Abstract
A panel of 10 monoclonal antibodies (MAbs) to recombinant Norwalk virus (NV) capsid protein were tested in competition enzyme-linked immunosorbent assays. Patterns of competition indicated that these MAbs recognize six to eight epitopes covering five nonoverlapping regions of the capsid protein. A single epitope, recognized by NV MAbs NV3901, NV3912, and NV2461 was found to occur in the majority of genogroup 1 (G1) but not genogroup 2 (G2) “Norwalk-like viruses” (NLVs). This observation supports the subdivision of human NLVs into two genogroups and provides an assay for the rapid identification of G1 NLVs in fecal specimens.
“Norwalk-like viruses” (NLVs) represent one of four genera within the Caliciviridae and are a genetically and antigenically diverse group of agents that cause acute gastroenteritis in adults and children (5, 8). Morphologically identical viruses that are genetically related to NLVs have also been identified in fecal specimens from cows (1, 3). Human caliciviruses have not yet been successfully grown in tissue cultures, which has prevented the application of classical virological methods to study these agents. However, the ability of recombinant Norwalk virus (rNV) capsid protein to spontaneously form virus-like particles (VLPs) when expressed in insect cells has exponentially increased the experimental approaches available to characterize these viruses (17). The genetic diversity of human NLVs has also been studied by the sequencing of full and partial genomes. Phylogenetic analyses reveal two major distinct clusters of NLVs, designated genogroup 1 (G1) and genogroup 2 (G2) (24). G1 NLV strains include Norwalk virus (NV), Southampton virus, Desert Shield virus, and Chiba virus (CV). G2 NLV strains include Hawaii virus, Lordsdale virus, Grimsby virus (GRV), Mexico virus (MXV), and the Snow Mountain agent (4, 9, 10, 14, 15, 16, 18–20, 23).
NV VLPs are morphologically and antigenically similar to native virus, and their atomic structure has been solved by X-ray crystallography (21). These 38-nm particles exhibit a T=3 icosahedral symmetry, and 90 dimers of the single capsid protein form distinctive arch-like structures. The structure has a shell domain, consisting of the N-terminal 225 residues of the 530-amino-acid capsid protein, and a protruding (P) domain (22). The central region of the sequence forms the topmost P2 domain of the arch-like structures, and the C terminus forms the P1 domain which connects the shell and P2 domains, comprising the body of the arch-like structures.
The process of antigenic mapping of NV began by the generation of 10 monoclonal antibodies (MAbs) to rNV VLPs (11). The MAbs reacted by Western blotting or immunoprecipitation with either the 58K full-length capsid protein or the C-terminal 32K product produced by trypsin cleavage of soluble capsid protein and were classified into three reactivity groups: group I, consisting of four MAbs (NV834, NV142, NV101, and NV813) that recognize discontinuous epitopes on the rNV capsid protein; group II, consisting of MAbs NV3901, NV3912, and NV2461 that recognize continuous epitopes on the C-terminal 74 amino acids of the capsid protein; and group III, consisting of three MAbs (NV7411, NV8812, and NV8301) that recognize discontinuous epitopes in the C terminus of the capsid protein (11, 12). The three group II MAbs reacted with the 32K form by Western blotting even when the protein was denatured by boiling prior to electrophoresis, whereas group I MAbs NV101 and NV813 and group III MAbs only recognized the nondenatured 32K capsid protein (11). Group I MAbs NV834 and NV142 could not be mapped to the C-terminal region (11). However, all 10 MAbs detected NV in the stools of NV-infected volunteers by an enzyme-linked immunosorbent assay (ELISA) (11, 15). None of these MAbs reacted by ELISA or Western blotting with recombinant G2 MXV VLPs (11, 15).
To further characterize the epitopes recognized by these MAbs, competition ELISAs were performed using antibodies purified on protein A or G columns (Pierce, Rockford, Ill.) as previously described (2, 13). One MAb was used to coat flat-bottomed polyvinylchloride microtiter plates (Dynatech Laboratories, Inc., Alexandria, Va.) overnight at 4°C at a concentration of 2 μg/ml in 0.05 M carbonate bicarbonate buffer (pH 9.6). In separate tubes, rNV VLPs, at a concentration of 5 to 500 ng/ml (depending on the coating MAb), were added to decreasing concentrations of competitor MAb (5, 1, 0.5, 0.1, and 0.05 mg/ml) in phosphate-buffered saline (PBS) (pH 7.2) containing 1% (wt/vol) BLOTTO (Carnation natural nonfat milk) and then incubated overnight at 4°C. A control of rNV in 1% BLOTTO without competitor MAb was also included in each plate. The antibody-coated microtiter plates were washed twice with PBS containing 0.05% Tween 20 (PBS-T) and blocked with 5% BLOTTO for at least 30 min at 37°C. Following two additional washes with PBS-T, 60 μl of each of the rNV-MAb reaction mixtures was added to duplicate wells, and the plates were incubated for 2 h at 37°C. After washing four times, 100 μl of a 1:5,000 dilution of rabbit anti-rNV hyperimmune serum in 1% BLOTTO was added to all wells. Plates were again incubated for 2 h at 37°C and washed four times, and a 1:5,000 dilution of goat anti-rabbit total immunoglobulins (Igs) (IgM, IgG, and IgA) conjugated to horseradish peroxidase (Cappel, West Chester, Pa.) was added. After a final incubation of 1 h at 37°C and four washes with PBS-T, 100 μl of 3,3′,5,5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was added, and the color reaction was stopped by the addition of 100 μl of 1 M phosphoric acid. The optical density at 450 nm (OD450) of wells was read, and the average of duplicates was calculated. The percentage of competition or enhanced binding was determined for all competitor MAb concentrations based on the value of the PBS control (i.e., the value of rNV binding to coating MAb in the absence of competitor MAb was defined as zero). Homotypic competition was included as a positive control for all coating MAbs.
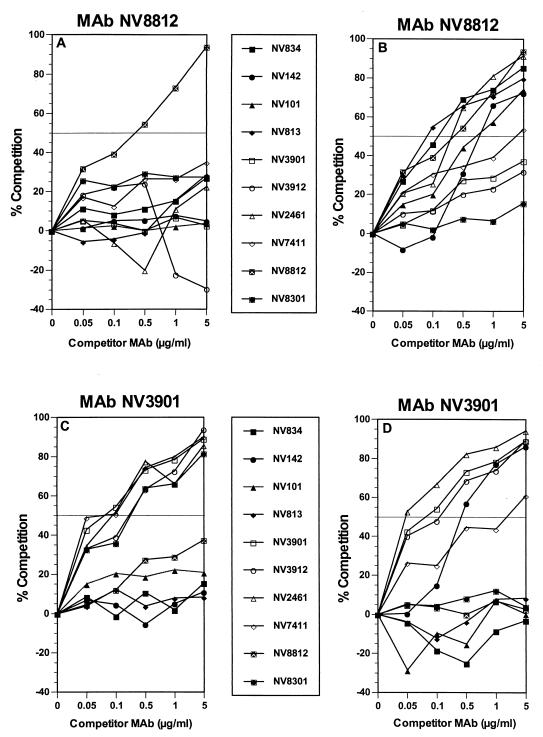
MAbs NV3901, NV3912, and NV2461 recognize a common epitope on rNV VLPs.
Initial experiments revealed that MAbs bound to the solid phase had different affinities for rNV capsid protein (data not shown). Thus, for the competition assays, the concentration of rNV VLPs used for each coating MAb was that which produced an OD450 of 0.5 to 1.5 when no competitor MAb was present. In agreement with other studies (2, 13), homotypic competition for the 10 MAbs was found to vary between 50 and 90% at the highest concentration of competitor antibody used in the ELISAs (data not shown). Significant competition was defined as at least a 50% reduction in detection signal at a concentration of 5 μg/ml of competitor antibody and when a concentration-dependent competition effect was observed.
No significant enhanced binding was observed, and the degree of heterotypic competition was found to vary from zero to over 90% (Fig. 1). The pattern of competition differed depending on whether a MAb was used as a coating or as a competitor antibody. When group III MAb NV8812 was used as the coating MAb, only homotypic competition was observed (Fig. 1A). However, when MAb NV8812 was used as the competitor antibody, MAb NV8812 competed with itself and with six additional MAbs (NV2461, NV834, NV813, NV101, NV142, and NV7411) (Fig. 1B). One-way competition was also observed when group I MAb NV142 was used as the coating MAb for all other MAbs except group I MAbs NV813 and NV834 (data not shown). Therefore, group I MAb NV142 is unique when compared to the other group I MAbs. This phenomenon may be due to a conformational change in the epitope recognized by the coating antibody, leading to a reduced affinity of binding. Alternatively, the epitopes may overlap, or one MAb may cause steric hindrance while the other may not. For some MAb combinations, two-way competition was observed. For example, group II MAb NV3901 competed with itself, group II MAbs NV3912 and NV2461, and group III MAb NV7411 (Fig. 1C and D). Group III MAb NV7411 showed a competition pattern similar to group II MAbs with the exception of the additional one-way competition of NV7411 with group I MAbs NV834 and NV813 (data not shown).
FIG. 1.
Competition ELISAs showing homotypic and heterotypic competition for MAb NV8812 (top) used either as coating antibody (A) or competitor antibody (B) or MAb NV3901 (bottom) either as coating antibody (C) or as competitor antibody (D). The 50% cutoff for significant competition is indicated by a horizontal line.
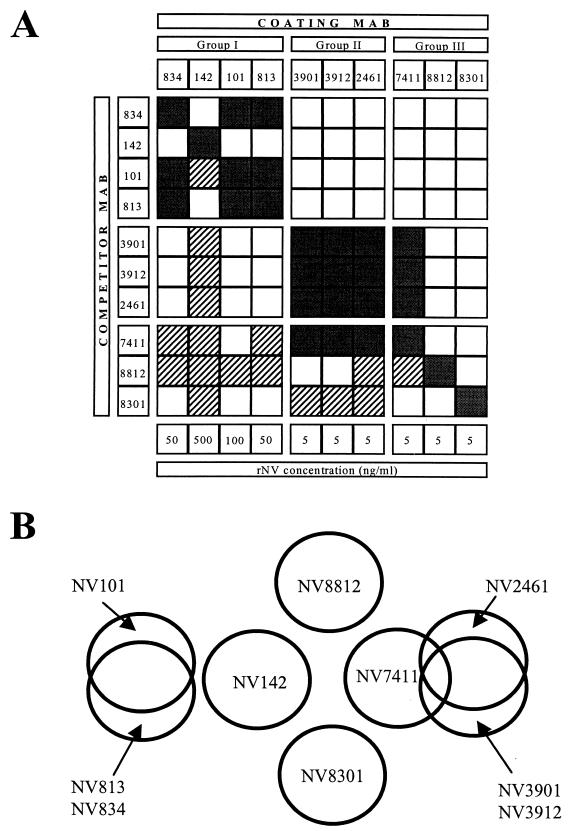
The results of the competition ELISAs for all MAb combinations are summarized in Fig. 2A. The patterns of competition were indistinguishable for a number of MAbs. Results obtained with group II MAbs NV3901 and NV3912 or MAb NV2461 were identical or similar, respectively (data not shown). Thus, group II MAbs likely recognize the same epitope or closely overlapping epitopes. Results with group I MAbs NV813, NV834, and NV101 were also identical except for the binding of group I MAb NV101 that was inhibited when group III MAb NV7411 was used as competitor antibody (data not shown). Since MAbs belonging to groups I and III recognize at least five discontinuous epitopes and two closely related discontinuous epitopes, the precise relationship of these MAbs to one another and to those in group II awaits further mapping on the rNV capsid protein. A schematic representation of the putative six to eight epitopes recognized by the 10 rNV MAbs is shown in Fig. 2B. The proposed map is compatible with the three groups described by Hardy et al. (11). In addition, group III MAb NV8812 was the only MAb to block binding of rNV to human intestinal epithelial CaCo-2 cells (25); in the present study, this MAb appears to recognize a unique epitope.
FIG. 2.
(A) Summary of epitope-mapping data. Pattern of competition of 10 anti-rNV MAbs, both as coating and competitor antibodies (as indicated). Combinations producing one-way competition are indicated by hatched lines and those producing two-way competition are indicated by black shading. MAbs are grouped according to Hardy et al. (11), and the concentration (5 to 500 ng/ml) of rNV VLPs used for each coating MAb is indicated at the bottom. (B) Proposed map of the putative six to eight epitopes on rNV VLPs defined by 10 rNV MAbs.
MAbs NV3901, NV3912, and NV2461 recognize a common epitope in G1 NLVs.
All MAbs were tested against recombinant capsid proteins of NV, CV, GRV, and MXV by indirect ELISA as described (17), with the exception that the plates were coated with antigen at a concentration of 1 μg/ml. All MAbs reacted with rNV VLPs but did not react with recombinant MXV or recombinant GRV (11, 15; data not shown). In addition, MAbs NV3901, NV3912, and NV2461 reacted with recombinant CV VLPs, a G1 NLV exhibiting 75% amino acid sequence identity over the entire capsid with NV, 66% amino acid sequence identity over the region spanning amino acids 228 to 530 (the 32K soluble protein), and 82% amino acid sequence identity over the region spanning amino acids 457 to 530 (the C-terminal 74 amino acids containing the common epitope for G1 NLVs) (11; data not shown). The fact that these MAbs were able to detect a common epitope present on the G1 CV was both unexpected and surprising because the NV polyclonal antigen capture ELISA that uses polyclonal antibodies to NV as capture and detection antibodies does not recognize CV. To determine whether MAb NV3901, NV3912, or NV2461 could be used to detect additional G1 NLVs, an antigen capture ELISA was developed essentially as described for the NV polyclonal antigen capture ELISA (6, 17) with minor modifications. Briefly, purified MAb NV3901 or rotavirus-specific MAb 3D8 (2) was used to coat flat-bottomed polyvinylchloride microtiter plates (Dynatech Laboratories, Inc.) at a concentration of 2 μg/ml in 0.05 M carbonate bicarbonate buffer (pH 9.6). A 1:5,000 dilution of guinea pig hyperimmune serum prepared against rNV VLPs (6, 17) in 1% BLOTTO was used as the detector antibody.
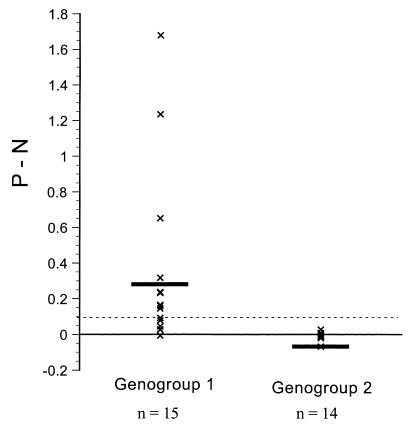
A panel of 29 fecal specimens containing G1 (n = 15) or G2 (n = 14) NLVs that had been characterized by reverse transcription-PCR and amplicon sequencing (7) were tested in the NV polyclonal and MAb NV3901 antigen capture ELISAs. None of the 29 fecal specimens reacted in the NV polyclonal antigen capture ELISA (data not shown), and the P − N values of the 29 fecal specimens in the MAb NV3901 ELISA are shown in Fig. 3. Nine of 15 (60%) fecal specimens containing strains from the G1 NLV genetic clusters (7) Musgrove, Southampton, Desert Shield, and Queens Arms (but not Winchester) were detected in the ELISA with MAb NV3901 (Table 1). None of the fecal specimens containing G2 NLV strains, represented by the Hawaii virus, Lordsdale virus, MXV, or Leeds G2 NLV genetic clusters (7), reacted in the MAb NV3901 ELISA (Table 1). The amino acid sequence identities, corresponding to the entire 539, 542, 544, 545, or 547 amino acids of the capsid protein of the G1 NLV strains tested, ranged from 63 to 70% when compared to the corresponding 530 amino acids of the capsid protein of NV (7). The level of amino acid sequence identity between NV and the G1 NLVs over the region spanning the C-terminal 74 amino acids of the capsid protein ranged from 79 to 90%. The two strains from the Winchester G1 NLV genetic cluster failed to react in the MAb NV3901 ELISA, possibly because they share the lowest capsid protein (63%) or C-terminal (79%) amino acid identity with NV. The amino acid identities of the different G2 NLV strains varied from 40 to 43% when compared to the corresponding capsid region of NV (7). Therefore, the ability of the MAb NV3901 antigen capture ELISA to detect a subset of G1 NLVs may possibly reflect the greater sensitivity of PCR over antigen detection by ELISA, differences in virus concentration among the fecal samples tested, or the result of genetic variation among the different G1 NLV genetic clusters. The main advantage of using the MAb3901 antigen capture ELISA to identify most G1 NLV strains over molecular techniques such as PCR is that large numbers of samples could be tested in a rapid and cost-effective manner.
FIG. 3.
Reactivity of 29 fecal specimens containing either G1 (n = 15) or G2 NLVs (n = 14) in an antigen ELISA based on MAb NV3901. The OD450 of MAb NV3901 (P) minus the OD450 of control rotavirus-specific MAb 3D8 (N) (2) is plotted for NLV G1 and G2. The cut off for reactivity (P − N >0.1) is indicated by a dashed line. Means are shown as horizontal bars.
TABLE 1.
Reactivity of 29 fecal specimens containing NLVs with the MAb NV3901 antigen ELISA
| Outbreak strain | Genogroup (designation)a | No. tested | No. positive |
|---|---|---|---|
| NLV/Win/94/UK | G1 (Winchester) | 2 | 0 |
| NLV/Hay/89/UK | G1 (Musgrove) | 1 | 1 |
| NLV/But/96/UK | G1 (Musgrove) | 3 | 2 |
| NLV/WR/96/UK | G1 (Southampton) | 2 | 1 |
| NLV/B291/93/UK | G1 (Desert Shield) | 1 | 1 |
| NLV/L&G/96/UK | G1 (Desert Shield) | 4 | 3 |
| NLV/QA/92/UK | G1 (Queens Arms) | 1 | 0 |
| NLV/Val/95/Malta | G1 (Queens Arms) | 1 | 1 |
| NLV/WGH/96/UK | G2 (Hawaii) | 2 | 0 |
| NLV/Elm/93/UK | G2 (Hawaii) | 1 | 0 |
| NLV/Grl/93/UK | G2 (Hawaii) | 1 | 0 |
| NLV/RBH/93/UK | G2 (Mexico) | 2 | 0 |
| NLV/RHCH/96/UK | G2 (Lordsdale) | 2 | 0 |
| NLV/ELH/96/UK | G2 (Lordsdale) | 1 | 0 |
| NLV/Bai/96/UK | G2 (Lordsdale) | 1 | 0 |
| NLV/Tru/96/UK | G2 (Lordsdale) | 1 | 0 |
| NLV/B288/93/UK | G2 (Lordsdale) | 1 | 0 |
| NLV/Col/93/UK | G2 (Leeds) | 2 | 0 |
| Total G1 NLVs | 15 | 9 | |
| Total G2 NLVs | 14 | 0 | |
| Total NLVs | 29 | 9 |
According to Green et al. (7).
In conclusion, MAbs NV2461, NV3901, and NV3912 appear to map to a single epitope that is common to G1 NLVs. This common epitope is present in monomeric forms of the 32K trypsin cleavage product and has been mapped to the C-terminal 74 amino acids of the capsid (11). Studies are underway to further map this epitope. These studies may allow determination of an equivalent epitope in G2 NLVs using sequence alignments directed by the rNV capsid atomic structure (21). Antibodies directed to the putative common epitope in G2 NLVs, in combination with MAb NV3901 that recognizes the common epitope in G1 NLVs, might allow the development of a broadly cross-reactive ELISA to rapidly and efficiently identify G1 and G2 NLVs in stool samples as a cause of outbreaks and sporadic cases of gastroenteritis worldwide.
Acknowledgments
We are extremely grateful to Sue E. Crawford, Robert L. Atmar, and Pamela J. Glass for helpful discussions.
This work was supported by Public Health Service grant AI38036 from the National Institute of Allergy and Infectious Diseases. Antony D. Hale undertook this work while on a fellowship from The Pathological Society of Great Britain and Ireland.
REFERENCES
- 1.Bridger J C, Hall G A, Brown J F. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect Immun. 1984;43:133–138. doi: 10.1128/iai.43.1.133-138.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns J W, Greenberg H B, Shaw R D, Estes M K. Functional and topological analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. J Virol. 1988;62:2164–2172. doi: 10.1128/jvi.62.6.2164-2172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dastjerdi A M, Green J, Gallimore C I, Brown D W G, Bridger J C. The bovine Newbury agent-2 is genetically more closely related to human SRSVs than to animal caliciviruses. Virology. 1999;254:1–5. doi: 10.1006/viro.1998.9514. [DOI] [PubMed] [Google Scholar]
- 4.Dingle K E, Lambden P R, Caul E O, Clarke I N. Human enteric Caliciviridae: the complete genome sequence and expression of virus-like particles from a genetic group II small round structured virus. J Gen Virol. 1995;76:2349–2355. doi: 10.1099/0022-1317-76-9-2349. [DOI] [PubMed] [Google Scholar]
- 5.Estes M K, Atmar R L, Hardy M E. Norwalk virus and other enteric caliciviruses. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1997. pp. 1009–1034. [Google Scholar]
- 6.Graham D Y, Jiang X, Tanaka T, Opekum A R, Madore H P, Estes M K. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 7.Green, J., J. Vinjé, C. Gallimore, M. Koopmans, A. D. Hale, and D. W. G. Brown. Capsid protein diversity among “Norwalk-like” viruses. Virus Genes, in press. [DOI] [PubMed]
- 8.Green, K. Y., T. Ando, M. S. Balayan, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neill, M. J. Studdert, and H.-J. Thiel. Caliciviridae. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. H. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh report of the International Committee on Taxonomy of Viruses, in press. Academic Press, Orlando, Fla.
- 9.Hale A D, Crawford S E, Ciarlet M, Green J, Gallimore C, Brown D W G, Jiang X, Estes M K. Expression and self-assembly of Grimsby virus: antigenic distinction from Norwalk and Mexico viruses. Clin Diagn Lab Immunol. 1999;6:142–145. doi: 10.1128/cdli.6.1.142-145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy M E, Kramer S F, Treanor J J, Estes M K. Human calicivirus genogroup II capsid sequence diversity revealed by analyses of the prototype Snow Mountain agent. Arch Virol. 1997;142:1469–1479. doi: 10.1007/s007050050173. [DOI] [PubMed] [Google Scholar]
- 11.Hardy M E, Tanaka T N, Kitamoto N, White L J, Ball J M, Jiang X, Estes M K. Antigenic mapping of recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology. 1996;217:252–261. doi: 10.1006/viro.1996.0112. [DOI] [PubMed] [Google Scholar]
- 12.Hardy M E, White L J, Ball J M, Estes M K. Specific proteolytic cleavage of recombinant Norwalk virus capsid protein. J Virol. 1995;69:1693–1698. doi: 10.1128/jvi.69.3.1693-1698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendry R M, Fernie B F, Anderson L J, Godfrey E, McIntosch K. Monoclonal capture antibody ELISA for respiratory syncytial virus; detection of individual viral antigens and determination of monoclonal antibody specificities. J Immunol Methods. 1985;77:247–258. doi: 10.1016/0022-1759(85)90037-7. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Matson D O, Ruiz-Palacios G M, Hu J, Treanor J, Pickering L K. Expression, self-assembly, and antigenicity of a Snow Mountain agent-like calicivirus capsid protein. J Clin Microbiol. 1995;33:1452–1455. doi: 10.1128/jcm.33.6.1452-1455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Matson D O, Velázquez F R, Calva J J, Zhong W M, Hu J, Ruíz-Palacios G M, Pickering L K. Study of Norwalk-related viruses in Mexican children. J Med Virol. 1995;47:309–316. doi: 10.1002/jmv.1890470404. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Wang M, Estes M K. Sequence and genomic organization of Norwalk virus. Virology. 1993;195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Wang M, Graham D Y, Estes M K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambden P R, Caul E O, Ashley C R, Clarke I N. Sequence and genome organization of a human small round structured (Norwalk-like) virus. Science. 1993;259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- 19.Lew J F, Kapikian A Z, Jiang X, Estes M K, Green K Y. Molecular characterization and expression of the capsid protein of a Norwalk-like virus recovered from a Desert Shield troop with gastroenteritis. Virology. 1994;200:319–325. doi: 10.1006/viro.1994.1194. [DOI] [PubMed] [Google Scholar]
- 20.Lew J F, Kapikian A Z, Valdesuso J, Green K Y. Molecular characterization of Hawaii virus and other Norwalk-like viruses: Evidence of genetic polymorphism among human caliciviruses. J Infect Dis. 1994;170:535–542. doi: 10.1093/infdis/170.3.535. [DOI] [PubMed] [Google Scholar]
- 21.Prasad B V V, Hardy M E, Dokland T, Bella J, Rossmann M G, Estes M K. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 22.Prasad B V V, Rothnagel R, Jiang X, Estes M K. Three-dimensional structure of baculovirus-expressed Norwalk virus capsids. J Virol. 1994;68:5117–5125. doi: 10.1128/jvi.68.8.5117-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utagawa E T, Takeda N, Inouye S, Kasunga K, Yamazaki S. 3′-Terminal sequence of a small round structured virus (SRSV) in Japan. Arch Virol. 1994;135:185–192. doi: 10.1007/BF01309777. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Jiang X, Madore H P, Gray J, Desselberger U, Ando T, Seto Y, Oishi I, Lew J F, Green K Y, Estes M K. Sequence diversity of small round structured viruses in the Norwalk virus group. J Virol. 1994;68:5982–5990. doi: 10.1128/jvi.68.9.5982-5990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White L J, Ball J M, Hardy M E, Tanaka T N, Kitamoto N, Estes M K. Attachment and entry of recombinant Norwalk virus capsids to cultured human and animal cell lines. J Virol. 1996;70:6589–6597. doi: 10.1128/jvi.70.10.6589-6597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]