute hypertensive crises, and palpitations.
α2-Adrenergic Receptor Agonists
α-Adrenergic receptors play a vital role in BP regulation. There are two main types of α-adrenergic receptors—α1 and α2. α1 receptors are found on vascular smooth muscle, and activation leads to smooth muscle contraction, vasoconstriction and elevated BP. α2 receptors are found on the presynaptic neuron and activation causes negative feedback inhibition of norepinephrine release and thus a decrease in BP. Examples of α-agonists commonly used include clonidine, guanfacine and methyldopa. The efficacy of clonidine as an antihypertensive was proved in a study in 28 patients with DBP above 110 mmHg who had either never received pharmacological treatment or for whom previous drug therapy had not yielded a meaningful BP reduction. Following treatment with clonidine, a significant decrease in both SBP and DBP in almost all patients was observed, with an average SBP drop of 54 mmHg and DBP drop of 22 mmHg [57].
A Cochrane systematic review of 12 trials was conducted in 2009 comparing the effects of methyldopa with placebo. The study showed a decrease in BP by 13/8 mmHg in the methyldopa group compared with the placebo group [58]. While clonidine, methyldopa and guanfacine are all approved by the US FDA for the management of hypertension, they are not recommended as a first-line therapy. In addition, methyldopa is also indicated in the management of hypertension in pregnancy. Common adverse effects of α2-agonists include hypotension, sedation and fatigue; α2 receptor agonists are contraindicated in patients with orthostatic hypotension or with any condition causing autonomic instability. Their use is also contraindicated in patients taking phosphodiesterase inhibitors.
Vasodilators
Vasodilators such as nitroglycerin and hydralazine are no longer mainstay treatments in hypertension but are used as an adjunct to control high BP. Vasodilators exert their action by acting at the level of the vessels by producing nitric oxide (NO), which increases cyclic guanosine monophosphate (cGMP), thus causing a relaxation in vascular smooth muscle. Nitroglycerin is primarily a venodilator and at higher doses also leads to arterial dilatation. The relaxant effect of nitroglycerin on the veins and arteries causes reduced preload and cardiac output, respectively. The main indication of nitroglycerin is angina pectoris. However, nitroglycerin is also used in special circumstances, including hypertensive emergencies associated with acute coronary syndromes or acute pulmonary oedema. Hydralazine is a direct-acting arterial vasodilator. In the Cochrane meta-analyses, it was found that after 3–6 weeks of treatment, hydralazine reduced SBP by approximately 5–20 mmHg and DBP by 5–15 mmHg [59]. Hydralazine has been replaced by other agents due to its unwanted adverse effects, including the lupus-like syndrome, which can even occur in low-dose treatment. In a longitudinal study, after 3 years of treatment, hydralazine 100 mg/day resulted in 5.4% of patients developing lupus-like syndrome. Moreover 10.4% developed lupus-like syndrome with hydralazine 200 mg/day [60]. Other adverse effects include reflex tachycardia, immune-mediated hemolytic anemia, glomerulonephritis and vasculitis. However, hydralazine is still indicated in special circumstances such as pregnancy and heart failure.
Emerging Antihypertensive Agents
Currently available antihypertensive agents have certainly proven effective in controlling high BP; however, there is still a large proportion of patients with inadequate BP control or ‘resistant hypertension’. In addition, many current treatments have some intolerable adverse effects.
Our expanding knowledge of the RAAS has led to the development of novel therapeutics, currently in both preclinical and clinical studies. Unfortunately, the coronavirus disease 2019 (COVID-19) pandemic has placed incredible strain on the conception, development and delivery of these novel drugs. Around 80% of non-COVID-19 clinical trials have been stopped or interrupted, with patient enrolments halted, laboratories closed and supply chains lost [61]. Funding and human resources have been diverted away from antihypertensive research, which will have lasting effects for patients, academic scholars and institutions.
Despite this hindrance, several drugs have shown promising safety and efficacy profiles in preclinical trials and are now in phase II/III testing (Table 2). These have a variety of mechanisms, targeting newly elucidated pathways implicated in the pathophysiology of hypertension. There are also other promising candidates that are still in the preclinical stages, including insulin-resistant aminopeptidase (IRAP), endothelin receptor antagonists (ERAs), dual-acting bispecific peptides and soluble guanylyl cyclase A (sGC) stimulators.
Table 2.
Emerging agents for the pharmacological management of hypertension
| Agent and developer | Mechanism of action | Trial details | Comments |
|---|---|---|---|
|
Esaxerenone (CS-3150) Daiichi Sankyo Co. |
Non-steroidal inhibitor of the mineralocorticoid receptor [63] Inhibits aldosterone-dependent sodium retention, causing decreased fluid reabsorption Reduces proteinuria in CKD patients |
Phase III [98] 368 patients with essential hypertension administered either esaxerenone 2.5 or 5 mg for 52 weeks Completed but findings not yet reported Phase III [99] 1001 patients with essential hypertension received either esaxerenone 2.5 or 5 mg or eplerenone 50 mg for 12 weeks Esaxerenone 5 mg reduced SBP by 4.8 mmHg, compared with 2.3 mmHg in the eplerenone group, with significant decrease in 24-h BP |
Esaxerenone has a longer (18.6 h) half-life than eplerenone (5 h), and thus causes less fluctuations in 24 h BP reduction. It also does not have the unwanted effect of hyperkalemia seen with eplerenone No difference in the incidences of adverse effects in any groups Also shown to be well tolerated and effective in lowering SBP in patients with primary aldosteronism [100] Currently approved in Japan for the treatment of hypertension |
|
Firibastat (RB 150) [68] Quantum Genomics SA |
Inhibits aminopeptidase A, which converts angiotensin II into brain angiotensin III [67] Decreased vasopressin release, increasing diuresis Reduction in sympathetic tone and hence vascular resistance Inhibition of the baroreflex response |
Phase IIb [101] 256 overweight or obese hypertensive patients (54% Black or Hispanic) received firibastat 250 mg bid for 8 weeks. No placebo arm Significant reduction in SBP by 10.5 mmHg in Black patients and 8.9 mmHg in non-Black patients Phase III [102]—expected completion December 2021 502 patients will receive firibastat 250 mg bid for 12 weeks or placebo in addition to current antihypertensives |
Adverse effects were headache (4%) and skin reactions (3%). 7.5% recipients stopped the drug due to adverse effects (one serious) Potential alternative therapy for Black populations, with an 18% greater SBP decrease compared with non-African Americans |
|
Bexagliflozin (EGT0001442) Theracos [103] |
Inhibits sodium-glucose co-transporter-2. Several hypothesized mechanisms [79, 81] Glycosuria-accompanied osmotic diuresis Inhibits sodium reuptake in the proximal tubule Upregulation of angiotensin 1–7 Reduction of serum uric acid |
Phase III [104] 1700 patients with T2DM across 10 countries received either bexagliflozin 20 mg or placebo over 30 months Modest reduction in SBP by 9.8 mmHg in the treatment arm, compared with 6.9 mmHg in the placebo arm Phase III [105] 680 hypertensive patients received either bexagliflozin 20 mg or placebo over 24 weeks |
Incidence of adverse effects in the treatment group (36.7%) were similar to placebo (33%) The BEST trial was not specifically powered or designed to evaluate BP effects and the results of trials evaluating this are pending |
|
Nesiritide Oslo University Hospital |
Recombinant B-type natriuretic peptide [74] Leads to vasodilation, natriuretic and diuretic effects |
Phase I and II (TENSE1) [106] 15 patients will receive gradually increasing doses of either nesiritide or placebo bid Estimated completion in 2030 |
Degraded quickly by tissue proteases and therefore needs to be administered by continuous IV infusion |
|
Vitamin D3 and Omega-3 fatty acids (antioxidants) Brigham and Women's Hospital |
Vitamin D [107] Negative regulator of the RAS by suppressing renin gene expression Regulates vascular smooth muscle intracellular calcium concentrations Omega-3 fatty acids [108] Vasorelaxant effects on vascular endothelial and smooth muscle cells through nitrous oxide Inhibit inflammatory processes in arteries |
Phase NA [109] 25,875 men and women with no prior history of hypertension will receive vitamin D3 2000 IU or omega-3 fatty acid 1 g |
Ongoing trial investigating whether supplementation prevents the development of hypertension in people with normal BP |
|
RLD2001-1 and HCP1904-1 Hanmi Pharma |
NA |
Phase III [110] 116 participants with hypertension will take either HCP1904-1 or RLD2001-1 once daily for 8 weeks |
Estimated completion September 2021 |
|
Amlodipine/candesartan cilexetil (CKD-330) and D086 Chong Kun Dang Pharmaceutical |
NA |
Phase III [111] 154 patients with hypertension will either receive amlodipine/candesartan cilexetil and D086 combination therapy or placebo |
Completed, no results posted. Also targeting dyslipidemias and T2DM |
bid twice daily, BP blood pressure, CKD chronic kidney disease, IU international units, IV intravenous, NA not available, RAS renin-angiotensin system, SBP systolic blood pressure, T2DM type 2 diabetes mellitus
Non-Steroidal Mineralocorticoid Receptor Antagonists
A frequent adverse effect of steroid mineralocorticoid receptor (MR) antagonists, such as spironolactone, is hyperkalemia. One interesting feature of the MRs is that they have essentially equivalent affinity for a range of other steroids, including progesterone, cortisol and corticosterone, at a level equivalent to that of aldosterone [62]. Novel non-steroidal selective MR blockers have been developed and esaxerenone was licensed for the treatment of hypertension in Japan in 2019 [63]. In one in vitro study using pituitary GH3 cells, esaxerenone inhibited the transient, late and persistent components of the voltage-gated Na+ current in a concentration-, time-, state- and hysteresis-dependent manner [64]. This was shown to be independent and upstream of its action on the MR and further in vivo studies are in progress to assess its benefit in related conditions, such as primary hyperaldosteronism, CKD and refractory hypertension [65, 66].
Aminopeptidase Inhibitors
Aminopeptidase (APA) is an enzyme that cleaves the N-terminal aspartate reside from AT II, converting it to AT III (and AT IV) in the brain. Here, AT III contributes to the regulation of BP through three AT1 receptor-phospholipase C (PLC)-dependent pathways—sympathetic nerve activation and subsequent noradrenaline release, inhibition of the baroreflex in the nucleus of the tractus solitarius, and stimulation of vasopressin release [67]. As such, there is interest in selective inhibitors of brain APA, such as the prodrug firibastat, which crosses the blood–brain barrier, and the active products that inhibit brain APA activity [68].
Natriuretic Peptide
Not only does the heart pump blood but it also secretes cardiac natriuretic peptides (NPs), which play a crucial role in maintaining cardiovascular homeostasis and regulating BP and glucose and lipid metabolism. These NPs are released in response to atrial or ventricular muscular wall stress from increased intravascular pressure and/or transmural pressure (e.g. due to heart failure, MI or cardiomyopathies) [69]. One of these NPs is B-type NP (BNP), which interacts with membrane-bound guanylyl cyclase A to activate protein kinase G via cGMP. The downstream effects include vasodilation and natriuretic and diuretic effects, which all contribute to lowering BP. [70]
Although ANP deficiency causes hypertension (and its overexpression hypotension), ANP levels are elevated in essential hypertension. However, this represents a protective and compensatory response to cardiac wall stress and the increased ANP may also attenuate the upregulation of renin synthesis, therefore buffering renin-dependent hypertension [71, 72]. The serine protease Corin was identified as the enzyme responsible for converting pro-ANP to active ANP. Its importance in regulating BP is currently being evaluated, especially in managing pregnancy-induced hypertension [73].Neprilysin is a membrane-bound zinc endopeptidase present in various organs that breaks down BNP. As such, there is interest in using neprilysin inhibitors (NEPi) to prevent its degradation and extend its beneficial effects on lowering BP [74]. Dual inhibition of AT II receptor and neprilysin (ARNI) led to a greater BP reduction compared with blocking the AT II receptor alone [75]. NEPi such as sacubitril in combination with valsartan have been approved for the use of both heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) [76]. Recently, Chua et al. [77] conducted a systematic review of RCTs comprising 5931 patients. The group reported that when compared with placebo, sacubitril/valsartan provided a mean reduction of SBP and DBP by 6.52 mmHg and 3.32 mmHg, respectively. Despite sacubitril/valsartan being effective and well tolerated in patients with hypertension, the drug is not currently approved for use in this indication.
Sodium Glucose Co-Transporter 2 Inhibitors
Sodium glucose co-transporter 2 inhibitors block glucose reabsorption in the renal proximal tubule, increasing urinary glucose excretion, and are primarily prescribed for the management of type 2 diabetes mellitus (T2DM); however, several studies have demonstrated the cardiovascular benefits of SGLT2 inhibitors [78]. The glycosuria-accompanied osmotic diuresis increases urine output and may therefore decrease intravascular volume [79]. In addition, SGLT2 inhibitors may inhibit the Na+/H+ exchanging channel isoform 3, increasing Na+ excretion and reducing preload. Their mechanism of action has been shown to be independent of GFR, suggesting that factors independent of volume depletion also contribute, including upregulation of AT 1–7, decreased arterial stiffness and reductions in serum uric acid [80]. The SGLT2 inhibitor dapagliflozin has also been shown to significantly improve the peripheral microvascular endothelial function leading to reduced BP. This mechanism is postulated to be through a reduction in the oxidative stress, improving the functional recovery of impaired microcirculation and abnormal vasomotion of arterioles with increased vascular tone [81]. With a considerable overlap of patient populations with T2DM and hypertension, interest is growing in the antihypertensive effects of SGLT2 inhibitors.
Soluble Guanylate Cyclase Stimulators
Soluble guanylate cyclase (sGC) is the primary target of NO in the cardiovascular system, eliciting many of the biological actions of NO via cGMP [82]. The NO/sGC/cGMP pathway is involved in the regulation of vasodilation and our understanding of its role in hypertension is evolving. Stimulators of sGC replace the oxidised hem cofactor, the loss of which causes sGC to become NO-insensitive [83]. sGC stimulators have been shown to ameliorate pulmonary and portal hypertension through stopping transforming growth factor (TGF)-β/connective tissue growth factor (CTGF) upregulation and subsequent hepatic stellate cell activation [84]. Other sGC stimulators effectively inhibited leukocyte recruitment and the adhesive properties of neutrophils to fibronectin in sickle cell mice. These agents significantly reduced the frequency of vaso-occlusive episodes, complementing their established dose-dependent blood pressure-lowering effects [85]. Olinciguat, another sGC stimulator that is currently in phase II clinical development for use in patients with sickle cell anemia, not only reduces BP in humans and in hypertensive and normotensive rats but also successfully reduces inflammatory mechanisms in TNF-stimulated mice. [86]
Endothelin Receptor Antagonists
The endothelin axis is involved in the physiological regulation of vascular tone through the G protein-coupled receptors ETA and ETB, and overproduction of endothelin has been implicated in the pathology of pulmonary arterial hypertension [87]. Trials with ERAs have shown BP reduction in models of salt-sensitive hypertension and patients with resistant hypertension [88, 89]. The SONAR trial also supported a potential role of ERAs in protecting renal function in patients with T2DM at high risk of developing end-stage kidney disease [90]. Furthermore, in vitro placental studies have shown direct transfer of ERAs across human placenta at term, and that the ETA receptor mediates endothelin-induced constriction in the fetoplacental vasculature, suggesting a role for ERAs in preventing pre-eclampsia [89]. Several large clinical trials have been completed to explore their potential use in treatment-resistant hypertension.
The first major investigation was the DORADO trial, where 379 patients with resistant hypertension treated with darusentan had an absolute reduction in BP compared with placebo, with only fluid accumulation as main adverse side effect [89]. However, the larger DORADO-AC trial, which included an active control agent (guanfacine), showed no significant BP difference between darusentan and placebo after 14 weeks of treatment [88]. Consequently, the manufacturer put further development of this agent on hold in treatment-resistant hypertension. More recently, aprocitentan is currently under investigation in the PRECISION phase III trial in 1971 patients with resistant hypertension [91, 92]. Previous studies have shown promising results; aprocitentan significantly reduced BP compared with placebo and lisinipril [93] and was shown to potentiate the antihypertensive effect of RAS blockers, suggesting a role in combination therapy [94]. The results of the PRECISION trial are estimated to be published in mid-2022. Bosentan is approved for the treatment of PAH and is currently in clinical trials studying its pharmacodynamics, and a further phase II trial in patients with hypertension is expected to be complete in November 2022 [95, 96].
Management of Hypertension in Specific Population Subgroups
Hypertension is a complex, multifactorial disorder that often exists with other comorbidities. Consequently, managing hypertension in specific groups of patients requires different strategies and combinations of pharmacological agents. Some of these populations yet to be discussed include patients with CKD, diabetes mellitus or obesity.
The majority (67–92%) of patients with CKD have comorbid hypertension [112]. The JNC-8 recommends a BP goal of < 140/90 mmHg [7], while the Kidney Disease Improving Global Outcomes (KDIGO) BP work group, which analysed systematic reviews and meta-analyses, suggested considering a lower target of < 130/80 mmHg in patients with CKD and albuminuria > 300 mg/day or a urine albumin-to-creatinine ratio of > 30 mg/g [113]. The ACC/AHA guidelines advocate in favour of the Systolic Blood Pressure Intervention Trial (SPRINT) results and therefore recommend a BP goal of > 130/80 mmHg for all patients with CKD [4]. ACEi (or ARBs if ACEi are not tolerated) are recommended as the first-line agents for the management of hypertension in patients with CKD stage 3 or higher, or stage 1 or 2 with albuminuria > 300 mg/day. As ACEi/ARBs reduce albuminuria through reduction of intra-glomerular pressure leading to a decrease in GFR, serum creatinine may rise up to 30% from baseline; however, a higher rise should warrant further investigation for acute renal failure [114]. Due to the risk of hyperkalemia and acute kidney injury, combination of an ACEi/ARB or ACEi/ARB with aliskiren should be avoided. If BP still remains poorly controlled then diuretics may be a rational option, especially in settings of volume overload, and non-dihydropyridine CCBs could be considered for patients with persistent proteinuria [115, 116]. A number of epidemiologic studies have demonstrated a 1.5- to 2-fold greater prevalence of hypertension in patients with diabetes mellitus when compared with non-diabetic patients [117]. When pharmacologic therapy becomes necessary for the management of high BP, clinical practice guidelines published jointly by the AHA, ACC and a number of other organizations suggest diuretics, CCBs, ACEi and ARBs [4]. The ALLHAT study found that ACEi provided a bigger reduction in mean fasting glucose levels (change in mean fasting glucose level for the diuretic, CCB and ACEi groups were +2.8 mg/dL, +0.6 mg/dL and −1.4 mg/dL, respectively) [18, 118]. Consequently, ACEi have become the initial mainstay choice for managing hypertension in diabetic patients struggling with glycemic control. Moreover, meta-analysis of controlled trials comparing antihypertensives in diabetic patients with kidney disease revealed a lower incidence of end-stage renal disease with the use of ACEi and ARBs, and therefore recommended these agents for managing hypertension in patients with diabetes and albuminuria [119]. In both obese and morbidly obese patients, pharmacological treatment requires a flexible approach given the metabolic and hemodynamic abnormalities present in this group. Causes of hypertension are multifactorial but include chronic insulin resistance, impaired renal pressure natriuresis and extracellular fluid volume expansion [120]. RAAS blockers, such as ACEi or ARBs are considered first-line, although monotherapy is seldom sufficient to control BP. Often, thiazide diuretics and BBs are combined with RAAS inhibitors; however, given their metabolic adverse effects [121], dihydropyridine CCBs may be more beneficial as they are metabolically neutral [122]. The excess adipose tissue in these patients also secretes aldosterone-releasing factors, stimulating aldosterone secretion and causing MR activation [123]. This hyperaldosteronism causes salt-sensitive hypertension independent of the systemic RAAS. Findings by Buglioni et al. [124] and Morales et al. [125] indicate a significant decrease in plasma aldosterone levels and mean BP in obese patients taking mineralocorticoid receptor antagonists (MRA) versus with RAAS blockers, suggesting combination therapy with MRA may improve BP control.
Conclusion
Over the last four decades, there has been significant progress in developing pharmacological agents to control hypertension via various mechanisms and pathways. Despite these advancements, heart disease remains the number one cause of mortality worldwide, a large proportion of which can be attributed to poorly controlled BP. Several novel pathways have been targeted in order to lower BP. However, given the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, progress in developing these agents has been slow. Nevertheless, with many of these agents in phase III clinical trials, the data emerging from recent studies has been encouraging and could hold promise in adequately controlling BP. Indeed, further trials must then establish which combination of current and novel agents provide not only the most therapeutic value but also pose the greatest risks of adverse effects.
Acknowledgements
Images were created and adapted from Smart Servier Medical Art (https://smart.servier.com/).
Declarations
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interests
Utkarsh Ojha, Sanjay Ruddaraju, Navukkarasu Sabapathy, Varun Ravindran, Pitchaya Worapongsatitaya, Jeesanul Haq, Raihan Mohammed and Vinod Patel have no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions
UO, SR, NS, RM, PW, VR and JH were involved in the literature review, interpretation of data, and drafting and editing of the manuscript. Vinod Patel was involved in the conception, revision and supervision of the manuscript. All authors approved the final manuscript and agree to be accountable for all aspects.
References
- 1.Chobanian AV, Bakris GL, Black HR, for the National Heart, Lung, and Blood Institute et al. Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Alcocer L, Cueto L. Hypertension, a health economics perspective. Ther Adv Cardiovasc Dis. 2008;2:147–155. doi: 10.1177/1753944708090572. [DOI] [PubMed] [Google Scholar]
- 3.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 4.Whelton P, Carey R, Aronow W, Casey D, Collins K, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 6.Aurell M, Rudin A, Tisell LE, Kindblom LG, Sandberg G. Captopril effect on hypertension in patient with renin-producing tumour. Lancet. 1979;2(8134):149–150. doi: 10.1016/S0140-6736(79)90031-X. [DOI] [PubMed] [Google Scholar]
- 7.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based guideline for the management of high blood pressure in adults: report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 8.Gavras H, Gavras I. Angiotensin converting enzyme inhibitors. Properties and side effects. Hypertension. 1988;11(3 Pt 2):II37–41. doi: 10.1161/01.hyp.11.3_pt_2.ii37. [DOI] [PubMed] [Google Scholar]
- 9.Israili ZH. Clinical pharmacokinetics of angiotensin II (AT 1) receptor blockers in hypertension. J Hum Hypertens. 2000;14(Suppl 1):S73. doi: 10.1038/sj.jhh.1000991. [DOI] [PubMed] [Google Scholar]
- 10.Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2008;4:CD003822. doi: 10.1002/14651858.CD003822.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148(1):16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers JE, Patterson JH. Angiotensin II-receptor blockers: clinical relevance and therapeutic role. Am J Health Syst Pharm. 2001;58(8):671–683. doi: 10.1093/ajhp/58.8.671. [DOI] [PubMed] [Google Scholar]
- 13.Johansen TL, Kjaer A. Reversible renal impairment induced by treatment with the angiotensin II receptor antagonist candesartan in a patient with bilateral renal artery stenosis. BMC Nephrol. 2001;2:1. doi: 10.1186/1471-2369-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman MB. ACE inhibitor-related angioedema: are your patients at risk. P T. 2013;38(3):170–172. [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen C, Herold P, Brunner HR. Aliskiren: the first renin inhibitor for clinical treatment. Nat Rev Drug Discov. 2008;7(5):399. doi: 10.1038/nrd2550. [DOI] [PubMed] [Google Scholar]
- 16.Staessen JA, Li Y, Richart T. Oral renin inhibitors. The Lancet. 2006;368(9545):1449–1456. doi: 10.1016/S0140-6736(06)69442-7. [DOI] [PubMed] [Google Scholar]
- 17.Musini VM, Lawrence KAK, Fortin PM, Bassett K, Wright JM. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database Syst Rev. 2017;4:CD007066. doi: 10.1002/14651858.CD007066.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 19.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 20.Laurent S. Antihypertensive drugs. Pharmacol Res. 2017;124:116–125. doi: 10.1016/j.phrs.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Dougall HT, McLay J. A comparative review of the adverse effects of calcium antagonists. Drug Saf. 1996;15(2):91–106. doi: 10.2165/00002018-199615020-00002. [DOI] [PubMed] [Google Scholar]
- 22.Elliott WJ, Ram CV. Calcium channel blockers. J Clin Hypertens (Greenwich) 2011;13(9):687–689. doi: 10.1111/j.1751-7176.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musini VM, Nazer M, Bassett K, Wright JM. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst Rev. 2014;5:CD003824. doi: 10.1002/14651858.CD003824.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther. 2014;19(1):5–13. doi: 10.1177/1074248413497257. [DOI] [PubMed] [Google Scholar]
- 25.Dahlöf B, Lindholm LH, Hanson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients With Hypertension (STOP-Hypertension) Lancet. 1991;338:1281–1285. doi: 10.1016/0140-6736(91)92589-T. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson S, Olofsson BO, Wester PO. Atenolol in the secondary prevention after stroke. Cerebrovasc Dis. 1995;5:21–25. doi: 10.1159/000107813. [DOI] [Google Scholar]
- 27.Dahlöf B, Devereux R, Kjeldsen S, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. The Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 28.Dahlöf B, Sever P, Poulter N, Wedel H, Beevers D, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. The Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 29.Khan N. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. Can Med Assoc J. 2006;174(12):1737–1742. doi: 10.1503/cmaj.060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 31.Ciulla MM, Paliotti R, Esposito A, Dìez J, López B, Dahlöf B, et al. Different effects of antihypertensive therapies based on losartan or atenolol on ultrasound and biochemical markers of myocardial fibrosis: results of a randomized trial. Circulation. 2004;110(5):552–557. doi: 10.1161/01.CIR.0000137118.47943.5C. [DOI] [PubMed] [Google Scholar]
- 32.Lithell HO. Effect of antihypertensive drugs on insulin, glucose, and lipid metabolism. Diabetes Care. 1991;14(3):203–209. doi: 10.2337/diacare.14.3.203. [DOI] [PubMed] [Google Scholar]
- 33.Wright JM, Musini VM. First-line drugs for hypertension. Cochrane Database Syst Rev. 2009;3:CD001841. doi: 10.1002/14651858.CD001841.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Ripley T, Saseen J. β-Blockers. Ann Pharmacother. 2014;48(6):723–733. doi: 10.1177/1060028013519591. [DOI] [PubMed] [Google Scholar]
- 35.Elliott W, Childers W. Should β blockers no longer be considered first-line therapy for the treatment of essential hypertension without comorbidities? Curr Cardiol Rep. 2011;13(6):507–516. doi: 10.1007/s11886-011-0216-z. [DOI] [PubMed] [Google Scholar]
- 36.Messerli F, Bell D, Fonseca V, Katholi R, McGill J, Phillips R, et al. Body weight changes with β-blocker use: results from GEMINI. Am J Med. 2007;120(7):610–615. doi: 10.1016/j.amjmed.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Heran BS, Galm BP, Wright JM. Blood pressure lowering efficacy of alpha blockers for primary hypertension. Cochrane Database Syst Rev. 2012;8:CD004643. doi: 10.1002/14651858.CD004643.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vidt DG. Alpha-blockers and congestive heart failure: early termination of an arm of the ALLHAT trial. Cleve Clin J Med. 2000;67(6):429–433. doi: 10.3949/ccjm.67.6.429. [DOI] [PubMed] [Google Scholar]
- 39.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 40.Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, CombAT Study Group et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57(1):123–131. doi: 10.1016/j.eururo.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 41.Tuncel M, Ram VC. Hypertensive emergencies. Etiology and management. Am J Cardiovasc Drugs. 2003;3(1):21–31. doi: 10.2165/00129784-200303010-00003. [DOI] [PubMed] [Google Scholar]
- 42.Garg MK, Kharb S, Brar KS, Gundgurthi A, Mittal R. Medical management of pheochromocytoma: Role of the endocrinologist. Indian J Endocrinol Metab. 2011;15 Suppl 4(Suppl4):S329–S336. doi: 10.4103/2230-8210.86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaman F, Bach C, Junaid I, Papatsoris AG, Pati J, Masood J, et al. The floppy iris syndrome - what urologists and ophthalmologists need to know. Curr Urol. 2012;6(1):1–7. doi: 10.1159/000338861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prihadi JC, Kusumajaya C. Priapism secondary to tamsulosin: a case report. Int J Surg Case Rep. 2020;72:460–463. doi: 10.1016/j.ijscr.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong GW, Laugerotte A, Wright JM. Blood pressure lowering efficacy of dual alpha and beta blockers for primary hypertension. Cochrane Database Syst Rev. 2015;8:CD007449. doi: 10.1002/14651858.CD007449.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann SJ. Combined α/β blockade: An underused approach to the treatment of resistant hypertension. J Clin Hypertens (Greenwich) 2007;9(9):663–666. doi: 10.1111/j.1524-6175.2007.07225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prichard BNC, Richards DA. Comparison of Labetalol with other antihypertensive drugs. Br J Clin Pharmacol. 1982;13(Suppl 1):41S–47S. doi: 10.1111/j.1365-2125.1982.tb01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohn JN, Mehta J, Francis GS. A review of the haemodynamic effects of labetalol in man. Br J Clin Pharmacol. 1982;13(1 Suppl):19S–26S. doi: 10.1111/j.1365-2125.1982.tb01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidt DG. Emergency room management of hypertensive urgencies and emergencies. J Clin Hypertens (Greenwich) 2001;3(3):158–164. doi: 10.1111/j.1524-6175.2001.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muzzi DA, Black S, Losasso TJ, Cucchiara RF. Labetalol and esmolol in the control of hypertension after intracranial surgery. Anesth Analg. 1990;70(1):68–71. doi: 10.1213/00000539-199001000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74(3):283–296. doi: 10.1007/s40265-014-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–1390. doi: 10.1016/S0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 53.Packer M, Fowler MB, Roecker EB, Coat AJ, Katus HA, Krum H, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194–2199. doi: 10.1161/01.CIR.0000035653.72855.BF. [DOI] [PubMed] [Google Scholar]
- 54.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292(18):2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 55.Torp-Pedersen C, Metra M, Charlesworth A, Spark P, Lukas MA, Poole-Wilson PA. Effects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure: data from the Carvedilol Or Metoprolol European Trial (COMET) Heart. 2007;93(8):968–973. doi: 10.1136/hrt.2006.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moser M, Frishman W. Results of therapy with carvedilol, a beta-blocker vasodilator with antioxidant properties, in hypertensive patients. Am J Hypertens. 1998;11(1 Pt 2):15S–22S. doi: 10.1016/S0895-7061(97)00424-X. [DOI] [PubMed] [Google Scholar]
- 57.MacDougall AI, Addis GJ, MacKay N, et al. Treatment of hypertension with clonidine. BMJ. 1970;3(5720):440–442. doi: 10.1136/bmj.3.5720.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mah GT, Tejani AM, Musini VM. Methyldopa for primary hypertension. Cochrane Database Syst Rev. 2009;4:CD003893. doi: 10.1002/14651858.CD003893.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kandler MR, Mah GT, Tejani AM, Stabler SN, Salzwedel DM. Hydralazine for essential hypertension. Cochrane Database Syst Rev. 2011;11:CD004934. doi: 10.1002/14651858.CD004934.pub4. [DOI] [PubMed] [Google Scholar]
- 60.Cameron HA, Ramsay LE. The lupus syndrome induced by hydralazine: a common complication with low dose treatment. Br Med J (Clin Res Ed) 1984;289(6442):410–412. doi: 10.1136/bmj.289.6442.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Dorn A. COVID-19 and readjusting clinical trials. Lancet. 2020;396(10250):523–524. doi: 10.1016/S0140-6736(20)31787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Funder JW Mineralocorticoid receptor antagonists: emerging roles in cardiovascular medicine. Integr Blood Press Control. 2013;6:129–138. doi: 10.2147/IBPC.S13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito S, Itoh H, Rakugi H, Okuda Y, Yamakawa S. Efficacy and safety of esaxerenone (CS-3150) for the treatment of essential hypertension: a phase 2 randomized, placebo-controlled, double-blind study. J Hum Hypertens. 2019;33(7):542–551. doi: 10.1038/s41371-019-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang WT, Wu SN. Characterization of direct perturbations on voltage-gated sodium current by esaxerenone, a nonsteroidal mineralocorticoid receptor blocker. Biomedicines. 2021;9(5):549. doi: 10.3390/biomedicines9050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res. 2019;42(12):1932–1941. doi: 10.1038/s41440-019-0314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Capelli I, Gasperoni L, Ruggeri M, Donati G, Baraldi O, Sorrenti G, et al. New mineralocorticoid receptor antagonists: update on their use in chronic kidney disease and heart failure. J Nephrol. 2020;33(1):37–48. doi: 10.1007/s40620-019-00600-7. [DOI] [PubMed] [Google Scholar]
- 67.Llorens-Cortes C, Touyz RM. Evolution of a new class of antihypertensive drugs: targeting the brain renin-angiotensin system. Hypertension. 2020;75(1):6–15. doi: 10.1161/HYPERTENSIONAHA.119.12675. [DOI] [PubMed] [Google Scholar]
- 68.Fournie-Zaluski MC, Fassot C, Valentin B, Djordjijevic D, Reaux-Le Goazigo A, Corvol P, et al. Brain renin-angiotensin system blockade by systemically active aminopeptidase A inhibitors: a potential treatment of salt-dependent hypertension. Proc Natl Acad Sci USA. 2004;101(20):7775–7780. doi: 10.1073/pnas.0402312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 70.Kato Y, Mori K, Kasahara M, et al. Natriuretic peptide receptor guanylyl cyclase-A pathway counteracts glomerular injury evoked by aldosterone through p38 mitogen-activated protein kinase inhibition. Sci Rep. 2017;7:46624. doi: 10.1038/srep46624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yasumoto K, Takata M, Ueno H, Tomita S, Tomoda F, Inoue H. Relation of plasma brain and atrial natriuretic peptides to left ventricular geometric patterns in essential hypertension. Am J Hypertens. 1999;12(9 Pt 1):921–924. doi: 10.1016/S0895-7061(99)00062-X. [DOI] [PubMed] [Google Scholar]
- 72.Demerath T, Staffel J, Schreiber A, Valletta D, Schweda F. Natriuretic peptides buffer renin-dependent hypertension. Am J Physiol Renal Physiol. 2014;306(12):F1489–F1498. doi: 10.1152/ajprenal.00668.2013. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y, Jiang J, Cui Y, Wu Q. Corin, atrial natriuretic peptide and hypertension. Nephrol Dial Transplant. 2009;24(4):1071–1073. doi: 10.1093/ndt/gfn727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campbell DJ. Long-term neprilysin inhibition—implications for ARNIs. Nat Rev Cardiol. 2017;14(3):171–186. doi: 10.1038/nrcardio.2016.200. [DOI] [PubMed] [Google Scholar]
- 75.Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375(9722):1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 76.Pericas P, Mas-Lladó C, Ramis-Barceló MF, Valadrón I, Noris Mora M, Pasamar Márquez L, et al. Impact of sacubitril-valsartan treatment on diastolic function in patients with heart failure and reduced ejection fraction. High Blood Press Cardiovasc Prev. 2021;28(2):167–175. doi: 10.1007/s40292-021-00437-x. [DOI] [PubMed] [Google Scholar]
- 77.Chua SK, Lai WT, Chen LC, Hung HF. The Antihypertensive effects and safety of LCZ696 in patients with hypertension: a systemic review and meta-analysis of randomized controlled trials. J Clin Med. 2021;10(13):2824. doi: 10.3390/jcm10132824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 inhibition in the diabetic kidney—from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12(4):700–710. doi: 10.2215/CJN.06080616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ojha U, Reyes L, Eyenga F, Oumbe D, Watkowska J, Saint-Jacques H. Diabetes, heart failure and beyond: elucidating the cardioprotective mechanisms of sodium glucose cotransporter 2 (SGLT2) inhibitors. Am J Cardiovasc Drugs. 2021 doi: 10.1007/s40256-021-00486-6. [DOI] [PubMed] [Google Scholar]
- 80.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–473. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varzideh F, Kansakar U, Santulli G. SGLT2 inhibitors in cardiovascular medicine. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):e67–e68. doi: 10.1093/ehjcvp/pvab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sips PY, Buys ES. Genetic modification of hypertension by sGCα1. Trends Cardiovasc Med. 2013;23(8):312–318. doi: 10.1016/j.tcm.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buys E, Sips P. New insights into the role of soluble guanylate cyclase in blood pressure regulation. Curr Opin Nephrol Hypertens. 2014;23(2):135–142. doi: 10.1097/01.mnh.0000441048.91041.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen PJ, Kuo LM, Wu YH, Chang YC, Lai KH, Hwang TL. BAY 41–2272 attenuates CTGF expression via sGC/cGMP-independent pathway in TGFβ1-activated hepatic stellate cells. Biomedicines. 2020;8(9):330. doi: 10.3390/biomedicines8090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferreira WA, Jr, Chweih H, Lanaro C, et al. Beneficial effects of soluble guanylyl cyclase stimulation and activation in sickle cell disease are amplified by hydroxyurea: in vitro and in vivo studies. J Pharmacol Exp Ther. 2020;374(3):469–478. doi: 10.1124/jpet.119.264606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmer DP, Shea CM, Tobin JV, Tchernychev B, Germano P, Sykes K, et al. Olinciguat, an oral sGC stimulator, exhibits diverse pharmacology across preclinical models of cardiovascular, metabolic, renal, and inflammatory disease. Front Pharmacol. 2020;11:419. doi: 10.3389/fphar.2020.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Enevoldsen FC, Sahana J, Wehland M, Grimm D, Infanger M, Krüger M. Endothelin receptor antagonists: status quo and future perspectives for targeted therapy. J Clin Med. 2020;9(3):824. doi: 10.3390/jcm9030824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakris GL, Lindholm LH, Black HR, Krum H, Linas S, Linseman JV, et al. Divergent results using clinic and ambulatory blood pressures: report of a darusentan-resistant hypertension trial. Hypertension. 2010;56(5):824–830. doi: 10.1161/HYPERTENSIONAHA.110.156976. [DOI] [PubMed] [Google Scholar]
- 89.Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, et al. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9699):1423–1431. doi: 10.1016/S0140-6736(09)61500-2. [DOI] [PubMed] [Google Scholar]
- 90.Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, SONAR Committees and Investigators et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393(10184):1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 91.A Research Study to Show the Effect of Aprocitentan in the Treatment of Difficult to Control (Resistant) High Blood Pressure (Hypertension) and Find Out More About Its Safety (PRECISION). ClinicalTrials.gov [cited 24 Oct 2021]. https://clinicaltrials.gov/ct2/show/NCT03541174.
- 92.Angeli F, Verdecchia P, Reboldi G. Aprocitentan, a dual endothelin receptor antagonist under development for the treatment of resistant hypertension. Cardiol Ther. 2021;10(2):397–406. doi: 10.1007/s40119-021-00233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verweij P, Danaietash P, Flamion B, Ménard J, Bellet M. Randomized dose-response study of the new dual endothelin receptor antagonist aprocitentan in hypertension. Hypertension. 2020;75(4):956–965. doi: 10.1161/HYPERTENSIONAHA.119.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trensz F, Bortolamiol C, Kramberg M, Wanner D, Hadana H, Rey M, et al. pharmacological characterization of aprocitentan, a dual endothelin receptor antagonist, alone and in combination with blockers of the renin angiotensin system, in two models of experimental hypertension. J Pharmacol Exp Ther. 2019;368(3):462–473. doi: 10.1124/jpet.118.253864. [DOI] [PubMed] [Google Scholar]
- 95.Endothelin Receptor Function and Acute Stress-Full Text View-ClinicalTrials.Gov [Internet]. Clinicaltrials.gov. [cited 2021 Oct 24]. https://clinicaltrials.gov/ct2/show/NCT02116335.
- 96.Vascular and renal impact of endothelin-1 receptor blockade in patients with resistant arterial hypertension. ClinicalTrials.gov [cited 24 Oct 2021]. https://clinicaltrials.gov/ct2/show/NCT04388124.
- 97.Hitzerd E, Neuman RI, Broekhuizen M, Simons SHP, Schoenmakers S, Reiss IKM, et al. Transfer and vascular effect of endothelin receptor antagonists in the human placenta. Hypertension. 2020;75(3):877–884. doi: 10.1161/HYPERTENSIONAHA.119.14183. [DOI] [PubMed] [Google Scholar]
- 98.Long-term Study of CS-3150 as Monotherapy or in Combination With Other Antihypertensive Drug in Japanese Patients With Essential Hypertension. ClinicalTrials.gov. [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT02722265.
- 99.Study of CS-3150 in Patients With Essential Hypertension. ClinicalTrials.gov [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT02890173.
- 100.Satoh F, Ito S, Itoh H, Rakugi H, Shibata H, Ichihara A, Omura M, Takahashi K, Okuda Y, Iijima S. Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res. 2021;44(4):464–472. doi: 10.1038/s41440-020-00570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Novel Evaluation With QGC001 in Hypertensive Overweight Patients of Multiple Ethnic Origins (NEW-HOPE). ClinicalTrials.gov. 2021 [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT03198793.
- 102.Firibastat in Treatment-resistant Hypertension (FRESH). ClinicalTrials.gov. 2021 [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT04277884.
- 103.Allegretti AS, Zhang W, Zhou W, Thurber TK, Rigby SP, Bowman-Stroud C, et al. Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD. Am J Kidney Dis. 2019;74(3):328–337. doi: 10.1053/j.ajkd.2019.03.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bexagliflozin Efficacy and Safety Trial (BEST) (BEST). ClinicalTrials.gov. 2021 [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT02558296.
- 105.An Integrated Assessment of the Safety and Effectiveness of Bexagliflozin for the Management of Essential Hypertension. ClinicalTrials.gov. 2021 [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT03514641.
- 106.Nesiritide in Hypertension (TENSE1). ClinicalTrials.gov. 2021 [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT02608996.
- 107.Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012;61(4):450–458. doi: 10.1016/j.metabol.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cao Y, Lu L, Liang J, Liu M, Li X, Sun R, Zheng Y, Zhang P. Omega-3 fatty acids and primary and secondary prevention of cardiovascular disease. Cell Biochem Biophys. 2015;72(1):77–81. doi: 10.1007/s12013-014-0407-5. [DOI] [PubMed] [Google Scholar]
- 109.Vitamin D and Omega-3 Hypertension Trial (VITAL Hypertension). ClinicalTrials.gov. 2021 [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT01653678.
- 110.Clinical Efficacy and Safety Evaluation of HCP1904-1 in Essential Hypertension Patients. ClinicalTrials.gov. 2021 [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT04820907.
- 111.Clinical Trial to Evaluate the Efficacy and Safety of CKD-333 Tablet. ClinicalTrials.gov. 2021 [cited 21 Jun 2021]. https://clinicaltrials.gov/ct2/show/NCT03583905.
- 112.Cai G, Zheng Y, Sun X, Chen X. Prevalence, awareness, treatment, and control of hypertension in elderly adults with chronic kidney disease: results from the survey of Prevalence, Awareness, and Treatment Rates in Chronic Kidney Disease Patients With Hypertension in China. J Am Geriatr Soc. 2013;61(12):2160–2167. doi: 10.1111/jgs.12551. [DOI] [PubMed] [Google Scholar]
- 113.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group Clinical practice guideline for the evaluation and management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2(5):337–414. [Google Scholar]
- 114.Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–287. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 115.Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74(1):120–131. doi: 10.1053/j.ajkd.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 116.Steuber TD, Lee J, Holloway A, Andrus MR. Nondihydropyridine calcium channel blockers for the treatment of proteinuria: a review of the literature. Ann Pharmacother. 2019;53(10):1050–1059. doi: 10.1177/1060028019843644. [DOI] [PubMed] [Google Scholar]
- 117.Simonson DC. Etiology and prevalence of hypertension in diabetic patients. Diabetes Care. 1988;11(10):821–827. doi: 10.2337/diacare.11.10.821. [DOI] [PubMed] [Google Scholar]
- 118.Ong HT, Rozina G. Selecting antihypertensive medication in patients with essential hypertension in Malaysia. Med J Malaysia. 2009;64(1):3–11. [PubMed] [Google Scholar]
- 119.Palmer SC, Mavridis D, Navarese D, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385(9982):2047–2056. doi: 10.1016/S0140-6736(14)62459-4. [DOI] [PubMed] [Google Scholar]
- 120.Jiang SZ, Lu W, Zong XF, Ruan HY, Liu Y. Obesity and hypertension. Exp Ther Med. 2016;12(4):2395–2399. doi: 10.3892/etm.2016.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grassi G. How to treat hypertension in the obese. e-J Cardiol Pract. 2013; 12(2).
- 122.Scholze J, Grimm E, Herrmann D, Unger T, Kintscher U. Optimal treatment of obesity-related hypertension: the Hypertension-Obesity-Sibutramine (HOS) study. Circulation. 2007;115(15):1991–1998. doi: 10.1161/CIRCULATIONAHA.106.625400. [DOI] [PubMed] [Google Scholar]
- 123.Kawarazaki W, Fujita T. The role of aldosterone in obesity-related hypertension. Am J Hypertens. 2016;29(4):415–423. doi: 10.1093/ajh/hpw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Buglioni A, Cannone V, Cataliotti A, Sangaralingham SJ, Heublein DM, Scott CG, et al. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension. 2015;65(1):45–53. doi: 10.1161/HYPERTENSIONAHA.114.03936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morales E, Gutiérrez E, Caro J, Sevillano A, Rojas-Rivera J, Praga M. Beneficial long-term effect of aldosterone antagonist added to a traditional blockade of the renin-angiotensin-aldosterone system among patients with obesity and proteinuria. Nefrologia. 2015;35(6):554–561. doi: 10.1016/j.nefro.2015.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.