Abstract
Background & aims
Immunotherapy with hepatitis B virus (HBV)-specific TCR redirected T (HBV-TCR-T) cells in HBV-related hepatocellular carcinoma (HBV-HCC) patients after liver transplantation was reported to be safe and had potential therapeutic efficacy. We aim to investigate the safety of HBV-TCR-T-cell immunotherapy in advanced HBV-HCC patients who had not met the criteria for liver transplantation.
Methods
We enrolled eight patients with advanced HBV-HCC and adoptively transferred short-lived autologous T cells expressing HBV-specific TCR to perform an open-label, phase 1 dose-escalation study (NCT03899415). The primary endpoint was to evaluate the safety of HBV-TCR-T-cell therapy according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) during the dose-escalation process. The secondary endpoint was to assess the efficacy of HBV-TCR-T-cell therapy by evaluating the anti-tumor responses using RECIST criteria (version 1.1) and the overall survival.
Results
Adverse events were observed in two participants among the 8 patients enrolled. Only one patient experienced a Grade 3 liver-related adverse event after receiving a dose of 1 × 105 HBV-TCR-T cells/kg, then normalized without interventions with immunosuppressive agents. Among the patients, one achieved a partial response lasting for 27.7 months. Importantly, most of the patients exhibited a reduction or stabilization of circulating HBsAg and HBV DNA levels after HBV-TCR-T-cell infusion, indicating the on-target effects.
Conclusions
The adoptive transfer of HBV-TCR-T cells into advanced HBV-HCC patients were generally safe and well-tolerated. Observations of clinical efficacy support the continued development and eventual application of this treatment strategy in patients with advanced HBV-related HCC.
Clinical trials registration
This study was registered at ClinicalTrials.gov (NCT03899415).
Keywords: HBV, HBV-TCR-T cells, HCC, Safety, Chronic hepatitis B, Clinical trial, Phase 1, Immunotherapy, Overall survival, Time-to-progression
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and is characterized by rapid progression and poor prognosis with 5-year survival rates of less than 5% [1, 2]. Surgical resection, chemotherapy and radiotherapy are conventional treatments on HCC, but, in the end, most patients die from disease recurrence or metastasis. Tyrosine kinase inhibitors, such as sorafenib, lenvatinib and cabozantinib, had been approved for HCC, but poor improvement in overall survival of advanced HCC patients was observed in the clinic [3–5]. Immune checkpoint blockade (ICB) therapy is a newly developed treatment for HCC. The efficacy of ICB therapy alone or in combination with kinase inhibitors has been reported with varying response rates and survival benefits in HCC patients [6–13]. Despite progress in available therapies, effective and durable systemic treatment options for HCC are still limited.
Adoptive immune cell therapies against HCC have been used by administrating living immune cells with or without antigen specificity. For example, non-specific adoptive cell therapy, including lymphokine-induced killer cells, autologous cytokine-induced killer (CIK) cells and natural killer cells, has captured increasing attention [14–16]. These immune-cell-based therapies, in combination with conventional treatments, and/or kinase inhibitors, demonstrate good safety in HCC patients. However, the lack of specificity restricts these immunotherapies primarily as adjuvant treatments. Specific adoptive cell therapy, such as chimeric antigen receptor engineered T-cell and T cell receptor-engineered T-cell (TCR-T) therapy, was hence developed to enhance the anti-tumor effect and improve treatment efficacy [17].
Hepatitis B-related HCC (HBV-HCC) is estimated to account for approximately 75–80% HCC cases in China [18, 19]. Furthermore, more than 90% of HBV-HCC have HBV DNA integrations and may express the whole or truncated forms of HBV antigens [20–22], so it is reasonable to presume that HBV T cell epitopes are processed and presented on HBV infected hepatocytes or HCC cells. Thus, it seems feasible to treat HBV-HCC using T cells engineered with HBV-specific T cell receptors (HBV-TCR T cells). Indeed, immunotherapy with HBsAg-specific TCR redirected T cells in three HBV-HCC patients after liver transplantation was reported to be safe and had potential therapeutic efficacy [22, 23]. However, at the moment there are no evidences to support whether HBV-TCR T cell could be implemented safely and with sufficient efficacy in advanced HBV-HCC patients due to the increased risk of severe liver inflammation from the destruction of HBV-infected normal hepatocytes (on-target off-tumor effects) under the general poor physical condition of these patients.
In the current phase I clinical trial, we examined the safety of T cell therapy using short-lived HBV-TCR-T cells in eight patients with advanced HBV-HCC. Our study supports the notion that HBV-TCR-T cell therapy is safe and well tolerated. On this basis, we also preliminarily evaluated the potential clinical efficacy of the treatment.
Patients and methods
Workflow and clinical design
Here, we adoptively transferred autologous short-lived mRNA HBV-TCR-T cells to perform an open-label, phase 1 dose-escalation study (NCT03899415) in eight advanced HBV-HCC patients from the Fifth Medical Center of Chinese PLA General Hospital, Beijing, China. This study was approved by the Ethics Committee of the Fifth Medical Center of PLA General Hospital and was conducted according to the principles of the Declaration of Helsinki.
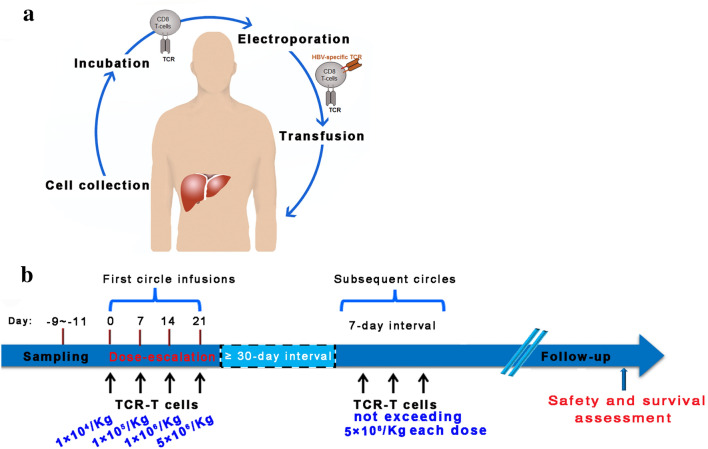
The workflow was showed in Fig. 1a. In this study, patient B004 was HLA-Cw0801 type and the remaining patients (B001-B003 and B005-B008) were HLA-A0201 type (Table 1). TCR-A2-HBs183-191 (TCR-A02/HBs) was used for the HLA-A0201 patients, and TCR-HBV s171 (TCR-C08/HBs) was used for HLA-Cw0801 patients (Table 1). The production of HBV-TCR-T cells were performed as follows: peripheral blood mononuclear cells (PBMCs) were harvested from 80 mL anti-coagulated whole blood of enrolled subjects, followed by the activation and expansion for 8 days with IL-2 (600 IU/mL, Miltenyi) and OKT-3 (50 ng/mL, Miltenyi) in AIM-V (Invitrogen) with 5% CTS Serum Replacement (Invitrogen) as demonstrated in the previous study [15]. Then, activated T cells were manufactured to express HBV-specific TCR through electroporation [22], and cultured overnight in AIM-V with IL-2 (100 IU/mL, Miltenyi). HBV-TCR-T cells were collected by centrifugation for 20 min at 1500 r/min, washed twice in saline (containing 5 g/L human albumin), resuspended in the same solution with 400–500 mL, then transfused back into patients intravenously.
Fig. 1.
Workflow and schematic of study design. a The Workflow of HBV-TCR-T-cell therapy. PBMCs are harvested, expanded, redirected short-lived mRNA HBV-Env-specific-TCR by electroporation and transferred back into the same patient. b The clinical protocol showing the overall study design included the infusion cycle, infusion dose and the main outcomes
Table 1.
Characteristics of patients
| Patients No | B001 | B002 | B003 | B004 | B005 | B006 | B007 | B008 |
|---|---|---|---|---|---|---|---|---|
| Gender | Male | Male | Male | Male | Male | Male | Male | Male |
| Age (years) | 49 | 51 | 59 | 48 | 46 | 67 | 56 | 59 |
| AST (IU/L) at baseline | 28 | 44 | 71 | 71 | 26 | 42 | 80 | 19 |
| ALT (IU/L) at baseline | 22 | 36.5 | 40 | 48 | 26 | 27.5 | 28 | 13 |
| Total bilirubin (μmol/L) at baseline | 8.5 | 43.3 | 18 | 21.7 | 7.5 | 11.1 | 15.3 | 10.1 |
| Platelet counts (109/L) at baseline | 230 | 24 | 77 | 173 | 160 | 192 | 276 | 147 |
| HBV‐TCRs (HBV‐Env) | HLA‐A0201 | HLA‐A0201 | HLA‐A0201 | HLA‐Cw0801 | HLA‐A0201 | HLA‐A0201 | HLA‐A0201 | HLA‐A0201 |
| HBV genotype | C | – | C | C | – | – | – | |
| HBsAg serum (IU/ml) | 202 | 1444 | 929.9 | 210.9 | 942.6 | 1549 | 231.5 | 303.8 |
| HBeAg status | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| HBeAb status | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| Circulating HBV DNA (IU/ml) at baseline | 499 | < 40 | 458 | 991 | < 20 | 44.2 | < 20 | < 20 |
| Epitope sequence at baseline | FLLTRILTI | – | FLLTRILTI | FLGPLLVLQA | – | – | – | – |
| Circulating HBV DNA (IU/ml) after TCR-T-cell infusions | 30.5 | < 40 | 318 | 492 | < 40 | < 20 | < 20 | < 20 |
| Epitope sequence after TCR-T-cell infusions | – | – | FLLTRILTI | FLGPLLVLQA | – | – | – | – |
| AFP ≥ 400 ng per milliliter | No | No | No | Yes | No | No | Yes | No |
| ECOG performance status score | 1 | 1 | 2 | 2 | 0 | 1 | 1 | 1 |
| Child–Pugh classification (score) | A5 | B8 | A6 | B9 | A5 | A6 | A6 | A6 |
| BCLC stage | C | B | C | C | C | A | C | C |
| Tumor in liver (size, max, cm & number) | Multiple (13.9 × 6.9) | Multiple (3.5 × 1.9) | Multiple (4.8 × 13.1) | Multiple (17.9 × 12.1) | 2 (3.9 × 3.4) | 1 (3.2 × 3.07) | Multiple (11.5 × 9.3) | 2 (3.8 × 3.2) |
| Presence of Macrovascular invasion | Yes | No | Yes | Yes | No | No | No | No |
| Presence of Extrahepatic spread | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| Antiviral treatment | Entecavir | Entecavir | Entecavir | Entecavir | Entecavir | Entecavir | Entecavir | Entecavir |
| Prior treatment for HCC | TACE + Sorafenib | TACE + Sorafenib | RFA | Sorafenib | TACE + Sorafenib | TACE + RFA | Sorafenib | Argon-helium knife therapy for HCC + RFA + Sorafenib |
| Combination therapy | Sorafenib | Sorafenib | Sorafenib | Sorafenib | Sorafenib + 1 RFA | 1 Microwave ablation | Sorafenib | Sorafenib |
| Therapy after TCR-T-cell infusion | Sorafenib | Sorafenib + Lenvatinib + 2 TACE | Sorafenib | Sorafenib | Sorafenib + 1 RFA | no | Sorafenib | Sorafenib |
AST aspartate aminotransferase, ALT alkaline phosphatase; HBV hepatitis B virus, HBsAg hepatitis B surface antigen, HBeAg hepatitis B e-antigen, AFP α-fetoprotein; ECOG Eastern Cooperative Oncology Group, BCLC Barcelona clinic liver cancer (BCLC) stage, TACE transcatheter arterial chemoembolization; RFA radiofrequency ablation
The patients were admitted to the hospital before cell therapy. No lymphodepletion was performed prior to the adoptive transfer of HBV-TCR-T cells. The treatment schedule with HBV-TCR-T cells was shown in Fig. 1b: the first cycle included 4 escalating doses (1 × 104–5 × 106 CD8+Vβ+ T-cells/kg) at one-week interval, and the subsequent infusions were given with variable quantities of HBV-TCR-T cells at a maximum dose of 5 × 106 CD8+Vβ+ T-cells /kg, each given one-week apart.
The primary endpoint was to evaluate the safety of HBV-TCR-T-cell therapy, and the dose-limiting toxicity of TCR-T cells was defined as follows: Grade 3/4 adverse events not related to patient’s underlying malignancy or preexisting comorbidities or any unexpected toxicity of any grade according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) during dose-escalation process and follow-up period. The secondary endpoint was to assess the efficacy of HBV-TCR-T-cell therapy by measuring tumor response as follows: the first evaluation of the tumor response was performed at the 21st to 24th post days from the first TCR-T-cell infusion by MRI or CT according to RECIST criteria (version 1.1), and every 3 months thereafter. Overall survival (OS) was assessed and analyzed [24].
Enrollment criteria of patients
Eligible patients were (1) aged 18–70 years; (2) expressing either HLA-A0201 or HLA-Cw0801; (3) Barcelona clinic liver cancer (BCLC) stage A-C HCC patients with a positive test for HBsAg; (4) at least 1 month after a surgical intervention or 2 weeks after transhepatic arterial chemotherapy and embolization (TACE) (may include other type of resection/ablation); (5) Child–Pugh < 7 points and Eastern Cooperative Oncology Group (ECOG) ≤ 1; (6) alanine aminotransferase (ALT) < 200 IU/L, aspartate aminotransferase (AST) < 200 IU/L, total bilirubin < 17.1 umol/L and creatinine clearance ≥ 60 ml/minute; (7) without clinically significant abnormality in chest X-ray, cardiac enzymes and electrocardiograph; (8) received antiviral treatment > 1 year prior to enrollment; (9) willing to use an acceptable method of contraception who was during child-bearing period; and (10) provided written informed consent.
Exclusion criteria were as follows: (1) patients experiencing acute infection or gastric bleeding within 30 days; (2) with positive hepatitis A/C/delta virus, human immunodeficiency virus, or a chronic liver disease other than CHB (e.g. autoimmune hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease and drug-induced liver disease; (3) with any other serious physical and mental illnesses; (4) women who are pregnant or breast-feeding; (5) with a history of allergic reaction to blood products or other investigational products; (6) patients s who are receiving systemic medications, such as steroids during the study treatment; (7) any cell therapy such as, but not limited to natural killer, cytokine-induced killer, dendritic cells, cytotoxic T lymphocyte, stem cells therapy 6 months prior to study treatment.
Detection of engineered HBV-TCR-T cells
To detect the frequencies of HBV-TCR-T cells, the following antibodies were used: anti-CD3-APC-Cy7, anti-CD8-BV510, and anti-TCR-Vβ. Cells were labelled with above-mentioned antibodies on ice for 30 min and then thoroughly washed and fixed for further analysis by flow cytometry.
Quantification of circulating HBsAg and HBV DNA
HBsAg was quantified using a Roche Cobas e601 electrochemistry luminescence immunity analyzer and an elecsys for HBsAg quantitation (Roche Diagnostics, Mannheim, Germany) with a lower limit of detection: 0.05 IU/mL [25]. HBV DNA was measured from 200-μl plasma using HBV-DNA assay kit (SANSURE BIOTECH INC., Hunan, China) with a lower limit of detection: 40 IU/mL, or COBAS AmpliPrep/COBAS TaqMan (Roche Diagnostics, Mannheim, Germany) with a lower limit of detection: 20 IU/mL.
HBV genotype and epitope identification
HBV genotype was determined as in previous study [26], and HBV DNA was sequenced to analyze HBV epitope sequences according to previous protocols [27].
Statistical analysis
AE terms were coded using the Medical Dictionary for Regulatory Activities (version 21.1). Time-to-Progression (TTP) was analyzed from the date of HBV-TCR-T-cell infusion to the date of disease progression. TTP and OS were analyzed with the Kaplan–Meier method.
Results
Patient characteristics
The enrolled patients with advanced HCC were all male and had a median age of 53.5 (ranging from 46 to 67) years. Baseline characteristics of the patients were listed in Table 1. Liver function of all patients met the enrollment criteria prior to treatment. Six patients had Child–Pugh class A liver function (B001, B003, B005-B008) and two patients were of Child–Pugh class B (B002 and B004). As for BCLC staging, 6 patients had stage C (B001, B003-B005, B007 and B008); two patients had stage B (B002) and stage A (B006) HCC, respectively. B006 had a single liver lesion while the remaining patients had more than 1 hepatic tumors. In addition, three patients had macrovascular invasion and six patients had extrahepatic disease (Table 1). Two subjects (B004 and B007) had AFP ≥ 400 ng/mL, the ranges of serum HBsAg were from 202 IU/mL to 1549 IU/ml, and four patients (B001, B003, B004 and B006) had circulating HBV DNA level > 40 IU/mL (Table 1). Meanwhile, HBV genotypes in three patients (B001, B003 and B004) were C, and the corresponding HBV Epitope sequences of B001 and B003 were FLLTRILTI and B004 was FLGPLLVLQA (Table 1). The results suggest that the amino acid sequences recognized by TCR-T were conserved in B003 and B004 with the same sequence at baseline and after TCR-T-cell infusions (Table 1) Due to the low levels of circulating HBV DNA in the remaining six patients, we failed to test HBV genotypes in these patients. Other clinical parameters of enrolled patients including peripheral platelet counts, serum HBeAg and HBeAb status were also shown in Table 1.
All patients had a history of chronic HBV infection and received entecavir treatment. Apart from B003 and B006, other patients were administrated with sorafenib before TCR-T-cell therapy. Moreover, all other patients, with the exception of B004 and B007, received local therapy for liver tumor including TACE or liver lesion microwave ablation before TCR-T-cell therapy. Patients B001, B002, B003, B004, B005, B007 and B008 received Sorafenib treatment in combination with TCR-T cell infusion (Table 1). More detailed, B002 received Sorafenib first, then followed by Lenvatinib after 1 year of the last infusion, and underwent twice TACE after 1 and 2 year of the last infusion during the follow-up period; B005 received RFA at 1 month after the last infusion; and B006 underwent 1 microwave ablation after the first TCR-T-cell infusion (Table 1).
Short-term HBV-TCR expression on T cells
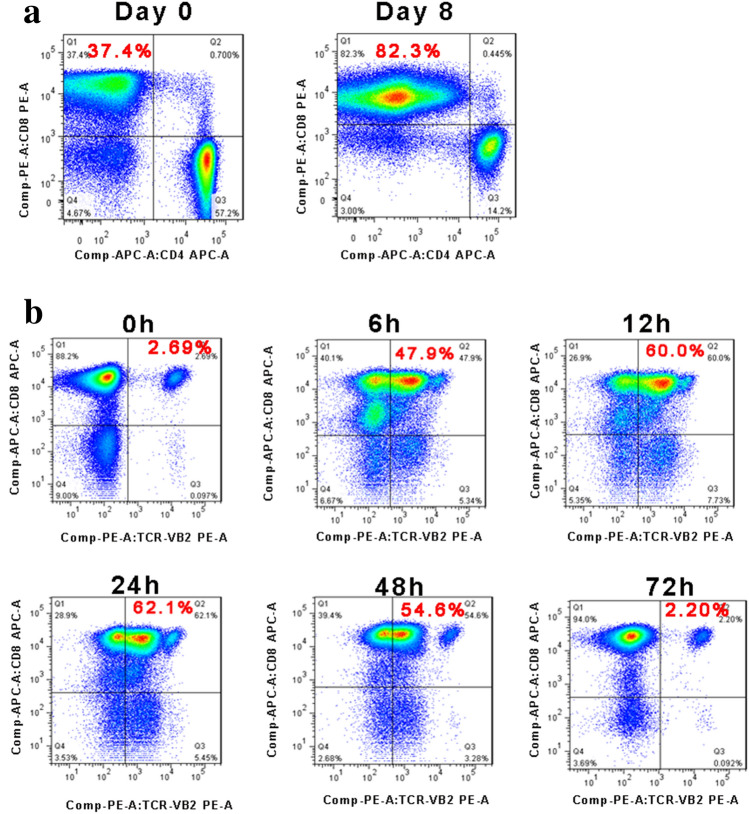
We successfully generated HBV-TCR-T cells for all eight patients. The median time to manufacture the cell products for clinical use was 10 (range from 9 to 11) days. Before electroporation, CD8 T cells accounted for 85% (range from 79 to 93%) of expanded cells after in vitro expansion. Representative dot plots of the percentages of CD8+ T cells in CD3+ T cells from B001 were shown in Fig. 2a. The percentages of HBV-TCR-T (CD8+Vβ+) cells in CD3+ T cells remained high between 12 and 48 h post electroporation, and then decreased to normal levels after 72 h. Representative dot plots of the dynamic percentages of HBV-TCR-T (CD8+Vβ+) cells in CD3+ T cells from B001 were shown in Fig. 2b. Given the clinical application, we performed infusions with HBV-TCR-T cells obtained 24 h after electroporation.
Fig. 2.
Characteristics of HBsAg-TCR-T cells. a Proliferation profiles of CD8 T cells from one HBV-HCC patient. Values in quadrant indicate percentage of CD8 T cells in cultured lymphocytes (gated on CD3 + T lymphocytes). b Electroporation efficiency. Values in quadrant indicate TCR-Vβ percentages expressed on T cells at different times after electroporation
Adverse effects
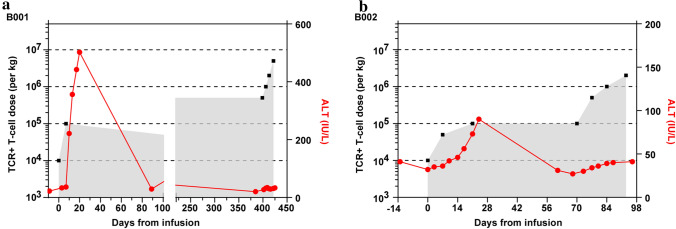
The median numbers of cell infusions were 8 (range from 4 to 12), B001 received 12 cell infusions, B002, B007 and B008 8 infusions, and B003-B006 received 4 infusions, respectively. During and immediately after T cell infusions, none of patients had acute adverse events of vomiting, allergies, coma, or graft-versus-host disease. The incidence and severity of all observed toxicities and their associated dose are displayed in Table 2. Two patients (B001 and B002) experienced adverse events during the first cycle of HBV-TCR-T infusion. B001 experienced Grade 3 adverse events with a rapid elevation of liver enzymes (a max ALT of 506 IU/ml) and jaundice (a max total bilirubin of 70.3 µmol/L) after receiving the second dose (1 × 105 cells/kg). These indexes peaked at ~ 16 days and resolved at ~ 80 days after the second TCR-T infusion (Fig. 3a). B002 had a mild and gradual elevation of ALT with a maximum of 90 IU/ml at ~ 10 days and resolved at ~ 50 days after the second infusion (5 × 104 cells/kg) (Fig. 3b). In both patients, liver enzymes normalized without interventions with glucocorticoid or other immunosuppressive agents such as IL-6 receptor antagonist tocilizumab. No liver-related adverse events were observed during the subsequent second cycle HBV-TCR-T infusions. Adverse events were not observed for the rest of the patients even at the maximum planned dose of 5 × 106 cells/kg. There were also no abnormal changes in renal function among the eight patients during the whole treatment period. Taken together, these data indicate that HBV-TCR-T-cell therapy for advanced HBV-HCC patients was safe and well tolerated.
Table 2.
Safety evaluation
| Patients No | The number of infusions and maximum dose | HBV TCR-T cell related AE | Best overall response | TTP (months) |
OS (months) |
Current status | |
|---|---|---|---|---|---|---|---|
| AE | Associated dose | ||||||
| B001 | 12 (5 × 106/kg) |
Grade 3 ALT increase; Grade 3 GGT increase; Grade 3 AST increase; Grade 3 bilirubin increase |
1 × 105/kg | PR | 27.7 |
37.4 (censored) |
Loss to follow-up |
| B002 | 8 (2 × 106/kg) | Grade 1 ALT increase | 5 × 104/kg | SD | 2.97 | 33.1 | Death |
| B003 | 4 (1 × 106/kg) | None | NA | PD | 0.93 | 3.53 | Death |
| B004 | 4 (2 × 105/kg) | None | NA | SD | 0.9 | 2.53 | Death |
| B005 | 4 (5 × 106/kg) | None | NA | SD | 9.4 |
18.8 (censored) |
Loss to follow-up |
| B006 | 4 (1.53 × 106/kg) | None | NA | NE | 23.8 |
23.8 (censored) |
Alive |
| B007 | 8 (5 × 106/kg) | None | NA | PD | 0.2 | 6.7 | Death |
| B008 | 8 (5 × 106/kg) | None | NA | NE | 16.9 |
20.0 (censored) |
Alive |
HBV hepatitis B virus, AE adverse reaction, TTP time-to-progression, OS overall survival, ALT alanine transaminase, GGT gamma-glutamyltransferase, AST aspartate aminotransferase, PR partial response, SD stable disease, PD progressive disease, NE not evaluated, NA not available
Fig. 3.
The alterations of ALT levels in B001 and B002 after TCR-T-cell infusion a B001 and b B002
Antitumor activity of HBV-TCR-T-cell therapy
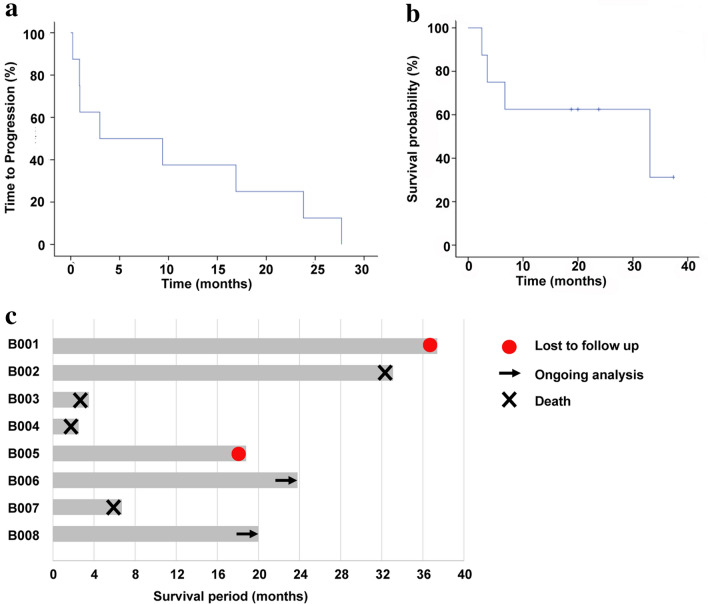
The impact on overall survival was then evaluated. The median TTP for patients enrolled was 6.18 months (ranging from 0.2 to 27.7 months) (Fig. 4a) and the median OS was 33.1 months (ranging from 2.5–37.4 months) (Fig. 4b). B003, B004 and B007 patients exhibited the shortest TTP (0.93, 0.9 and 0.2 months, respectively). The high tumor load and AFP values at baseline of these 3 patients together with extrahepatic and macrovascular spread at the inception of the study indicates a more advanced disease state before treatment (Table 1). B003 and B004 passed away within 4 months after one cycle of TCR-T-cell infusion and B007 died within 7 months after two cycles of TCR-T-cell infusion (Fig. 4c). On the other hand, B005, B006 and B008, showed a longer TTP (9.4, 23.8 and 16.9 months, respectively) and survival (18.8, 23.8 and 20.0 months, respectively) (Table. 2 and Fig. 4b). While B001 was lost to follow-up, B006 and B008 were alive at the time the data were analyzed (Table. 2 and Fig. 3c). The good outcomes of the three patients could be associated with the lower tumor load at study inception (Table. 1). This was especially pertinent for patient B006 who not only had a lower tumor load but was also at an earlier stage of the disease (BCLC stage A). Despite the high tumor load at baseline, B001 and B002 had a longer TTP (27.7 and 2.97 months, respectively) and survival (37.4 and 33.1 months, respectively) than those of B003, B004 and B007 after receiving 4 and 2 cycles of TCR-T-cell therapy, respectively (Fig. 4c). Importantly, B001 achieved a partial response lasting with 27.7 months, with a significant shrinkage of liver tumor size, and B002 achieved stable disease, supporting a significant benefit of HBV-TCR T-cell therapy on long-term OS. Overall, there was a trend towards increased survival after TCR-T treatment.
Fig. 4.
Disease response. a Median TTP for patients after infusions was 6.18 months n = 8, and b median OS for patients after infusions was 33.1 months (n = 8). c Outcome for evaluable patients by Swimmer plot
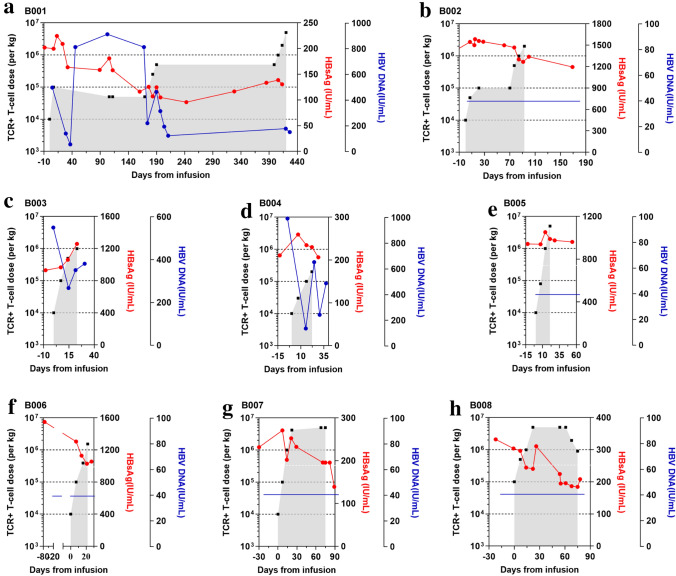
Having evaluated the efficacy aspects of the treatment, we next assessed whether the adoptive transfer of the short-lived HBV-TCR-T cells have an on-target effect. By comparing the serum HBsAg levels before and after the last HBV-TCR-T cell infusion, we observed either a decline or stabilization of serum HBsAg in 7 out of 8 patients (Fig. 5a–h). Simultaneously, the circulating HBV DNA loads were reduced or stable at undetectable levels in all 8 patients after TCR-T-cell infusions (Fig. 5a–h). Collectively, these results suggest that the treatment could indeed target HBV-expressing cells.
Fig. 5.
Serum levels of HBsAg and HBV DNA before and after TCR-T-cells infusion. HBsAg and HBV DNA levels of every patient treated with HBV-TCR-T cells a B001; b B002; c B003; d B004; e B005; f B006; g B007 and h B008 before and after HBV-TCR-T-cell infusion. The numbers of HBV-TCR -T cells are indicated in black, HBsAg and HBV DNA levels are expressed in red and blue, respectively. The blue horizontal lines in b, e, f, g and h meant that the levels of HBV DNA levels were lower the limit of detectable levels
Discussion
Previous studies reported that HBV-TCR-T-cell immunotherapy in HBV-HCC patients after liver transplantation with disseminated HCC metastasis in lung, bones and neck or with HCC recurrence had no signs of acute toxicities and only had a largely unremarkable alteration of ALT levels. However, the safety of HBV-TCR-T-cell immunotherapy had not been evaluated in HBV-HCC patients who had not performed liver transplantation. Herein we conducted a dose escalation, phase I trial using short-lived HBV-TCR-T cells in 8 advanced HBV-HCC patients and found that HBV-TCR-T-cell infusions were well-tolerated with one patient (12.5%) experiencing Grade 3 increase of liver enzymes and one patient experiencing Grade 1 AEs with mild elevation of liver enzymes during the first treatment cycle. The AEs resolved without intervention with glucocorticoid and immunosuppressive agents. Compared to CAR-Glypican-3 T-Cell therapy for advanced HCC, the incidence and severity of AEs after HBV-TCR-T-cell immunotherapy in our study was lower and less frequent, and had no neurotoxicity, cytokine release syndrome, or WBC, lymphocyte and platelet count reduction. Comparing to autologous CIK cells for HCC, the rates of AEs were 62%, and the rates of SAEs were 6%. The lower rates of SAEs in CIK therapy than that of HBV-TCR-T-cell immunotherapy may be due to the lower tumor load of patients who had undergone curative treatment. Overall, these data suggest that HBV-TCR-T-cell immunotherapy in advanced HBV-HCC patients without prior liver transplantation was safe and tolerable.
Consistent with the previous study in HBV-HCC patients after liver transplantation, we observed therapeutic efficacy of HBV-TCR-T-cell immunotherapy in the recruited advanced HBV-HCC patients. Three patients showed radiological responses with a shrinkage of tumor, especially in B001 whose tumor size decreased from 13.9 × 6.9 cm to 8.6 × 5.8 cm after four cycles of HBV-TCR-T-cell therapy. This anti-tumor efficacy was likely due to the on-target effects of the adoptively transferred HBV-TCR-T cells as serum HBsAg levels declined in all but one patient after the last HBV-TCR-T cell infusion. Additionally, the median OS duration and the median of time-to-progression in patients were 33.1 and 6.18 months, respectively. Compared to the median OS of advanced patients in our study, the median OS achieved after CAR-Glypican-3 T-Cell therapy (10.0 months) was lower.
There were some limitations in our study. For example, it was very difficult to obtain liver tumor tissues from the enrolled patients due to potential risks that included promoting tumor metastasis and/or bleeding. In addition, there was a small number of enrolled patients. Future studies are warranted to confirm the safety and efficacy of such short-lived HBV-TCR-T cells in a larger cohort of advanced HBV-HCC patients.
In conclusion, adoptive T cell therapy using autologous short-lived HBV-TCR-T cells is a technically feasible and safe treatment with tolerable toxicity and with potential therapeutic efficacy in advanced HBV-HCC patients. These data accumulated thus far support the continued development of this approach as an alternative treatment for HCC.
Acknowledgements
We thank the staff from the Department of liver disease and all the patients for their participation in this study.
Abbreviations
- ALT
Alanine aminotransferase
- HBV-TCR-T cells
HBV-specific TCR-redirected T cells
- BCLC
Barcelona clinic liver cancer stage
- CIK
Cytokine-induced killer cells
- ECOG
Eastern cooperative oncology group
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- ICB
Immune checkpoint blockade
- OS
Overall survival
- TTP
Time-to-progression
- TACE
Transhepatic arterial chemotherapy and embolization
- PBMCs
Peripheral blood mononuclear cells
Author contribution
JZ, JL, TW and RWW collected the data and performed data analysis; FM, S-YW, YL, LS, J-LF, SY, YS, LL and MS managed the patients; C-BZ, YX, AB and JW revised the figures and tables; J-YZ, JZ and ATT drew the figures and wrote the manuscript; WL-E, SK and JJ developed the investigational HBV-TCR-T cell product; J-YZ, F-SW designed and supervised the clinical trial. All authors approved the final manuscript.
Funding
Supported by Grants from the National Science and Technology Major Project [2018ZX10301202] and the Innovative Research Team in the National Natural Science Foundation of China [81721002].
Declarations
Conflict of interest
Antonio Bertoletti is a co-founder of Lion TCR Pte. Ltd. a biotech company developing T cell receptors for treatment of virus-related cancers and chronic viral diseases. Anthony T. Tan is a scientific consultant for Lion TCR Pte. Ltd. Regina Wanju Wong, Wai Lu-En, Sarene Koh and Tingting Wang are employees of Lion TCR Pte. Ltd. Fanping Meng, Jinfang Zhao, Wei Hu, Si-Yu Wang, Jiehua Jin, Juan Wu, Yuanyuan Li, Lei Shi, Jun-Liang Fu, Shuangjie Yu, Yingjuan Shen, Limin Liu, Junqing Luan, Ming Shi, Yunbo Xie, Chun-Bao Zhou, Ji-Yuan Zhang, Fu-Sheng Wang have no conflicts of interest to disclose.
Consent to participate
This study was approved by the ethics committees of our hospital (2018096D) and fully complied with the Declaration of Helsinki and the Guideline for Good Clinical Practice, informed consent was obtained from eight subjects who participated in the study.
Consent for publication
Informed consent for publication was obtained from all authors.
Human and animal research
This article does not contain any studies with animals performed by any of the authors.
Availability of data and plant reproducibility
Supporting data for this study are available from the corresponding author and the first author upon reasonable request. The measurements of HBsAg levels were repeated three times.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fanping Meng, Jinfang Zhao, and Anthony Tanoto Tan have contributed equally to this study.
Contributor Information
Tingting Wang, Email: tinawang@liontcr.com.
Ji-Yuan Zhang, Email: uniquezjy@163.com.
Fu-Sheng Wang, Email: fswang302@163.com.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Hao XS, Wang PP, Chen KX, Li Q, He M, Yu SB, Guo ZY, et al. Twenty-year trends of primary liver cancer incidence rates in an urban Chinese population. Eur J Cancer Prev. 2003;12:273–279. doi: 10.1097/00008469-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: An open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25:515–523. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 7.Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, Kikuchi H, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71:1247–1261. doi: 10.1002/hep.30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang M, He M, Guo Y, Li H, Shen S, Xie Y, Li X, et al. The influence of immune heterogeneity on the effectiveness of immune checkpoint inhibitors in multifocal hepatocellular carcinomas. Clin Cancer Res. 2020;26:4947–4957. doi: 10.1158/1078-0432.CCR-19-3840. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, Shao G, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27:1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack SP, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 12.Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L, Zhang Y, et al. Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer. 2020;9:338–357. doi: 10.1159/000505695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng HHM, Lee RY, Goh S, Tay ISY, Lim X, Lee B, Chew V, et al. Immunohistochemical scoring of CD38 in the tumor microenvironment predicts responsiveness to anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J Immunother Cancer. 2020;8:e000987. doi: 10.1136/jitc-2020-000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–13911386. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 15.Shi M, Zhang B, Tang ZR, Lei ZY, Wang HF, Feng YY, Fan ZP, et al. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol. 2004;10:1146–1151. doi: 10.3748/wjg.v10.i8.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barkholt L, Alici E, Conrad R, Sutlu T, Gilljam M, Stellan B, Christensson B, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1:753–764. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- 17.Ecsedi M, McAfee MS, Chapuis AG. The anticancer potential of T cell receptor-engineered T cells. Trends Cancer. 2020;7:48–56. doi: 10.1016/j.trecan.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491e471. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu YT, Wong JK, Choi SW, Sze KM, Ho DW, Chan LK, Lee JM, et al. Novel pre-mRNA splicing of intronically integrated HBV generates oncogenic chimera in hepatocellular carcinoma. J Hepatol. 2016;64:1256–1264. doi: 10.1016/j.jhep.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Furuta M, Tanaka H, Shiraishi Y, Unida T, Imamura M, Fujimoto A, Fujita M, et al. Characterization of HBV integration patterns and timing in liver cancer and HBV-infected livers. Oncotarget. 2018;9:25075–25088. doi: 10.18632/oncotarget.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan AT, Yang N, Lee Krishnamoorthy T, Oei V, Chua A, Zhao X, Tan HS, et al. Use of expression profiles of HBV-DNA integrated into genomes of hepatocellular carcinoma cells to select T cells for immunotherapy. Gastroenterology. 2019;156:1862–1876e1869. doi: 10.1053/j.gastro.2019.01.251. [DOI] [PubMed] [Google Scholar]
- 23.Qasim W, Brunetto M, Gehring AJ, Xue SA, Schurich A, Khakpoor A, Zhan H, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62:486–491. doi: 10.1016/j.jhep.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Liao H, Liu Y, Li X, Wang J, Chen X, Zou J, Li Q, et al. Monitoring of serum HBV RNA, HBcrAg, HBsAg and anti-HBc levels in patients during long-term nucleoside/nucleotide analogue therapy. Antivir Ther. 2019;24:105–115. doi: 10.3851/IMP3280. [DOI] [PubMed] [Google Scholar]
- 26.Xu Z, Liu Y, Xu T, Chen L, Si L, Wang Y, Ren X, et al. Acute hepatitis B infection associated with drug-resistant hepatitis B virus. J Clin Virol. 2010;48:270–274. doi: 10.1016/j.jcv.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Jin B, Zhang JY, Xu B, Wang H, Shi M, Wherry EJ, et al. Dynamic decrease in PD-1 expression correlates with HBV-specific memory CD8 T-cell development in acute self-limited hepatitis B patients. J Hepatol. 2009;50:1163–1173. doi: 10.1016/j.jhep.2009.01.026. [DOI] [PubMed] [Google Scholar]