Abstract
The presence of neutralizing antibodies against SARS-CoV-2 in a large number of people is – besides cellular immunity – important to overcome the SARS-CoV-2 pandemic. While determination of neutralizing antibodies via virus neutralization tests are laborious, assays to determine the antibody levels serologically are fully automated and widely available. Correlations between these methodologies were recently given by the manufacturers, however performance in samples close to the cut off value have not yet been fully validated.
Thus, we analysed 22 borderline and low positive (<100 BAU/ml) samples and 9 high positive (≥ 100 BAU/ml) from infected and/or vaccinated individuals and compared the SARS-CoV-2 IgG II Quant assay (Abbott), LIAISON SARS-CoV-2 TrimericS IgG (Diasorin), Elecsys Anti-SARS-CoV-2 S (Roche), and SARS-CoV-2 IgG (Siemens) with results obtained from a virus neutralization test.
Based on the cut off values given by Abbott, Diasorin, Roche, and Siemens, the positive serologic results were concordant with the virus neutralization test in 100%, 76%, 88%, and 71%, respectively, while in turn, negative ones were in agreement in 29%, 79%, 93%, and 86%, respectively.
In conclusion, weakly positive, serologic results are challenging to correctly predict the presence of neutralizing antibodies. Our study suggests, that different cut off values (for positivity vs. presence of neutralizing antibodies) could improve the test's performance, but determination thereof requires more samples to be analysed.
Keywords: SARS-CoV-2, Virus neutralization test, Automated antibody assays, Correlation, Serology, Neutralizing antibodies
Brief communication
A key-step to overcome the corona pandemic is a high percentage of people having recovered from an infection or have been vaccinated in order to get a sufficient cellular and/or humoral immune response. Gold-standard to assess the extent of humoral response are virus neutralisation tests using viable viruses to quantify the presence of neutralizing antibodies. As they require specialized laboratory equipment and biosafety measures (level 3), they cannot be performed at large scale for population wide studies. Modifications thereof, such as pseudovirus neutralization tests or surrogate virus neutralisation tests can be conducted with a reduced workload under lower safety levels but are still more time consuming than fully automated high throughput SARS-CoV-2 serologic assays to quantify antibodies against SARS-CoV-2. However, the latter not necessarily measure neutralizing antibodies, thus manufacturers have recently evaluated the correlation of their quantitative antibody assays with virus neutralization titres (VNT), and have subsequently provided this information and cut-offs as surrogate for virus neutralization tests. Furthermore, external clinical correlation studies were performed and published [1–7]. The correlation studies were usually performed across a broad range of (mainly high) antibody levels against SARS-CoV-2. Interpretation of borderline reactive and weakly positive antibody results remains challenging in clinical practice. As of yet the correlation between antibody test results and neutralization titres stated by the manufacturers apply only for convalescent patients after COVID-19 infection but do not necessarily apply to subjects after vaccination. For both groups of subjects the exact cut-off of the different antibody tests which grossly guarantees a positive VNT has to be established urgently and harmonized among laboratories.
Therefore, as part of our clinical routine assay validation, we briefly investigated the agreement between antibody levels especially in the borderline range and a VNT, including samples of vaccinees. We first applied our routinely used assay, the SARS-CoV-2 IgG II Quant assay (Abbott) to identify negative, borderline, and (high) positive samples (Table 1 ) in respect to the manufacturer's cut off value for positivity (7.1 BAU/ml), above which a 100% positive agreement with positive neutralization titres is predicted by Abbott. In addition, we also analysed in parallel these samples with the following quantitative assays: LIAISON SARS-CoV-2 TrimericS IgG (Diasorin), Elecsys Anti-SARS-CoV-2 S (Roche), and SARS-CoV-2 IgG (Siemens) using the cut off values of 33.8 BAU/ml, 15 BAU/ml, and 21.6 BAU/ml, respectively. A virus neutralization test with authentic, live SARS-CoV-2 virus was conducted as described [8] and TCID50 virus neutralisation titres equal or higher than 4 were considered as positive. Details on sample characteristics are given in Table 1.
Table 1.
Patient sample characteristics.
| Gender |
Female | 18 |
|---|---|---|
| Male | 13 | |
| Patient age | median (IQR) | 46 (36;57) |
| Sample composition* | total | |
| Negative (<3 BAU/ml) | 1 | |
| Borderline (6–16 BAU/ml) | 17 | |
| positive (30–100 BAU/ml) | 4 | |
| Highly positive (> 100 BAU/ml) | 9 | |
| Total | 31 |
Abbreviations: IQR, interquartile range.
Sample selection based on the Abbott test. Antibody levels in a range from 6 to 16 BAU/ml around the off value of the manufacturer (7.1 BAU/ml) were considered borderline in our setting.
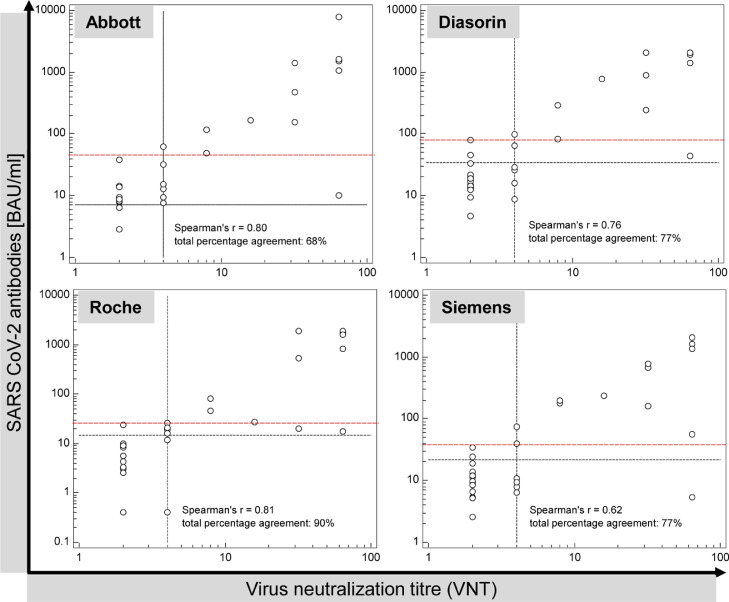
Based on the above-mentioned cut off values for positivity, the correlations between the serologic tests and the VNT are shown in Fig. 1 , respective pivot tables and calculations are given in supporting Table S1. Under the restriction of the small number of samples, the assays from Abbott, Roche and Diasorin exhibited similar Spearman's correlations (rS) with the virus neutralization test (rS = 0.80, rS = 0.81, and rS = 0.76, respectively) while the correlation was weaker in the case of Siemens (rS = 0.62). These correlation coefficients of the small cohort are in line with the recently published correlation of the Siemens assay with the VNT [1] in a larger cohort (rS = 0.843). The virus neutralization test, which was used as the reference method in our study, identified the presence of neutralizing antibodies (i.e.VNT ≥ 4) in 17 out of 31 samples. Out of the 17 samples with neutralizing antibodies, 17, 15, 13, and 12 samples exhibited serological levels above the respective cut off values for Abbott, Roche, Diasorin, and Siemens, respectively. The 14 negative samples in the VNT were determined negative serologically in 4, 13, 11, and 12 cases, respectively. Thus, Abbott exhibited the highest PPA (100%, 17/17 positive samples) but in turn the lowest NPA (29%, i.e. 4/14 negative samples) when using the manufacturer's cut off. In turn, Roche gave the highest NPA (93%) while still possessing a high PPA (88%) in our setting. This is also reflected in the TPA, where Roche performed best (90%) followed by Diasorin (77%), Siemens (77%) and Abbott (68%).
Fig. 1.
Correlation of the Abbott (SARS-CoV-2 IgG II Quant), Diasorin (TrimericS), Roche (Elecsys Anti-SARS-CoV-2 S) and Siemens (SARS-CoV-2 IgG) antibody assay results with virus neutralization titres in 31 borderline or weakly positive samples in the Abbott assay. The black dotted lines depict the manufacturer's cut-off for detection of neutralizing antibodies (7.1, 33.8, 15 BAU/ml, respectively) or the cut off for positivity if no statement from the manufacturer was given (21.6 BAU/ml, Siemens) and for the VNT (titre ≥ 4). The red dashed line exhibits the experimentally found values, above which all samples of this cohort showed a positive VNT.
Briefly, we compared the performance for the low positive samples only (i.e. exclusion of the 9 samples above 100 BAU/ml). In this case (Table S1) the TPA is less affected in the case of Roche (86%) compared to Diasorin (68%), Siemens (68%) and Abbott (55%).
In a last step, we identified for each assay the sample with the lowest antibody concentration above which all samples resulted in a positive VNT in our setting (in comparison to the manufacturers’ cut off): Abbott ≥ 48 BAU/ml (7.1 BAU/ml), Diasorin ≥ 82 BAU/ml (33.8 BAU/ml), Roche ≥ 26 BAU/ml (15 BAU/ml), Siemens ≥ 39 BAU/ml (21.6 BAU/ml). Due to the small number of samples, these values should not be considered as suggestions for improved cut off values, but raise concern that – for all assays – weakly/low positive results can be challenging to interpret in regard to their clinical meaning although in general a good qualitative agreement has been reported for various antibody tests with VNT in larger populations of COVID-19 patients or vaccinees [[2], [3], [4], [5], [6],7]. False positive serologic results are more critical than false negative ones as they pretend the presence of neutralizing antibodies. To resolve this, establishment of different cut off values, one to detect antibodies and (a higher) to correlate with neutralizing antibodies, is suggested as already given only by Roche (15 BAU/ml compared to 0.8 BAU/ml as cut-off for reactivity). In addition, the Roche assay detects all classes of high affinity antibodies. Sterlin et al. showed that IgA dominates the neutralizing response especially in the early stage (< 28 d after symptom onset) [9]. These may be the reasons why Roche exhibited the best qualitative agreement in our setting while the other manufacturers suggest the same cut-off value for both questions and only detect IgG antibodies. Nevertheless, discrepant results persisted to a different extend for all assays. In our point of view, this is due to the lack of standardized procedures to validate the correlation to neutralization antibodies, including (1) limited information on sample selection and range of antibody concentration in the manufacturers’ cohorts, (2) use of different methodologies (virus neutralization test in the case of Abbott, Diasorin and Siemens vs. an in vitro surrogate ELISA for neutralizing antibodies in the case of Roche) and (3) different targets and antibody populations evaluated by the various serologic assays: total, high affinity antibodies (including IgG) against S1-RBD in the case of Roche, IgG against the entire trimeric S-protein (Diasorin), IgG against the S1-RBD domain only (Abbott, Siemens). Virus neutralization tests on the other hand are able to detect all antibody isotypes with neutralizing activity targeting the S1-RBD but also other neutralizing epitopes on the spike protein: Non-RBD spike antibodies, such as the N-terminal directed ones were found to be protective in convalescent plasma donors [10] and thus these antibodies should contribute to the results obtained with the Diasorin assay (entire Spike protein) and VNTs.
In conclusion, SARS-CoV-2 antibody tests are valuable to indicate previous infection as well as a serological response to previous infection or vaccination against COVID-19.
However, especially weakly positive antibody levels should be interpreted with caution regarding the presence of neutralizing antibody levels based on the manufacturer's cut off values. Doing so, false positive results may be predicted and therefore, re-evaluation of these cut off based on a larger number of borderline samples is recommended. In addition, all these correlations are only in vitro-based and neither a clinically protective (lowest) antibody level nor a protective virus neutralization titre is yet established.
Ethical statement
All procedures were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki declaration and its later amendments. We only used retrospective data, which were part of the routine diagnostics (determination of antibodies against SARS-COV-2, e.g.), and analysed remnant samples (no additional blood draw) for comparative assay validation purposes during the ongoing COVID-19 pandemic. This investigation had no effect on the treatment of the patients studied.
Research funding
None declared.
CRediT authorship contribution statement
Alexander E. Egger: Conceptualization, Investigation, Validation, Formal analysis, Visualization, Writing – original draft. Christian Irsara: Investigation, Formal analysis, Writing – review & editing. Barbara Holzer: Investigation, Writing – review & editing. Christoph Winkler: Conceptualization, Investigation. Rosa Bellmann-Weiler: . Günter Weiss: . Boris Hartmann: Investigation, Writing – review & editing. Wolfgang Prokop: . Gregor Hoermann: Conceptualization, Validation, Writing – original draft, Supervision. Andrea Griesmacher: Conceptualization, Writing – review & editing, Resources, Supervision. Markus Anliker: Conceptualization, Writing – review & editing, Resources, Supervision.
Declaration of Competing Interest
No potential conflict of interest relevant to this article.
Acknowledgements
The authors acknowledge Robert Kiechl (Abbott), Harald Schwarz (Diasorin), Erik Alk (Roche), and Gernot Osterer (Siemens) for technical support.
References
- 1.Irsara C., Egger A.E., Prokop W., Nairz M., Loacker L., Sahanic S., et al. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin. Chem. Lab. Med. 2021;59:1453–1462. doi: 10.1515/cclm-2021-0214. [DOI] [PubMed] [Google Scholar]
- 2.Padoan A., Bonfante F., Cosma C., Di Chiara C., Sciacovelli L., Pagliari M., et al. Analytical and clinical performances of a SARS-CoV-2 S-RBD IgG assay: comparison with neutralization titers. Clin. Chem. Lab. Med. 2021;59:1444–1452. doi: 10.1515/cclm-2021-0313. [DOI] [PubMed] [Google Scholar]
- 3.Grenache D.G., Ye C., Bradfute S.B. Correlation of SARS-CoV-2 neutralizing antibodies to an automated chemiluminescent serological immunoassay. J. Appl. Lab. Med. 2021;6:491–495. doi: 10.1093/jalm/jfaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gundlapalli A.V., Salerno R.M., Brooks J.T., Averhoff F., Petersen L.R., McDonald L.C., et al. SARS-CoV-2 serologic assay needs for the next phase of the US COVID-19 pandemic response. Open Forum Infect. Dis. 2021;8:ofaa555. doi: 10.1093/ofid/ofaa555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riepler L., Rössler A., Falch A., Volland A., Borena W., von Laer D., et al. Comparison of four SARS-CoV-2 neutralization assays. Vaccines. 2021;9:13. doi: 10.3390/vaccines9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang M.S., Case J.B., Franks C.E., Chen R.E., Anderson N.W., Henderson J.P., et al. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin. Chem. 2020;66:1538–1547. doi: 10.1093/clinchem/hvaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoud S.A., Ganesan S., Naik S., Bissar S., Zamel I.A., Warren K.N., et al. Serological assays for assessing postvaccination SARS-CoV-2 antibody response. Microbiol. Spectr. 2021;9 doi: 10.1128/Spectrum.00733-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klausberger M., Duerkop M., Haslacher H., Wozniak-Knopp G., Cserjan-Puschmann M., Perkmann T., et al. A comprehensive antigen production and characterisation study for easy-to-implement, specific and quantitative SARS-CoV-2 serotests. EBioMedicine. 2021;67 doi: 10.1016/j.ebiom.2021.103348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claer L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voss W.N., Hou Y.J., Johnson N.V., Delidakis G., Kim J.E., Javanmardi K., et al. Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes. Science. 2021;372:1108–1112. doi: 10.1126/science.abg5268. [DOI] [PMC free article] [PubMed] [Google Scholar]