Abstract
Objectives
Venous sinus thrombosis (VST) after vaccination with the mRNA-based vaccine produced by Pfizer is comparatively rare and usually occurs after the first dose. VST after the second dose has been reported in only few patients.
Case report
The patient is a 33yo male who experienced an episode of sudden onset focal, stabbing headache in the left temporal region for about 4 h. Three days later a similar episode occurred accompanied by a focal seizure. Twenty days before he had received the second dose of the Pfizer vaccine. Clinical exam was normal but MRI of the brain revealed a VST of the left transverse and sigmoid sinuses. Anticoagulation with low molecular weight heparin followed by dabigatran resulted in complete recovery.
Conclusions
This case shows that also the second dose of the Pfizer vaccine can be followed by a VST. Currently, there are more arguments in favour of a causal relation between the vaccination and the VST than against it.
Keywords: SARS-CoV-2, COVID-19, Immune-mediated, Vaccination, Headache, Seizures, Thrombosis
1. Introduction
Venous sinus thrombosis (VST) after SARS-CoV-2 vaccinations is increasingly reported as a putative complication of SARS-CoV-2 vaccinations.1 In the vast majority of cases VST occurs after the first dose of the vaccine [submitted]. VST particularly develops after application of vector-based vaccines.2 VST after vaccination with the mRNA-based vaccine produced by Pfizer is comparatively rare and usually occurs after the first dose [submitted]. VST after the second dose has been reported in only few patients as per the end of July 2021 [submitted]. Here, we present another patient with VST following the second dose of the Pfizer vaccine.
2. Case report
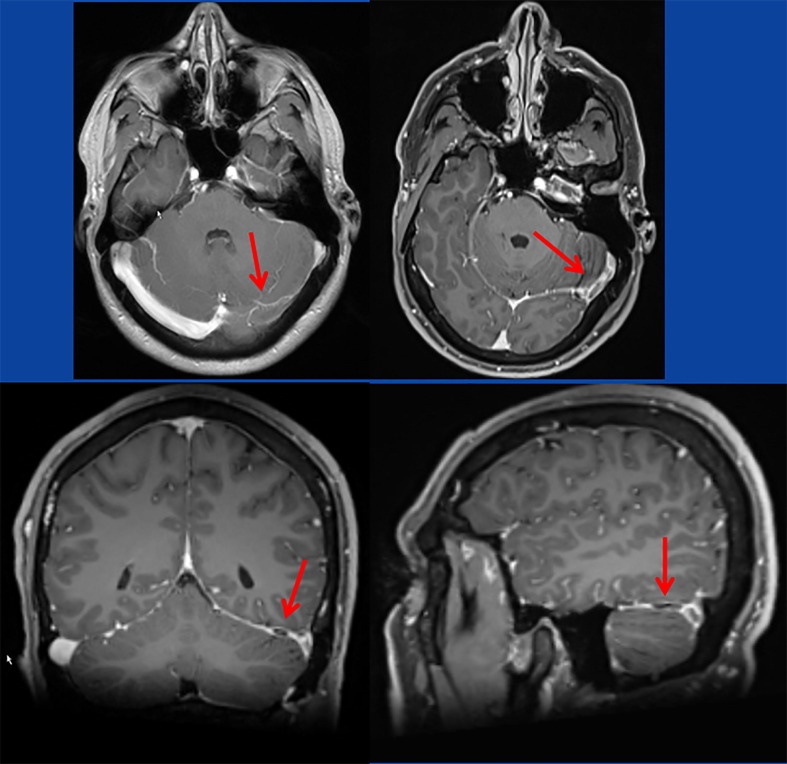
The patient is a 33yo male who experienced sudden onset focal, stabbing headache in a left temporal projection shortly before falling asleep, which was associated with nausea and vomiting and did not respond to ordinary analgesics but subsided spontaneously after four hours. Since this episode he noted enhanced fatigability. Three days after the first episode headache recurred but was associated with visual hallucinations followed by an episode of fixed gaze and loss of consciousness for two minutes without cloni or secessus but post-ictal speech disturbance. The previous history was noteworthy for the second dose with an mRNA-based SARS-CoV-2 vaccine (Pfizer) 20d prior and polytrauma including vertebral bone fracture and traumatic brain injury about 15y years ago with complete recovery. The patient had tolerated the first dose of the same vaccine without any complications. He was not taking any medical or illicit drugs. On admission he only complained about mild headache. Clinical neurologic exam was normal. Blood tests did not show abnormal deflections. An initial cerebral CT scan was non-informative, however, multimodal cerebral MRI revealed a long-stretched VST in the left transverse and sigmoid sinuses (Fig. 1 ). The electroencephalogram did not reveal seizure activity. Low-molecular weight heparin was started and replaced by dabigatran (300 mg/d) three days after admission. Additionally, the patient received levetiracetam 1000 mg/d. Under this regimen he recovered completely and no further seizures occurred. A second MRI had not been performed.
Fig. 1.
T1-weighted images with contrast medium showing flow voids and contrast medium recesses in the left transverse sinus and the left sigmoid sinus.
3. Discussion
The case is interesting for VST after the second dose with the Pfizer vaccine. VST after the second dose of the Pfizer vaccine has been only reported in a few cases.3 Whether the VST was causally related with the vaccination or not remains speculative. Arguments in favour of a causal relation are that >300 cases with post-SARS-CoV-2 vaccination associated VST have been reported as per the end of July 20214 [submitted], that VST also occurs after infection with the SARS-CoV-2 virus, and that VST occurred time-linked to the vaccination. Arguments against a causal relation, however, are that the coagulation parameters and the thrombocyte count were normal throughout hospitalisation, that VST has not been reported as a complication of vaccinations against viruses other than SARS-CoV-2, and that all risk factors for thrombosis (e.g. exsiccosis, thrombocytosis, activation of coagulation) were negative. Anyhow, coagulopathy (hyper- as well as hypo-coagulability)5 has been repeatedly reported as a complication of SARS-CoV-2 infections/vaccinations. Hypercoagulability is explained by direct activation of platelets, enhancing coagulation, by indirect activation of endothelial cells by SARS-CoV-2, shifting endothelium from an anti-thrombotic to a pro-thrombotic state, by direct activation of complement pathways, promoting thrombin generation, by endothelialitis, and by immune thrombocytopenia with dysfunctional thrombocytes.6
In conclusion, this case shows that also the second dose of the Pfizer vaccine can be followed by a VST. Currently, there are more arguments in favour of a causal relation between the vaccination and the VST than against it.
Funding
None received.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Ethical approval
The study was approved by the ethics committee of the Messerli Institute. All clinical practices and observations were conducted in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from patient included in the study.
Patient consent
Informed consent was obtained from patient for publication of this manuscript.
Consent for publication
All the authors have consented for publication of this manuscript.
References
- 1.Palaiodimou L., Stefanou M.I., Katsanos A.H., et al. Cerebral venous sinus thrombosis and thrombotic events after vector-based COVID-19 vaccines: a systematic review and meta-analysis. Neurology. 2021 doi: 10.1212/WNL.0000000000012896. [DOI] [PubMed] [Google Scholar]
- 2.Krzywicka K.M.R., HeldnerSánchez van Kammen M., et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021 doi: 10.1111/ene.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccone A., Zanotti B. working group on cerebral venous thrombosis after COVID-19 vaccination. The importance of recognizing cerebral venous thrombosis following anti-COVID-19 vaccination. Eur J Intern Med. 2021;89:115–117. doi: 10.1016/j.ejim.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finsterer J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol Scand. 2022;145:5–9. doi: 10.1111/ane.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kircheis R. Coagulopathies after vaccination against SARS-CoV-2 may be derived from a combined effect of SARS-CoV-2 spike protein and adenovirus vector-triggered signaling pathways. Int J Mol Sci. 2021 Oct 6;22(19):10791. doi: 10.3390/ijms221910791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steadman E., Fandaros M., Yin W. SARS-CoV-2 and plasma hypercoagulability. Cell Mol Bioeng. 2021;14(5):513–522. doi: 10.1007/s12195-021-00685-w. [DOI] [PMC free article] [PubMed] [Google Scholar]