Abstract
Objective
While adjuvant treatment regimens have been modified for older patients with glioblastoma (GBM), surgical strategies have not been tailored.
Methods
Clinical data of 48 consecutive patients aged 70 years or older, who underwent surgical resection for GBM with intraoperative ultrasonography (IoUS) alone or combination with intraoperative MRI (IoMRI) at Yale New Haven Hospital were retrospectively reviewed. Variables were analyzed, and comparative analyses were performed.
Results
The addition of IoMRI was not superior to IoUS alone in terms of overall survival (OS) (P = 0.306), Karnofsky Performance Score (KPS) at postoperative 6 weeks (P = 0.704) or extent of resection (P = 0.263). Length of surgery (LOSx), however, was significantly longer (P = 0.0002) in the IoMRI group. LOSx (P = 0.015) and hospital stay (P = 0.025) were predictors of postoperative complications. Increased EOR (GTR or NTR) (P = 0.030), postoperative adjuvant treatment (P < 0.0001) and postoperative complications (P = 0.006) were predictive for OS. Patients with relatively lower preoperative KPS scores (<70) showed significant improvement at postoperative 6 weeks (P<0.0001). Patients with complications (P = 0.038) were more likely to have lower KPS at postoperative 6 weeks.
Conclusions
Aggressive management with surgical resection should be considered in older patients with GBM, even those with relatively poor KPS. The use of ioMRI in this population does not appear to confer any measurable benefit over ioUS in experienced hands, but prolongs the length of surgery significantly, which is a preventable prognostic factor for impeding care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11060-021-03862-z.
Keywords: Glioblastoma, Surgical strategies, Neuronavigation, Intraoperative imaging
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults. The incidence of GBM increases with advanced age, with a peak in the 75–84 age group [1]. Studies show that more than one fourth of GBM patients are older than 70 years of age at the time of diagnosis [2]. Recent studies centered on the use of radiation and chemotherapy in this older population have garnered results and recommendations to help specifically guide treatment of GBM [3–8] in this population, however strategies for surgical management have yet to be optimized.
Though there is compelling evidence that maximal safe resection and chemoradiation are associated with improved survival in younger GBM patients, many physicians have traditionally followed more conservative approaches when treating septuagenarians and octogenarians [9–11]. This paradigm, however, is recently challenged by a growing body of evidence demonstrating that elderly patients benefit from maximal safe resection and chemoradiation [3, 4, 5, 6, 7, 8, 12, 13, 14, 15, 16, 17, 18, 19, 20]. Indeed, improved survival with increased EOR in older GBM patients occurs in patients without postoperative complications [21] and prolonged surgery is an independent predictor of complications in elderly patients undergoing craniotomies [22–29].
More sophisticated intraoperative image guidance techniques have been used more routinely in GBM surgery to achieve maximal EOR. Though the use of intraoperative MRI (IoMRI) may now be considered the “gold standard”, it prolongs surgical time [30]. On the other hand, IoUS remains a reliable, real-time imaging tool, which improves surgical resection [31–35], while reducing the length of surgery (LOSx) compared with IoMRI. Surgeon expertise with its interpretation, however, is important for its success. Given the notion that increased surgical time could potentially be associated with postoperative complications, we sought to determine whether there were indeed any differences in outcome with these intraoperative image guidance techniques in older patients with GBM.
Methods
Clinical data acquisition
This study was approved by the Yale School of Medicine Institutional Review Board. Forty-eight consecutive patients, age 70 years and older, who underwent surgical resection by the senior author for histologically confirmed GBM between November 2015 and January 2021 were included. Demographic and clinical variables were retrospectively collected. All patients were followed until death or through submission of this manuscript with none lost during follow-up. Patients not meeting the aforementioned criteria and/or those who underwent biopsy were excluded.
Functional assessment
Functional assessment was done using the Karnofsky Performance Scale (KPS) score, as determined by retrospective review of detailed medical records. Preoperative KPS was determined at the time of admission, while postoperative KPS was assessed at approximately 6 weeks follow-up. Functional improvement was determined based on the difference. To further evaluate the clinical improvement of patients, we also used The Neurologic Assessment in Neuro-Oncology (NANO) scale [36, 37].
Tumor location, volume and extent of resection
Tumor volume was calculated using the modified ellipsoidal formula, V = (4/3) × π × (D1/2) × (D2/2) × (D3/2) based on the maximum tumor diameters in perpendicular dimensions. All patients underwent contrast enhanced T1-weighted MRI before surgery and within 48 h after surgery when possible and were used to determine the amount of tumor removed. All surgeries were performed by the senior author (JM). The extent of resection (EOR) was calculated using the formula 1 − [((Pre-Op Tm Volume – Post-Op Tm Volume) / Pre-Op Tm Volume) * 100]. In accordance with prior literature, EOR was independently determined by a board certified neuroradiologist (RKF) as: (1) gross total resection (i.e. GTR: ≥98% tumor removal), (2) near total resection (i.e. NTR: 90–98% tumor removal) or (3) subtotal resection (i.e. STR: <90% of tumor removal) [38]. For patients who were unable to undergo postoperative MRI, EOR was determined based on intraoperative imaging, post-operative CT and operative report.
Intraoperative image guidance techniques
The use of the Brainlab Neuronavigation system and an operating microscope was standard in all surgeries. The 3D intraoperative ultrasound (IoUS) system (BK Flex Focus 800 or bk5000 Neurosurgical System) and 3T intraoperative magnetic resonance imaging (IoMRI) (3T MRI Scanner, Siemens MAGNETOM) were used as intraoperative image guidance modalities.
Tumor pathology
Histological diagnosis of all tumors was determined by board-certified neuropathologists in accordance with WHO guidelines. MGMT status was determined in forty-seven patients (Table 1).
Table 1.
General patient characteristics and comparative analysis between ioUS and combined ioMRI + ioUS groups
| Characteristics | Total | IoUS | ioUS+IoMRI | P value |
|---|---|---|---|---|
| Age in Years (Mean ± SD) | 75.98 ± 4.99 | 80.16 ± 5.99 | 74.43 ± 3.36 | 0.0053 |
| Gender | ||||
| Female | 20 | 3 (23.08%) | 17 | 0.188 |
| Male | 28 | 10 (76.92%) | 18 | |
| Presentation | ||||
| Altered mental status | 17 (35.42%) | 5 (38.46%) | 12 (34.29 %) | 1 |
| Facial strength | 9 (18.75%) | 2 (15.38%) | 7 (20 %) | 1 |
| Language/Speech deficit | ||||
| Seizures | 5 (10.42%) | 2 (15.38%) | 3 (8.57%) | 0.602 |
| Motor deficit and Ataxia | 16 (33.33%) | 6 (46.15%) | 10 (28.57%) | 0.311 |
| Visual field cut | 10 (20.83%) | 1 (7.69%) | 9 (25.71%) | 0.248 |
| Past medical history | 44 (91.67%) | 12 (92.31%) | 32 (91.43%) | 1 |
| Hypertension | 27 (56.25%) | 7 (53.85%) | 20 (57.14%) | 1 |
| Diabetes Mellitus | 9 (18.75%) | 1 (7.69%) | 8 (22.86%) | 0.411 |
| Hyperlipidemia | 23 (47.92%) | 6 (46.1 %) | 17 (48.57%) | 1 |
| Thyroid disorder | 5 (10.42%) | 0 | 5 (14.29%) | 0.304 |
| DVT/PE | 4 (8.33%) | 1 (7.69 %) | 3 (8.57%) | 1 |
| Cardiovascular Disorders | 17 (35.42 %) | 8 (61.54 %) | 9 (25.71%) | 0.039 |
| Arrhythmia | 9 (18.75 %) | 4 (30.77 %) | 5 (14.29%) | 0.228 |
| Stroke | 4 (8.33 %) | 2 (15.38 %) | 2 (5.71%) | 0.294 |
| CAD | 4 (8.33%) | 2 (15.38%) | 2 (5.71%) | 0.294 |
| Aortic aneurysm | 3 (6.25%) | 3 (23.08%) | 0 | 0.017 |
| Aortic stenosis | 2 (4.16%) | 1 (7.69%) | 1 (2.86%) | 0.473 |
| Localization | ||||
| Right | 32 (66.67%) | 7 (53.85%) | 25 (71.43%) | 0.311 |
| Left | 16 (33.33%) | 6 (46.15%) | 10 (28.57%) | |
| Anatomic classification | ||||
| Supratentorial | 47 (97.92%) | 13 (100%) | 34 (97.14%) | 1 |
| Infratentorial | 1 (2.08%) | 0 | 1 (2.86%) | |
| Location | ||||
| Frontal | 15 (31.25 %) | 4 (30.77%) | 11 (31.43%) | 1 |
| Temporal | 22 (45.83 %) | 6 (46.15%) | 16 (45.71%) | 1 |
| Parietal | 16 (33.33 %) | 5 (38.46%) | 11 (31.43%) | 0.735 |
| Occipital | 3 (6.25%) | 1 (7.69%) | 2 (5.71%) | 1 |
| Multilobar | 8 (16.67%) | 3 (23.08%) | 5 (14.29%) | 0.665 |
| Multifocal | 1 (2.08 %) | 1 (7.69%) | 0 | 0.271 |
| Eloquent structure involvement | 16 (33.33 %) | 5 (38.46%) | 11 (31.43%) | 0.735 |
| Basal Ganglia | 6 (12.50%) | 3 (23.08%) | 3 (8.57%) | 0.323 |
| Insula | 8 (16.67%) | 1 (7.69%) | 7 (20%) | 0.418 |
| Thalamus | 3 (6.25%) | 2 (15.38%) | 1 (2.86%) | 0.265 |
| Motor cortex | 1 (2.08%) | 1 (7.69%) | 0 | 0.271 |
| Large arterial encasement by the tumor | 1 (2.08 %) | 0 | 1 (2.86%) | 1 |
| Brainstem | 1 (2.08%) | 0 | 1 (2.86%) | 1 |
| Corpus callosum | 0 | 0 | 0 | NA |
| Median tumor volume | 27.35 | 24.35 | 34.25 | 0.1454 |
| MGMT status | ||||
| Methylated | 17 (36.17%) | 3 (6.38%) | 14 (29.79%) | 1* |
| Partially methylated | 6 (12.77%) | 3 (6.38%) | 3 (6.38%) | |
| Non-Methylated | 24 (51.06%) | 6 (12.77%) | 18 (36.30%) |
Bold values indicate statistical significance at the p < 0.05 level
*Methylated and partially methylated MGMT have been grouped together for the comparative analysis
DVT Deep venous thrombosis, PE Pulmonary embolus
Statistical analysis
Statistical analyses were performed using SPSS (IBM SPSS Statistics, version 24) or Graphpad Prism version 8.3.0. Univariate analyses used either simple logistic regression model or one of the following: Fisher Exact test (categorical variables when two groups were compared); pairwise Fisher exact test (if more than two groups were compared); t-test with Welch correction (continuous variables with normal distribution); Mann Whitney-U test was conducted for any variables, which did not pass a Kolmogorov-Smirnov normality test; Wilcoxon Matched-Pairs Signed Rank test was used for paired variables with normal distribution. When the dependent variable was continuous, univariate and multivariate analyses were performed using linear regression models. For multivariate analysis, a binary logistic regression with a forward stepwise selection model was conducted using all variables tested in univariate analysis with an entry P value threshold 0.05 and removal threshold 0.1. Cox proportional hazards model was used to examine the impact of the variables on overall survival (OS). Kaplan Meier survival curves were performed to visualize OS for significant variables. P values < 0.05 were considered statistically significant.
Results
Cohort characteristics
Forty-seven patients had newly diagnosed GBM; one patient who presented with a “recurrent” GBM was operated on in another hospital 5 months prior to undergoing surgery with the senior author and had not received any adjuvant treatment. The average age was 75.98 years (range: 70–92). The median preoperative KPS score was 70 (range: 20–100), while the median KPS at 6-weeks follow-up was 90 (range: 20–100). The median tumor volume was 27.35 cm 3. These results are summarized in Tables 1 and 2.
Table 2.
Descriptive analysis of the surgical outcome and comparison between the ioUS and combined ioMRI + ioUS group
| Characteristics | Total | IoUS | ioUS+IoMRI | P value |
|---|---|---|---|---|
| Preoperative Karnofski performance scale (KPS) Score | ||||
| ≥70 | 26 (54.17 %) | 8 (61.54 %) | 18 (51.43 %) | 0.746 |
| <70 | 22 (45.83 %) | 5 (38.46 %) | 17 (48.57 %) | |
| Preoperative NANO scale (median) | 3 | 2.5 | 3 | 0.917 |
| Extent of resection | ||||
| Gross total resection (GTR) | 31 (64.58 %) | 7 (53.84 %) | 24 (68.58 %) | 0.498 |
| Near total resection (NTR) | 12 (25 %) | 3 (23.08 %) | 9 (25.71 %) | 1 |
| Subtotal resection (STR) | 5 (10.42 %) | 3 (23.08 %) | 2 (5.71 %) | 0.115 |
| Average length of surgery (minutes) | 169.7 ± 58.93 | 111.1 ± 51.32 | 189.7 ± 47.11 | 0.0002 |
| Postoperative complications * | 8 (16.67 %) | 1 (7.69 %) | 7 (20 %) | 0.418 |
| Altered mental status | 4 (8.33 %) | 0 | 4 (11.43 %) | 0.563 |
| Urinary tract infection | 3 (6.25 %) | 0 | 3 (8.57 %) | 0.553 |
| Wound infection | 2 (4.17 %) | 0 | 2 (5.71 %) | 1 |
| Ileus | 2 (4.17 %) | 0 | 2 (5.71 %) | 1 |
| Postoperative hematoma (Managed without evacuation) | 1 (2.08 %) | 1 (7.69 %) | 0 | 0.271 |
| Pulmonary embolus | 1 (2.08 %) | 0 | 1 (2.86 %) | 1 |
| Perforator infarct | 1 (2.08 %) | 0 | 1 (2.86 %) | 1 |
| Atrial fibrillation | 1 (2.08 %) | 0 | 1 (2.86 %) | 1 |
| Average length of the hospital stay after the surgery (Days) | 4.583 ± 3.451 | 4.769 ± 2.891 | 4.514 ± 3.673 | 0.803 |
| Disposition | ||||
| Home | 23 (47.92 %) | 6 (46.15 %) | 17 (48.57 %) | 1 |
| Skilled nursing facilities (Short-term) | 16 (33.33 %) | 5 (38.46 %) | 11 (31.43 %) | 0.735 |
| Rehabilitation centers | 9 (18.75 %) | 2 (15.38 %) | 7 (20 %) | 1 |
| Functional outcome (Median) | ||||
| Postoperative KPS score (at discharge) | 70 | 70 | 70 | 0.964 |
| Postoperative follow-up KPS score (at 6 weeks follow-up) | 90 | 80 | 90 | 0.382 |
| Median Follow-up NANO scale | 1 | 1 | 1 | 0.722 |
| Changes in functional outcome | ||||
| Improvement | 28 (58.33 %) | 6 (46.15 %) | 22 (62.86 %) | 0.339 |
| Unchanged | 12 (25 %) | 5 (38.46 %) | 7 (20 %) | 0.263 |
| Worsening | 8 (16.67 %) | 2 (15.38 %) | 6 (17.14 %) | 1 |
| Chemoradiation protocols | ||||
| Stupp protocol | 24 (50 %) | 4 (30.77 %) | 20 (57.14 %) | 0.193 |
| Perry protocol | 5 (10.42 %) | 0 | 5 (14.29 %) | 0.304 |
| Hypofractionated radiation treatment alone | 8 (16.67 %) | 4 (30.77 %) | 4 (11.43 %) | 0.187 |
| No therapy | 11 (22.92 %) | 5 (38.46 %) | 6 (17.14 %) | 0.14 |
Bold values indicate statistical significance at the p < 0.05 level
*Some patients developed more than one complication
GTR or NTR were achieved in forty-three cases (89.58%). Average length of hospital stay was 4.58 days, with 47.92% of patients being discharged home. Twenty-four patients (50%) received adjuvant treatment according to Stupp Protocol [39], five patients (10.42%) according to Perry Protocol [3] and eight patients (16.67%) received hypo-fractionated radiotherapy alone. Eleven patients (22.92 %) did not receive any adjuvant treatment.
Comparison of intraoperative imaging modalities
A combination of IoMRI and IoUS was used in 35 cases, while IoUS solely was used in 13 cases. There were no statistically significant differences between the IoUS and IoUS+IoMRI groups in terms of gender (Fisher Exact test, P = 0.188), pre-operative tumor volume (Mann-Whitney test, P = 0.15), involvement of eloquent structures (Fisher Exact Test; P = 0.74), MGMT status (Fisher Exact test, P = 1) or preoperative KPS score (Mann-Whitney test, P = 0.96). Patients in the IoUS group, however, were significantly older (t-test with Welch correction, P = 0.005) (Table 1).
While there was no difference in EOR (Pairwise Fisher Exact test, P = 0.263) between the groups, the LOSx was significantly longer (t-test with Welch correction, P = 0.0002) in the IoMRI group. There were no significant differences however with regards to postoperative complications (Fisher Exact test, P = 0.42), length of hospital stay (Mann-Whitney test, P = 0.80) or place of disposition (Pairwise Fisher Exact Test, P = 0.918). There were no differences in terms of number of patients who received adjuvant treatment with Stupp Protocol [39] (Fisher Exact test, P = 0.193), Perry Protocol [3] (Fisher Exact test, P = 0.304), hypo-fractionated radiotherapy alone (Fisher Exact test, P = 0.187) or who did not receive any adjuvant treatment (Fisher Exact test, P = 0.14) (Table 2). Patients in both groups showed significant functional improvement at 6 weeks postoperative follow-up (Wilcoxon matched-pairs signed Rank Test, P = 0.039 and P = 0.0003 in ioUS and ioMRI+ioUS, respectively).
Postoperative complications
Postoperative complications were observed in 8 patients (16.67%), some of whom experienced more than one issue. Several of these complications were related to medical care, including urinary tract infections and pulmonary embolus (Table 2). Prolonged surgery time (OR = 1.023, 95%, CI 1.005–1.043; P = 0.013), pre-operative KPS score less than 70 (OR = 11.67, 95% CI 1.305–104.337; P = 0.028) and prolonged hospital stay (OR = 1.249, 95% CI 1.025–1.522; P = 0.028) were among the significant predictors of complications.
The frequency of postoperative complications in the ioMRI+ioUS group was more than two-times as much as the ioUS-alone cases (20–7.69%), though this difference was not statistically significant (Fisher Exact Test, P = 0.42).
LOSx (OR = 1.033, 95% CI 1.006–1.06; P = 0.015) and hospital stay (OR = 1.422, 95% CI 1.046–1.932; P = 0.025) remained significant in multiple logistic regression analysis (Table 3), suggesting that longer time spent in surgery and the hospital increases the likelihood of postoperative complications.
Table 3.
Logistic regression analysis of postoperative complications
| Characteristics | Odds Ratio (95% CI) | P value |
|---|---|---|
| Univariate analysis simple logistic regression | ||
| Length of surgery | 1.023 (1.005–1.043) | 0.013 |
| Pre-operative KPS (KPS score ≥70 vs. KPS score <70) | 0.085 (0.010–0.767) | 0.028 |
| Length of hospital stay | 1.249 (1.025–1.522) | 0.028 |
| Tumor volume | 0.974 (0.934–1.016) | 0.226 |
| Pre-operative language deficit | 2.4 (0.471–12.22) | 0.292 |
| Gender | 0.36 (0.075–1.727) | 0.202 |
| Image guidance technique (ioUS vs. ioMRI + ioUS) | 0.333 (0.037–3.014) | 0.328 |
| Pre-operative motor deficit | 1.246 (0.258–6.031) | 0.784 |
| Age | 0.961 (0.810–1.139) | 0.644 |
| Pre-operative altered mental status | 0.556 (0.099–3.113) | 0.504 |
| Involvement of eloquent structures | 1.246 (0.257–6.031) | 0.784 |
| Stepwise-Forward Multiple Logistic Regression | ||
| Length of surgery | 1.033 (1.006–1.06 ) | 0.015 |
| Length of hospital stay | 1.422 (1.046 –1.932) | 0.025 |
Bold values indicate statistical significance at the p < 0.05 level
KPS Karnofski Performance Scale; ioUS, intraoperative ultrasonography, ioMRI intraoperative magnetic resonance imaging. NANO, The Neurologic Assessment in Neuro-Oncology
Discharge disposition
We analyzed the place of disposition as a reflection of immediate postoperative functional outcome. 23 patients (47.92 %) were discharged home, while 16 patients (33.33%) were discharged to short-term skilled nursing facilities (SNF) and 9 (18.75 %) to rehabilitation centers. The multiple logistic regression model demonstrated that patients with a preoperative KPS score less than 70 (OR = 0.211, 95% CI 0.061–0.732; P = 0.014) were less likely to be discharged home and more likely to SNF or rehabilitations centers.
Follow-up KPS score at postoperative six week and functional improvement
We next sought to elucidate the variables contributing to the follow-up KPS score at postoperative 6 weeks. Univariate analysis implicated postoperative complications (OR = 0.15, 95% CI 0.029–0.764; P = 0.022), and preoperative language deficit (OR = 0.194, 95 % CI 0.046–0.824; P = 0.026) as significant predictors of follow-up KPS score. In multivariate logistic regression model, postoperative complications (OR = 0.161, 95% CI 0.03- 0.904; P = 0.038) and preoperative language deficit (OR = 0.208, 95% CI 0.045–0.971; P = 0.046) remained significant to predict a follow-up KPS score less than 70 at postoperative 6 weeks.
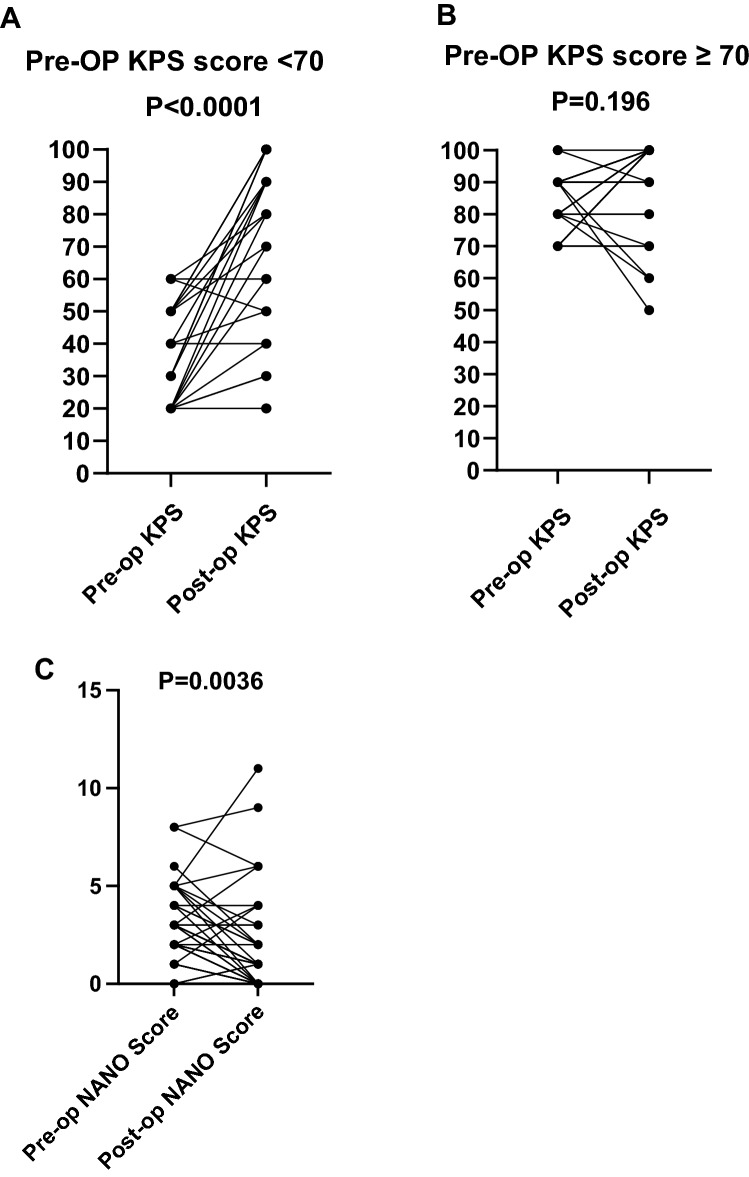
We further analyzed the factors associated with postoperative functional improvement, which we defined as an at least 10-point difference between the follow-up KPS and the preoperative KPS scores. We found that 28 patients (58.33%) showed functional improvement, while 12 patients (25%) remained the same and 8 patients (16.67%) worsened. Interestingly, 59% of patients with a preoperative KPS score less than 70 showed a follow-up KPS score 70 or higher and showed significant functional improvement (Wilcoxon matched-pairs signed Rank Test, P<0.0001), although patients with a preoperative KPS score 70 or higher did not show a significant change (P = 0.196) (Fig. 1). This result remained the same (P = 0.1578) when we excluded two patients with a preoperative KPS score of 100 from the analysis. Taken together, these results suggest that patients with relatively lower preoperative KPS scores can demonstrate significant functional improvement following surgery, while those with higher scores maintain them.
Fig. 1.
Patients with pre-operative KPS score <70 demonstrated significant improvement 6-weeks after surgical
resection. Paired parallel axis dot plot showing significant functional improvement for patients
with pre-op KPS score <70 following the surgical resection (p<0.0001) (A) ,while no significant changes were observed in pre-op KPS score ≥ 70 group (B). Preoperative NANO scale improved significantly at postoperative follow-up assessment (P = 0.0036) from a preoperative median score of 3 to 1 at follow-up (C). Abbreviations: Pre-op, preoperative; post-op, postoperative; KPS, Karnofski Performance Scale; NANO, The Neurologic Assessment in Neuro-Oncology
Forty-three patients, who had clear physical examination records necessary for all components of scoring were assessed using the NANO scale. Median preoperative and follow-up NANO scale scores were 3 and 1, respectively. A significant improvement was observed when the functional status of patients was evaluated using NANO scale (P = 0.0036, Wilcoxon Matched-Pairs Signed Rank test) (Fig. 1). 28 (65%) of the patients showed at least 1 level improvement in NANO scale, while 20 patients (46.5%) showed at least 2 levels, and 10 (23.3%) patients showed at least 3 levels improvement.
Postoperative adjuvant treatment
We next elucidated variables contributing to the likelihood patients went on to receive postoperative adjuvant treatment, including the Stupp protocol [39], Perry protocol [3] or hypofractionated RT only.
We found that a preoperative KPS score 70 or higher showed the most significant association in univariate analysis (OR = 8.307, 95% CI 1.557–44.32; P = 0.013) with more likelihood to proceed with adjuvant therapy, which was followed by the KPS score at postoperative 6 weeks (OR = 5.143, 95% CI 1.214–21.795; P = 0.026).
Preoperative KPS score was significantly associated with postoperative adjuvant treatment in multivariate analysis (OR = 16.251, 95% CI 2.048–128.944; P = 0.008). However, when we adjusted the analysis for the postoperative KPS score, we found that the preoperative KPS score is not a significant predictor of postoperative adjuvant treatment for the patients who had a follow-up KPS score less than 70 (P = 0.99), underscoring the importance of postoperative KPS score in decision-making for postoperative adjuvant treatment.
These findings suggest that the functional status of older GBM patients after surgery is an important determinant in whether patients are able to continue with postoperative adjuvant treatment, and not necessarily predictive pre-operatively.
Survival analyses
Two patients were excluded: One who was operated in another institution for GBM five months prior to the second surgery in our institution was excluded, and another with COVID19 during the radiation treatment, which resulted in interruption of the treatment and death soon thereafter.
Univariate analysis showed the presence of postoperative complications (HR = 4.119, 95% CI 1.753–9.679; P = 0.001) was predictive for overall survival (OS). In this cohort, we found that postoperative adjuvant therapy (any aforementioned type) is associated with improved OS compared to none (HR = 0.075, 95% CI 0.028–0.204, P<0.0001).
In multivariate analysis, we found that greater EOR (GTR or NTR vs. STR) (Median OS = 281 vs. 163 days; HR = 0.329, 95% CI 0.121–0.896; P = 0.030) and use of postoperative adjuvant treatment (Median OS = 335 vs. 110; HR = 0.144, 95% CI 0.057 – 0.362; P<0.0001), are associated with improved OS in this population of patients. Overall survival, however, was significantly shorter with the presence of postoperative complications (Median OS = 120 vs. 305 days; HR = 3.620, 95% CI 1.433 – 9.144, P = 0.006) (Table 4).
Table 4.
Univariate and Multivariate Analysis of overall survival with Cox proportional hazards model
| Characteristics | Hazard ratio (95% CI) | P value |
|---|---|---|
| Univariate analysis | ||
| Adjuvant treatment | 0.075 (0.028–0.204) | 3.47e–7 |
| Post-operative complications | 4.119 (1.753–9.679) | 0.001 |
| Pre-operative KPS (KPS score ≥70 vs. KPS score <70) | 0.559 (0.301–1.040) | 0.066 |
| EOR (GTR+NTR vs. STR) | 0.540 (0.209–1.400) | 0.205 |
| MGMT * (methylated and partially methylated vs. unmethylated) | 0.694 (0.366–1.317) | 0.264 |
| Gender | 1.431 (0.762–2.690) | 0.265 |
| Image guidance technique (ioUS vs. ioMRI + ioUS) | 1.450 (0.712–2.954) | 0.306 |
| Pre-operative altered mental status | 1.290 (0.677–2.460) | 0.439 |
| Involvement of eloquent structures | 1.192 (0.621–2.289) | 0.598 |
| Pre-operative motor deficit | 1.175 (0.606–2.281) | 0.633 |
| Tumor volume | 0.998 (0.990–1.008) | 0.802 |
| Follow-Up KPS (KPS score ≥70 vs. KPS score <70) | 1.044 (0.495–2.203) | 0.910 |
| Multivariate analysis | ||
| EOR (GTR+NTR vs. STR) | 0.329 (0.121–0.896) | 0.030 |
| Post-operative complications | 3.620 (1.433–9.144) | 0.006 |
| Adjuvant treatment | 0.144 (0.057–0.362) | 3.80e–5 |
Bold values indicate statistical significance at the p < 0.05 level
*An independent univariate analysis was further performed to assess the impact of MGMT status on overall survival in patients who had MGMT status determined as “methylated” or “unmethylated” and received temozolomide treatment (n = 29), (P = 0.172, HR:0.550, 95% CI 0.233 – 1.30)
Abbreviations: KPS, Karnofski Performance Scale; ioUS, intraoperative ultrasonography; ioMRI, intraoperative magnetic resonance imaging; EOR, Extent of resection; GTR, gross total resection; NTR, near total resection; STR, subtotal resection
Discussion
GBM remains a challenging disease with an overall poor prognosis despite the most aggressive, multimodal treatment. Surgery, followed by chemoradiation, can be quite demanding, particularly for older individuals, therefore raising the question of how aggressive to be. Paradigms have been modified with regards to other aspects of GBM treatment in this population [3, 39], and thus we sought to determine whether surgical strategies can be similarly optimized.
In support of previous reports [4–6, 12–19], we demonstrated that older patients with GBM who undergo maximal EOR and adjuvant chemoradiation have an improved OS, suggesting that surgical resection and postoperative adjuvant treatment should not be withheld from this patient population. Patients with relatively lower pre-op KPS scores (<70) were more likely to be discharged to short-term facilities but showed significant improvement at postoperative 6-weeks. In fact, 59% of these patients received a follow-up KPS score of 70 or more at that time, which was a prognostic threshold important for predicting whether patients received postoperative adjuvant treatment. Indeed, patients who received postoperative chemoradiation, tolerated the treatment well and demonstrated improved OS compared to patients who did not receive adjuvant treatment. Thus, older patients, even those with relatively low preoperative KPS, benefit from surgery and can improve from their baseline preoperative functional state to tolerate adjuvant treatment and reap the OS benefits. Survival and quality of life outcomes, however, were significantly impacted by the presence of preoperative language deficit and postoperative complications, the latter of which was predictive for poor OS and follow-up KPS score at postoperative 6-weeks.
Given the prognostic implications of postoperative complications, and the trend that they increase with longer surgical time, surgical techniques and intraoperative adjuncts should be carefully considered to minimize complication risk, while aiming for maximal EOR. Similar to others, when investigating the usefulness of the addition of ioMRI, we found it does not offer any advantage for OS or functional improvement in this population. Indeed, a recent meta-analysis compared IoUS, IoMRI and other image guidance techniques in high grade glioma surgery and similarly found no significant difference between these modalities in terms of EOR and survival, while ioMRI use was significantly more expensive [31]. However, these studies did not compare clinical outcome in older patients. Indeed, ioMRI use in older patients in our cohort, significantly prolonged surgery, which served as a risk factor for postoperative complications in these patients, which were predictive of follow-up KPS score at postoperative 6-weeks and poor overall survival. There was a trend toward an increased frequency of postoperative complications in combined ioMRI+ioUS group compared to ioUS-alone cases (20–7.69%) despite the latter group being older on average. Though this difference did not reach statistical significance, possibly due to sample size, this finding suggests that surgery in older patients with GBM should be aimed at minimizing the operative time and avoiding the use of unnecessary adjuncts. In experienced hands, ioUS can garner similar benefits as IoMRI, while potentially avoiding deleterious ones. While this is the strategy now employed by the senior author, it should be noted that older patients should be assessed on a case-by-case basis such that the addition of ioMRI could be justified in certain patients and circumstances.
Our study has limitations. First, it is retrospective and of a single center designed with a homogenous, but relatively small patient cohort. This could explain why no survival benefit with regards to MGMT status or pre-op KPS was observed, as well as the small number of those patients undergoing STR. Second, as expected, a significant association was found between EOR and improved OS in multivariate analysis, but oddly not observed in univariate analysis. While this may seem counterintuitive, other variables that impact OS (e.g., complications) may act as negative confounders for the association between GTR and OS in multivariate analysis, rendering the significance of EOR in multivariate, but not univariate analysis. As described before, the inclusion of negative confounders in the model would push the adjusted association away from the null, leading to the significance of EOR more significant in multivariate analysis [40]. Additionally, it is important to note that the average age of the patients in ioUS group was significantly older than the combined group. This difference was due to the senior’s author intention to reduce the duration of surgeries in this relatively more senior age group, based on our hypothesis. However, the relatively older age in the ioUS group, would render that group at a disadvantage from a prognostic standpoint, which further underscores the observation. This study can serve as a pilot for data building the reasoning for further prospective studies with larger study cohorts.
Conclusions
Aggressive surgical resection improves OS and functional outcomes in older patients with GBM, and therefore maximal interventions should be considered in this patient population. In experienced hands, ioMRI use does not add any significant benefit over ioUS in terms of OS and functional outcomes in older GBM patients, but does increase the length of surgery, a preventable prognostic factor for postoperative complications.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 573.2 kb)
Supplementary material 2 (DOCX 542.1 kb)
Acknowledgements
We are grateful to the patients who contributed to this study. This study was supported by the Gregory M. Kiez and Mehmet Kutman Foundation. This study was also supported by the Connecticut Brain Tumor Alliance.
Footnotes
The original online version of this article was revised due to a retrospective Open Access order.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/23/2021
A Correction to this paper has been published: 10.1007/s11060-021-03888-3
References
- 1.Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-oncology. 2019;21(Supplement_5):v1–v100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbro-Peray P, Zouaoui S, Darlix A, et al. Association of patterns of care, prognostic factors, and use of radiotherapy–temozolomide therapy with survival in patients with newly diagnosed glioblastoma: a French national population-based study. J Neuro-Oncol. 2019;142(1):91–101. doi: 10.1007/s11060-018-03065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. New England J Med. 2017;376(11):1027–1037. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 4.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. New England J Med. 2007;356(15):1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 5.Roa W, Brasher P, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 6.Roa W, Kepka L, Kumar N, et al. International Atomic Energy Agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. doi: 10.1200/JCO.2015.62.6606. [DOI] [PubMed] [Google Scholar]
- 7.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. JNCI: J Nat Cancer Institute. 1993;85(9):704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 8.Malmström A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. The Lancet Oncology. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto FM, Reiner AS, Nayak L, Panageas KS, Elkin EB, Abrey LE. Prognosis and patterns of care in elderly patients with glioma. Cancer. 2009;115(23):5534–5540. doi: 10.1002/cncr.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnholtz-Sloan JS, Williams VL, Maldonado JL, et al. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg. 2008;108(4):642–648. doi: 10.3171/JNS/2008/108/4/0642. [DOI] [PubMed] [Google Scholar]
- 11.Lutterbach J, Bartelt S, Momm F, Becker G, Frommhold H, Ostertag C. Is older age associated with a worse prognosis due to different patterns of care? A long-term study of 1346 patients with glioblastomas or brain metastases. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2005;103(6):1234–1244. doi: 10.1002/cncr.20895. [DOI] [PubMed] [Google Scholar]
- 12.Almenawer SA, Badhiwala JH, Alhazzani W, et al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro-oncology. 2015;17(6):868–881. doi: 10.1093/neuonc/nou349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu R, Komisarow JM, Agarwal VJ, et al. Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. Journal of neurosurgery. 2016;124(4):998–1007. doi: 10.3171/2015.4.JNS142200. [DOI] [PubMed] [Google Scholar]
- 14.Chaichana KL, Chaichana KK, Olivi A, et al. Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Journal of neurosurgery. 2011;114(3):587–594. doi: 10.3171/2010.8.JNS1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noorbakhsh A, Tang JA, Marcus LP, et al. Gross-total resection outcomes in an elderly population with glioblastoma: a SEER-based analysis. Journal of neurosurgery. 2014;120(1):31–39. doi: 10.3171/2013.9.JNS13877. [DOI] [PubMed] [Google Scholar]
- 16.Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people–a randomised study. Acta neurochirurgica. 2003;145(1):5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 17.Chaichana KL, Garzon-Muvdi T, Parker S, et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Annals of surgical oncology. 2011;18(1):239–245. doi: 10.1245/s10434-010-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE. Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center Experience (1997‐2007) Cancer: Interdisciplinary International Journal of the American Cancer Society. 2009;115(16):3758–3766. doi: 10.1002/cncr.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oszvald Á, Güresir E, Setzer M, et al. Glioblastoma therapy in the elderly and the importance of the extent of resection regardless of age. Journal of neurosurgery. 2012;116(2):357–364. doi: 10.3171/2011.8.JNS102114. [DOI] [PubMed] [Google Scholar]
- 20.Yin A-a, Zhang L-h, Cheng J-x, et al. Radiotherapy plus concurrent or sequential temozolomide for glioblastoma in the elderly: a meta-analysis. PLoS One. 2013;8(9):e74242. doi: 10.1371/journal.pone.0074242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karsy M, Yoon N, Boettcher L, et al. Surgical treatment of glioblastoma in the elderly: the impact of complications. Journal of neuro-oncology. 2018;138(1):123–132. doi: 10.1007/s11060-018-2777-9. [DOI] [PubMed] [Google Scholar]
- 22.Karhade AV, Fandino L, Gupta S, et al. Impact of operative length on post-operative complications in meningioma surgery: a NSQIP analysis. Journal of neuro-oncology. 2017;131(1):59–67. doi: 10.1007/s11060-016-2262-2. [DOI] [PubMed] [Google Scholar]
- 23.Golebiowski A, Drewes C, Gulati S, Jakola AS, Solheim O. Is duration of surgery a risk factor for extracranial complications and surgical site infections after intracranial tumor operations? Acta neurochirurgica. 2015;157(2):235–240. doi: 10.1007/s00701-014-2286-3. [DOI] [PubMed] [Google Scholar]
- 24.Valentini LG, Casali C, Chatenoud L, Chiaffarino F, Uberti-Foppa C, Broggi G. Surgical site infections after elective neurosurgery: a survey of 1747 patients. Neurosurgery. 2008;62(1):88–96. doi: 10.1227/01.NEU.0000311065.95496.C5. [DOI] [PubMed] [Google Scholar]
- 25.Oh T, Safaee M, Sun MZ, et al. Surgical risk factors for post-operative pneumonia following meningioma resection. Clinical neurology neurosurgery. 2014;118:76–79. doi: 10.1016/j.clineuro.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Chibbaro S, Di Rocco F, Makiese O, et al. Neurosurgery and elderly: analysis through the years. Neurosurgical Review. 2011;34(2):229–234. doi: 10.1007/s10143-010-0301-6. [DOI] [PubMed] [Google Scholar]
- 27.Khaldi A, Helo N, Schneck MJ, Origitano TC. Venous thromboembolism: deep venous thrombosis and pulmonary embolism in a neurosurgical population. J Neurosurgery. 2011;114(1):40–46. doi: 10.3171/2010.8.JNS10332. [DOI] [PubMed] [Google Scholar]
- 28.Browd SR, Ragel BT, Davis GE, Scott AM, Skalabrin EJ, Couldwell WT. Prophylaxis for deep venous thrombosis in neurosurgery: a review of the literature. Neurosurgical Focus. 2004;17(4):1–6. doi: 10.3171/foc.2004.17.4.1. [DOI] [PubMed] [Google Scholar]
- 29.Procter LD, Davenport DL, Bernard AC, Zwischenberger JB. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am College Surg. 2010;210(1):60–65. doi: 10.1016/j.jamcollsurg.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 30.Schroeck H, Welch TL, Rovner MS, Johnson HA, Schroeck FR. Anesthetic challenges and outcomes for procedures in the intraoperative magnetic resonance imaging suite: a systematic review. J Clini Anesthesia. 2019;54:89–101. doi: 10.1016/j.jclinane.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eljamel MS, Mahboob SO. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis Photodynamic Therapy. 2016;16:35–43. doi: 10.1016/j.pdpdt.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Coburger J, Scheuerle A, Kapapa T, et al. Sensitivity and specificity of linear array intraoperative ultrasound in glioblastoma surgery: a comparative study with high field intraoperative MRI and conventional sector array ultrasound. Neurosurg Rev. 2015;38(3):499–509. doi: 10.1007/s10143-015-0627-1. [DOI] [PubMed] [Google Scholar]
- 33.Mahboob S, McPhillips R, Qiu Z, et al. Intraoperative ultrasound-guided resection of gliomas: a meta-analysis and review of the literature. World Neurosurg. 2016;92:255–263. doi: 10.1016/j.wneu.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Saether C, Torsteinsen M, Torp S, Sundstrøm S, Unsgård G, Solheim O. Did survival improve after the implementation of intraoperative neuronavigation and 3D ultrasound in glioblastoma surgery? A retrospective analysis of 192 primary operations. J Neurol Surg Part A: Central European Neurosurg. 2012;73(02):073–078. doi: 10.1055/s-0031-1297247. [DOI] [PubMed] [Google Scholar]
- 35.Bander ED, Magge R, Ramakrishna R. Advances in glioblastoma operative techniques. World Neurosurg. 2018;116:529–538. doi: 10.1016/j.wneu.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Nayak L, DeAngelis LM, Brandes AA, et al. The neurologic assessment in neuro-oncology (NANO) scale: a tool to assess neurologic function for integration into the response assessment in neuro-oncology (RANO) criteria. Neuro-oncology. 2017;19(5):625–635. doi: 10.1093/neuonc/nox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ung TH, Ney DE, Damek D, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale as an assessment tool for survival in patients with primary glioblastoma. Neurosurgery. 2019;84(3):687–695. doi: 10.1093/neuros/nyy098. [DOI] [PubMed] [Google Scholar]
- 38.Abhinav K, Aquilina K, Gbejuade H, La M, Hopkins K, Iyer V. A pilot study of glioblastoma multiforme in elderly patients: treatments, O-6-methylguanine-DNA methyltransferase (MGMT) methylation status and survival. Clinical Neurol Neurosurg. 2013;115(8):1375–1378. doi: 10.1016/j.clineuro.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England J med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 40.Mehio-Sibai A, Feinleib M, Sibai TA, Armenian HK. A positive or a negative confounding variable? A simple teaching aid for clinicians and students. Annals of Epidemiol. 2005;15(6):421–423. doi: 10.1016/j.annepidem.2004.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 573.2 kb)
Supplementary material 2 (DOCX 542.1 kb)