Abstract
Cancer represents an important cause of disease-related death in children worldwide. Improved treatment and understanding of the ways in which cancer manifests has allowed for a greater prospect of survival in children of all ages. However, variation in childhood cancer experience exists based on factors at the individual, community and systems levels. Throughout the cancer care continuum these factors may influence the access and timeliness of care a child receives, leading to delays in diagnosis and treatment. The pejorative designation ‘delay in diagnosis and treatment’ is better characterised as lag time, representing an interval that is thought to influence survival and overall outcome. In recent decades, work has been done to expedite early childhood cancer diagnosis through the creation of screening and education-based programmes. Although systematic cancer screening in children poses risks and fails to achieve the goal of early diagnosis, a case has been made for risk-based surveillance that has been shown to improve outcome and reduce occurrence of advanced stage disease in targeted populations. The components of lag time are examined separately and individually. This review highlights the challenges of early diagnosis in childhood cancers and describes important contributors in the cancer care continuum.
Subject terms: Paediatric cancer, Diagnosis, Epidemiology
Introduction
Cancer is a leading cause of disease-related death in children worldwide. Although rare, by comparison with cancer in adults, childhood cancer, defined here as cancer in children aged 0–19 [1], carries a significant disease burden with almost 400,000 incident cases diagnosed globally every year [2]. Depending on the geographical setting, childhood cancer represents between 0.5 and 4.6% [3] of newly diagnosed cancers; however, these estimates often rely on data reported in population-based registries, which may not entirely capture cancer occurrence across the age span in low- and middle-income countries (LMICs) [4]. Results from a recent model-based study estimating global childhood cancer incidence suggest that nearly one in two (43%) children, aged 0–14 years, with cancer never receive a diagnosis, indicating significant underdiagnosis, which is not accounted for in incidence estimates [5].
Within paediatric populations, there is significant variation in the proportions of diagnostic groups and stages of disease at diagnosis [1, 2, 6]. Advances in cancer therapy have aided improved outcomes resulting in 5-year survival that exceeds 80% in many high-income countries (HICs). However, with occasional exceptions [7] similar improvements have not been established in paediatric populations living in LMICs, where more than 90% of children at risk of developing cancer reside [8]. These differences in survival are associated with factors at the patient/family, physician and health systems levels leading to prolonged diagnostic intervals and, ultimately, underdiagnosis of paediatric cancers in LMICs.
Malignancies in paediatric populations differ from those common in adults in whom the influence of modifiable risk factors—such as tobacco smoking, alcohol, obesity and certain infections—dominates. Such factors are poorly defined and understood in the context of childhood cancers [9]. Therefore, primary cancer prevention initiatives have had little impact at reducing childhood cancer incidence. Instead, interventions aimed at addressing timeliness of diagnosis and treatment, i.e. secondary prevention, are likely to provide opportunities for substantial improvement. It is well established that timing of cancer diagnosis can be an indicator of cancer outcome, with evidence of this relationship seen in some malignancies common to children and adolescents [10–12]. In this article we describe the challenges of early diagnosis and treatment of cancers in childhood and the impact that these challenges can have on affected children.
Descriptive epidemiology
In paediatric populations differences in disease occurrence are influenced primarily by age and sex [13]. Overall, leukaemias, central nervous system tumours and lymphomas predominate during childhood, yet some cancers are more common in certain age groups: children 0–4 years old frequently present with sympathetic nervous system tumours; 15–19 year olds have more epithelial, germ cell and gonadal tumours (Fig. 1) [2, 13, 14]. (N.B.: GLOBOCAN omits detailed data on sarcomas, which as a group would be ranked among the top five childhood cancer types in the 0–19 year group in the United States) [15]. Comparison of geographic locations also reveals differences in childhood cancer incidence; however, the paucity of population-based cancer registries may limit conclusions [4]. For example, retinoblastoma, a disease that is more frequent in young children living in LMICs than in HICs, has a known hereditary pattern, which makes disease incidence differ based on regional and community levels [16]. Correspondingly, disability adjusted life years—expressed as the number of years lost due to ill health, disability or death [17] are highest in LMICs, meaning that not only are more cases of disease found in these regions, but also the lasting effects of both disease and treatment are more drastic [18].
Fig. 1. Estimated incidence (numbers of cases, dark grey bars) of and mortality (numbers of deaths, light grey bars) from common paediatric cancers according to age group.
A: 0–4, B: 5–9, C: 10–14, D: 15–19 years. Combined frequencies for males and females in 2020. Source: GLOBOCAN 2020 [14]. N.B.: GLOBOCAN omits data on sarcomas.
Early diagnosis
Is there a rationale for cancer screening in children?
Early diagnosis of cancer is a foundational goal in cancer care as it improves the prospect of curable disease while also offering lower treatment morbidity and improved quality of life compared to later stage diagnosis [12]. Strong evidence of this effect exists in adults; early diagnosis and treatment of breast [19], cervix [20] and colon [21] cancers (or of their precancerous lesions)—via opportunistic or organised screening programmes—leads to reduction of cause-specific mortality. In part, success in early diagnosis comes from understanding the natural history of cancer development and its determinants, and may therefore be exploited through cancer screening. However, the influence of these determinants—particularly lifestyle—on the development of cancer in children is poorly understood and thought to be of lesser significance than tumour biology, which is estimated to contribute 60% of the prognosis [22]. The cancers common in children tend to be more “biologically active,” leading to diseases which advance quickly and are more aggressive than those in adults [23]. Therefore, screening for cancers in children, as a population level intervention, is not usually feasible because the onset of disease is indiscernible and the drivers of these cancers are not preventable or detectable using traditional screening methods. In fact, screening for childhood cancer fails to meet many of Wilson and Jungner’s classical screening criteria [24] used as guidance for policy decisions in cancer control. Most importantly, our lack of understanding of the natural history of specific childhood cancers, and the nonexistence of a latent or early symptomatic stage amenable to effective intervention, add to the potential harms which may come from screening. Likewise, except for neuroblastoma, a disease, which leads to increased excretion of homovanillic acid and vanillylmandelic acid—and thus potentially amenable to screening via a biochemical urine test—early detection of childhood cancers must rely on clinical recognition of signs and symptoms, e.g. retinoblastoma [11]. Standardised screening based on ascertaining altered morphology via radiological imaging or abnormal cytology is the primary means for early diagnosis of adult cancers, such as those of breast, colorectum and uterine cervix. Such conditions are not currently available for childhood cancers. In Table 1 we show how screening for two childhood cancers, taken as examples, fails to properly fulfill all of Wilson and Jungner’s screening criteria, in contrast to adult cancers for which mass screening is part of guidelines in many countries, i.e. cervix, breast and colorectal cancers [15, 24, 25].
Table 1.
Fulfillment of Wilson and Jungner’s criteria regarding established cancer screening programmes for adults and for two childhood cancers as comparison.
| Cervix | Breast | Colorectuma | Neuroblastomaa | Retinoblastomaa | |
|---|---|---|---|---|---|
| Incidenceb | +++ | ++++ | ++++ | + | + |
| Survivalb | +++ | + | +++ | ++ | + |
| Natural history is adequately understood | ++ | ++ | ++ | +/− | ++ |
| Latent/early stage of disease exists | ++ | ++ | ++ | +/− | +/− |
| Suitable test or examination availabled | ++ | ++ | ++ | ++ | ++ |
| Test acceptable to populationc | ++ | ++ | ++ | ++ | ++ |
| Accepted treatment for disease exists | Yes (conditional on availability of secondary and tertiary care facilities) | ||||
| Facilities for diagnosis and treatment available | |||||
| Agreed upon policy on whom to treat as patients | Yes | ||||
| Potential harms of undergoing screeningd | Yes (variable by disease and test) | ||||
| Cost-effectivenesse | + | + | + | − | + |
| Case-finding a continuous process (estimated number of screens during the age-eligible period depending on practice guidelines) | 8–15 (ages 25–69) | 12–35 (ages 40–75) | 2–8 (ages 50–75) | 1–2 (before age 1) | Twice (ages 0–7) |
Modified from Tota et al. [25].
aEstimates for males and females were collapsed because of comparability in incidence and survival statistics.
bIncidence and survival were used to reflect Wilson and Jungner’s first criterion (i.e. whether the disease is an important health problem [US as example [15]]). For incidence: up to 1 (+); 1–10 (++); 10–100 (+++); >100 (++++) per 100,000, assuming highest age-specific rate. For 5-year survival: >90% (+); 75–90% (++); 50–75% (+++); <50% (++++).
cIncludes only tests that have been in widespread use and/or have clinical application in specific circumstances. Diagnostic work-up procedures not considered.
dConsiders psychological harms, potential morbidity and risks during the entire screening process, including diagnostic work-up and treatment.
eCost-effective (+): cancers for which organised, guideline-driven screening programmes currently exist; Equivocal cost-effectiveness (+/−): uncertainty regarding the cost-effectiveness of screening; Not cost-effective (−): high certainty that the cost-effectiveness of screening is very low.
A valiant attempt at childhood cancer screening: neuroblastoma
Early diagnosis of neuroblastoma, for which detection is possible via an acceptable and simple screening test (a requirement for organised or opportunistic screening), has met important challenges and serves as a case study in ineffective childhood cancer screening. At least conceptually, neuroblastoma represents an important screening target as the disease exhibits characteristics which suggest that preclinical detection may improve outcome [26]. Notably, mass screening programmes in Japan [27], Quebec, [26] and Germany [28] were implemented, aiming to decrease the mortality and incidence of advanced stage disease. In Quebec, 425,816 children were screened and 82 possible tumours were identified, of which 39 (47.5%) proved to be false positive cases at clinical diagnosis [26, 29]. Quebec’s neuroblastoma incidence was 92% higher compared to control populations in Ontario and Minnesota where no screening was offered and similar treatment protocols were followed [26, 29]. In Germany, the incidence of advanced stage disease was comparable between the screening and control groups (3.7 per 100,000 versus 3.8 per 100,000, respectively), indicating that screening for neuroblastoma in children failed to meet the goals described at programme conception [28].
The questionable effectiveness of the neuroblastoma screening programmes became apparent when assessing the screening procedures. Although the estimated screening specificity (probability of a negative test result in the absence of disease) is high at 99.9%, as was the case in Japan, Quebec, and Germany, neuroblastoma is very rare and the disease prevalence is low within populations, meaning that the positive predictive value (the likelihood that a person who tests positive for the disease actually has the disease) remains low [26, 30]. Specifically, for every 100 children who tested positive for neuroblastoma in the German programme, only eight would have the disease, corresponding to a positive predictive value of 8.4% [31]. However, because screened populations are asymptomatic by definition, those detected by screening may therefore be more likely to have less aggressive disease. In the case of neuroblastoma, in which spontaneous disease regression without intervention is well documented [32], researchers determined that screening could not differentiate spontaneously regressing disease from disease which would progress clinically. As a result, favourable disease would be detected more frequently [29, 33], indicating the presence of length time bias.
Neuroblastoma screening has been abandoned since the results of these screening programmes were released; however, the impact they have had was profound. In the Quebec cohort, 45 children were diagnosed through screening; more than 10 of these patients experienced treatment complications with one patient developing a chemotherapy-induced secondary malignancy 5 years after initial diagnosis [34]. Fifty-five children in Germany who received false negative screening test results subsequently developed neuroblastoma; 14 of these children died [31]. It is estimated that 99 of the 149 German individuals identified through screening as having neuroblastoma would have never developed clinically relevant disease and/or required treatment [28]. Additionally, overdiagnosis was substantial in Germany, where seven cases per 100,000 were found in the screened group [28, 31]. Therefore, it is conceivable that without neuroblastoma screening the majority of these tumours would not have been detected, but they would not be clinically relevant or cause death, and would spontaneously regress without treatment [29]. Here, the harms of overdiagnosis, both physical and psychological, occur before treatment onset, as anxiety induced by a cancer diagnosis is compounded by uncertainty. Based on these findings, the small potential benefit of the screening programme was outweighed by insignificant reductions in mortality and a high number of cases for whom treatment may have actually caused more harm.
Another well-meaning attempt: screening for thyroid cancer
More recently, the issue of overdiagnosis—defined as the diagnosis of a disease that is unlikely to cause symptoms or death in a person’s lifetime [35]—has been raised in the case of thyroid cancer screening, with considerable changes in incidence observed in young and middle-aged populations. A 2016 publication described the growing thyroid cancer “epidemic” based on observations from 12 HICs, suggesting that the introduction of sensitive screening technologies, including computed tomography, ultrasonography and magnetic resonance imaging, have aided this change in disease incidence [36]. Abrupt changes in thyroid cancer incidence have also been observed in large-scale thyroid cancer surveillance programmes in targeted populations [36]. Following the 2011 nuclear accident in Japan’s Fukushima Prefecture systematic thyroid cancer screening using ultrasound was established, targeting children under the age of 18, and within months thyroid cancer incidence in children was nearly 30 times that of the national average [36, 37]. Importantly, because there is a long latency between radiation exposure and thyroid cancer development, it is not expected that this influx of cases is attributable to radiation exposure and is therefore thought to be the result of overdiagnosis. Increased access and availability of highly sensitive technologies used in thyroid cancer screening allows for detection of small lesions; however, the natural history of the most common forms of thyroid cancer, especially in paediatrics, is poorly defined, and the detection of clinically indolent lesions causes more harm than benefit [36]. With no evidence of new risk factors or changing exposures to known causes of thyroid cancer, it is apparent that overdiagnosis may account for increases in thyroid cancer incidence across all ages, and that systematic screening fails to reduce all potential harms [36, 38, 39].
Opportunistic and risk-based screening
Although systematic screening for childhood cancer has demonstrated little effect on the incidence of advanced stage disease, targeted programmes promoting early identification have been shown to be beneficial, particularly in LMICs. For retinoblastoma, disease identified early is often intraocular (contained within the eye) and curable with surgery, radiotherapy and chemotherapy in various combinations; whereas increased time to diagnosis is commonly associated with extraocular disease (outside of the eye) which is more advanced, less receptive to treatment, and can be fatal [11, 40]. Because 75% of retinoblastomas occur sporadically and, in the majority of children, manifest in the first year of life, screening should be concentrated in this population whose cancer risk may not be identified by genetic characteristics alone [41]. Moreover, unlike screening for neuroblastoma, which leads to substantial overdiagnosis and treatment, early diagnosis of retinoblastoma only leads to benefits with low harm potential (Table 1) [42].
In Brazil and Honduras, where it was discovered that retinoblastoma was detected predominantly in advanced stages, researchers identified that ignorance of common signs and symptoms facilitated delayed presentation to healthcare services [43–45]. In response, two educationally based public health programmes were created with the goal of expediting the early diagnosis of retinoblastoma. In Brazil [45], media sources displayed images of leukocoria (white pupil), the most common early sign of retinoblastoma, and encouraged engagement from community healthcare professionals. In Honduras, a programme linked to a vaccination campaign was created with the aim of decreasing advanced retinoblastoma incidence through the distribution of posters and flyers with similar leukocoria imagery, community programming, advertisements in communal areas, and addressing community members [44]. As a result of these interventions time to healthcare contact and referral decreased, leading to an increase in the diagnosis of intraocular disease in both countries [44, 45]. In Honduras, 73% of retinoblastomas were extraocular at diagnosis in the 8 years preceding study commencement, whereas only 35% showed extraocular spread after educational intervention [44]. Most importantly, survival of children with retinoblastoma increased from 14 to 48%, and the cost of the programme remained low with flyers and posters costing only $2700 per year [44]. This indicates a shift in the timing of presentation with potential opportunity to improve treatment outcome.
There are other instances in which investigation for early diagnosis in paediatric cancer is warranted, as in the case of childhood cancer survivors and individuals with cancer predisposition syndromes [46]. Rather than employing systematic screening, a risk-based care approach is employed, targeting a smaller population of high-risk individuals with more intensive investigation compared to traditional screening. In 2003, the Children’s Oncology Group Long Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancer recommended surveillance for subsequent malignancies in survivors of childhood cancers with known increased risk due to their treatment regimens [47]. Childhood cancer survivors without elevated risk for these conditions are recommended to follow screening guidelines for the general population [48, 49]. Further recommendations from the Institute of Medicine were released in 2006, supporting the need for childhood cancer survivors to receive a survivorship care plan (SCP) and/or a primary care provider plan (PCP) at completion of therapy to ensure continuity of care [50]. A recent study of childhood cancer survivors, looking at adherence to surveillance guidelines, determined that those with SCP and/or PCP plans had increased adherence to surveillance [48]. However, it was also shown that adherence to guidelines is suboptimal, as few childhood cancer survivors and primary care providers have these plans in place, and they have not been introduced retroactively for older cancer survivors. Challenges with screening and surveillance adherence are reflected in the Commission on Cancer’s decision to decrease the percentage of survivors with SCPs from 75 to 50% due to a lack of resources at most facilities [51].
With improvements in cancer care conferring increased survival, greater caseloads for oncologists can be expected, creating questions of feasibility of follow-up care given limited resources. In 1998 the Pediatric Oncology Group of Ontario (POGO) established population-based ratios for paediatric oncologists, recommending one oncologist to every 15 newly diagnosed malignancies [52]. In subsequent years the C17 Council of Canadian Pediatric Hematology/Oncology aimed to develop national standards for physician resources through assessment of programme trends, training, and physician demographics across the 16 paediatric cancer programmes operating in 17 Canadian facilities [53]. Like POGO, C17 described a ratio of 1:15 for optimal care; however, their findings indicated that 10 of the 17 Canadian paediatric cancer facilities had less than one oncologist per 15 newly diagnosed cancers, with the average oncologist to patient ratio being 1:17.8 [53]. Of these 17 facilities, three centres had one oncologist to more than 30 patients, indicating limited resources [53]. Describing oncologist caseload, however, has proven difficult as the scope of work and varying definition of what constitutes an oncologist changes. For this reason, and due to the variation in the roles in which haematologists and oncologists serve, ratios have been abandoned in favour of programme- and facility-specific descriptors. With an estimated 1700 new childhood cancers diagnosed every year in Canada and more than 30,000 childhood cancer survivors, availability and allocation of resources may limit surveillance and follow-up feasibility [54].
Risk-based care has also been studied in individuals with cancer predisposition syndromes. Importantly, such populations under surveillance are known to have high disease prevalence and increased risk of developing the disease of interest [55]. In turn, this ‘risk-enriched’ prevalence scenario increases the positive predictive value of tests used to detect disease in these populations while reducing the risk of overdiagnosis. To explore the effectiveness of surveillance, Kratz et al. [56] described the experience of a cohort of individuals with Li-Fraumeni Syndrome (LFS) who were followed for 11 years. LFS, one of the most aggressive cancer predisposition syndromes, is characterised by a high risk of early onset cancer. In order to reduce occurrence of advanced disease, participants either received whole-body magnetic resonance imaging (WBMRI) or general follow-up. In the surveillance group, 40 tumours were detected in 19 of 59 patients whereas 61 tumours presented in 43 of 49 patients who did not undergo surveillance [56]. Notably, 25 of the 40 tumours detected by surveillance were determined to be low grade, indicating that surveillance may be able to identify lesions before clinical manifestation and shorten time to diagnosis and treatment [56]. When comparing outcomes in surveillance versus non-surveillance, 5-year overall survival was higher in the surveillance group (88.9%) compared to the non-surveillance group (59.6%) [56]. To further assess the impact of surveillance, a meta-analysis of 578 participants who received WBMRI for LFS cancer surveillance between 2004 and 2016 was performed [57]. Seven percent of participants were found to have one or more new primary malignancies, with the proportion of cancers identified being highest in those younger than 18 (31%) compared to those 18–40 years old (16%) or those over 40 (18%) [57]. Overall, 1 in 14 individuals who received their first WBMRI had a tumour identified and were treated subsequently with curative intent [57]. Importantly, surveillance can be further enriched through refining eligibility criteria, as was the case in initial LFS screening protocols, when researchers identified screening candidates based on familial characteristics [58]. Table 2 depicts the impact this approach has on overall screening effectiveness. When eligibility criteria become more stringent (i.e. indicators of inherited cancer syndrome become stronger) the estimated positive predictive value of the screening test increases as a consequence of increased disease prevalence in the more stringent definitions.
Table 2.
Impact of patient selection criteria on the positive predictive value and relative sensitivity for p53 screening in childhood cancer (Adapted from Chompret et al. [58].).
| Degree of stringency | Criterion | Positive predictive value | Relative sensitivity |
|---|---|---|---|
| Very lenient | At least one first or second degree relative affected by any type of cancer. | 6% (14/223) | 93% (14/15) |
| Lenient | As above but neoplasms in the affected relatives restricted to the following types: sarcoma, brain tumours, breast cancer, adrenocortical carcinoma, haematological malignancies, stomach cancer, melanoma and germ cell tumours. | 9% (13/141) | 87% (13/15) |
| Stringent | As above but tumour types further restricted to sarcomas, brain tumours, breast cancer and adrenocortical carcinoma. | 15% (12/81) | 80% (12/15) |
| Very stringent | As above but the affected relative(s) must have been diagnosed before age 46 or, if sarcoma, at any age. | 43% (9/21) | 60% (9/15) |
Although international guidelines for the screening of cancer in children with predisposition syndromes exist, there remains uncertainty about the value of such procedures [59]. Limitations in the availability of evidence from large studies combined with the inherent challenges of interpreting, applying, and accessing these programmes are present [60]. A further expansion of the criteria used to measure screening efficacy is required to encompass the holistic benefits, such as improved quality of life and decreased treatment related morbidity, as equally important outcomes of screening to overall survival. With the availability of highly effective treatment regimens for many childhood cancers, the survival gap between early and advanced stage disease is narrowing, making overall survival of less importance clinically [61].
“Delay” in diagnosis
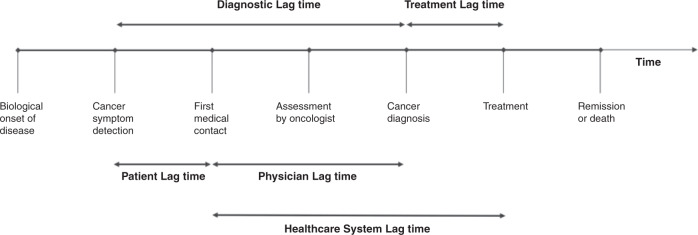
The term “delay in diagnosis” has been described as pejorative with the implicit assumption of fault; the well-established alternative designation “lag time” being preferred [62]. Tumours in children tend to have short latency periods, grow quickly and are invasive. In combination with the effects of personal and health system-level characteristics, differential time to diagnosis may ensue [63]. Referred to as lag time, this differential time to diagnosis is comprised of two components: point of initial symptom presentation to first healthcare contact; and from this contact to subsequent cancer diagnosis [62]. Importantly, increased lag time can occur when a person who presents with symptoms attributed to cancer is not investigated or referred for investigation, when a person is incorrectly diagnosed, or when positive test results are not acted upon [64]. With longer lag time, disease may progress and negatively affect patient outcome; however, describing and quantifying lag time is difficult, and its impact on survival is not well understood. A recently published systematic review and meta-analysis exploring mortality due to treatment “delays,” found that a 4-week lag in treatment is associated with increased mortality across all common forms of cancer in adults [65]. The impact of lag time in paediatric cancers has been described in several studies, although it is not clear what the overall effect is as there is significant heterogeneity within and between disease types [66, 67]. In some diseases longer lag time is associated with poorer survival, in others with better survival, while in the remainder the duration of lag time is unrelated to survival [62]. Figure 2 describes the continuum of cancer care and identifies the components of diagnostic and treatment lag times. Here we describe patient/family-level lag times (time from onset of symptoms to first healthcare contact), physician-level lag times (time from first healthcare contact to subsequent diagnosis) and healthcare system-level lag times (time from first healthcare contact through to treatment) across different milestones in the continuum of care.
Fig. 2. Lag time in diagnosis and treatment according to patient, physician and healthcare system influences across the cancer treatment continuum.
Adapted from Dang-Tam & Franco [67].
Patient- and family-level lag times
The influence of patient characteristics on lag time has been well described in paediatric and adult populations [68]. In paediatrics, patient delays are dictated by two main factors: the role of caregivers and the misattribution of symptoms [64]. Because children are typically under the supervision of a caregiver (i.e. parent, grandparent, guardian), there is a reliance on caregiver knowledge, behaviour, and attitude towards cancer care as these individuals must articulate health concerns and act on the child’s behalf [67]. In adults, patient directed lag times tend to be associated with factors at the individual level, including education and medical knowledge [64]. While an understanding of lag time exists in these populations, less focus has been directed toward adolescent and young adult populations, which represent a growing portion of new cancer cases [2]. Indication of a contrasting lag time experience between paediatric age groups is seen in a review of 23 studies exploring diagnostic “delays” in Canada, which reports a positive association between age and lag time [67]. As the authors describe, it is thought that, as children age and gain independence, they become more self-reliant concerning their health status compared to younger children, leading to an under reporting of symptoms. The underlying assumption of intrinsic health in young people compounded with unfamiliarity with common signs of cancer may foster ignorance and a sense of denial. It is, therefore, not unsurprising that teenagers and young adults are more likely than older adults to be diagnosed with cancer through emergency presentation [69]. Pointedly detailed in It’s Not About the Bike: My Journey Back to Life [70], Lance Armstrong describes how, as a very young adult, he dismissed and denied the cardinal signs and symptoms of his testicular cancer by attributing these to his cycling training. It was not until pain interfered with his ability to sit in his bike saddle that he consulted a physician, when he received a diagnosis and found that the disease had metastasised to his lungs and brain.
Patient-level lag times and geographic variation
In contrast to HICs, patient/family dictated lag times in LMICs manifest from different causes, for achieved caregiver education, knowledge of healthcare systems, access to private insurance, and access to healthcare facilities contribute significantly [9]. Overwhelmingly, children in LMICs present with more advanced stage disease at diagnosis. Physical barriers to accessing care, in combination with poor understanding of common signs and symptoms of cancers frequent in children, often result in diagnostic and treatment “delays” for children living in rural regions. As a result, individuals may only seek medical attention once it is perceived to be necessary, creating increased lag time. Additionally, a primary reliance on alternative medicine, rather than seeking immediate care in institutions specialised in childhood cancer care, has been shown to increase lag time and delay specialist contact and subsequent diagnosis. This is not to say that there may not be a role for complementary medicine approaches integrated with scientific medicine; however, sole reliance on the former is to be discouraged [71].
Physician-level lag times
The timing between first healthcare contact and subsequent cancer diagnosis can be affected by actions at the primary, secondary and tertiary healthcare levels. Because cancer frequently presents through non-specific symptoms, misattribution and resulting limited or lack of investigation for malignancy can cause significant physician-level delays [64]. In a survey conducted at an annual Teenage Cancer Trust “Find your Sense of Tumour” Conference for young people with cancer, over one third of respondents reported a lag in specialist referral, meaning that individuals with symptoms of cancer visited a general practitioner (GP) five or more times before receiving specialist care [72]. Although these survey results reflect the experience in the United Kingdom and therefore may not be generalisable, they suggest that GPs, even in regions with advanced cancer programmes, may be unfamiliar with paediatric cancers, leading to misdiagnosis and resulting in late emergency presentation. Several systematic reviews and meta-analyses have described the influence of the physicians’ specialisation on lag time after a child’s first healthcare contact. Brasme et al. [66] identified the longest diagnostic time in children who present to GPs, suggesting that unfamiliarity with symptom complexes may facilitate a “delay” and substantiating the Teenage Cancer Trust survey results. Further, time to diagnosis was examined in paediatricians and emergency physicians. Emergency physicians had shorter lag times; however, tumour biology may influence the severity of symptoms and, by extension, the type of physician a child first contacts (i.e. children presenting in the emergency room may have more pronounced symptoms and thus a more advanced disease than those attending the GP) [66].
Physician lag times, experience and professional education
Due to the uncommon nature of childhood cancer, GPs often see few paediatric cancer cases throughout their career. With children and young people representing between 15 and 30% of the population in many HICs, it is estimated that a single GP may only see one young person with cancer every 5–10 years [73]. For patients, the level of familiarity their healthcare contact has with cancer symptoms is critical, as this individual provides entry into the health system and ensures continuation of care through the diagnostic pathway. A survey of 791 advanced-year medical students from 12 Mexican universities assessed student knowledge of common signs and symptoms of retinoblastoma, their ability to identify the problem and their ability to make appropriate referrals [74]. The survey respondents represent an important population, as these emerging physicians are the primary healthcare contact in rural communities, and their knowledge of retinoblastoma is critical due to its commonality among children in this region. Only 39.7% of respondents properly identified the two most frequent signs and suspected retinoblastoma; 3.3% of respondents correctly answered more than three quarters of the questions; and only 35.7% knew that retinoblastoma can cause death, with 32.9% considering a watch-and-wait approach as appropriate intervention [74]. A similar study in Jordan assessed the knowledge of medical students, paediatricians, and ophthalmologists and yielded comparable findings, with poor knowledge exhibited even by ophthalmologists [75]. Notably, 94% of participants identified leukocoria as an abnormal sign; however, 45% of participants did not attribute it as a sign of a life-threating malignancy; 67% of participants failed to achieve the established sufficiency score (70%) in their questionnaire responses [75]. The results of these studies indicate that physicians may lack sufficient knowledge required to detect and provide timely referral for patients with retinoblastoma.
System-level lag times
Once a patient enters the healthcare system, diagnostic lag times may be influenced by access to medical care services, availability of suitable instrumentation and service from competent pathologists. The influence of these factors varies significantly, especially across LMICs. In India, where nearly 70% of the population lives in rural communities, 95% of cancer care facilities are located in urban regions [76, 77]. This represents a physical barrier to access to care as a result of poor infrastructure and high rates of poverty [9, 64]. Once contact with the healthcare system has been established, 1500 oncologists with training in paediatrics serve the over 1 billion people living in India, resulting in an oncologist to patient ratio of 1:1600 [78]. With fewer than 10 large facilities able to offer care for paediatric patients, many patients must travel over 100 km to urban regions in order to receive care. In HICs, system-level lag times are frequently affected by administrative setbacks which manifest as increased time to diagnostic procedures, surgery and treatment. A study exploring Canadian paediatric surgical wait times described the interval between clinical decision and date of surgery [79]. Collecting data from 15 paediatric academic health centres, results indicated that 28% of cancer surgeries occurred after the therapy target date, representing the third longest lag time across studied surgical specialisations. Although differences in health states may account for some of this variation, clinically derived targets found that 27% of children were kept waiting too long given their specific condition.
Differences in health systems may also account for variation in diagnostic time. As indicated by Brasme et al. [66] the longest diagnostic “delay” occurred in those who initially presented to GPs. Therefore, it is plausible that single-payer health systems similar to those in Canada and England may have an over-reliance on general practitioners for triaging health complaints compared to the United States where insured patients may access specialised care directly [66, 67]. To expedite cancer diagnosis and reduce lag time at the system level, the National Institute for Health and Care Excellence (NICE) in England created guidelines for the recognition and referral of suspected cancers in adult and paediatric populations [80]. With infographics targeting both healthcare professionals and individuals with suspected cancers, information regarding common signs and symptom complexes corresponding to specific cancers are described and categorised by priority, recommended healthcare referral, and next steps in the diagnostic pathway [81]. Introduced in 2005, improvements in care corresponding to timeliness and outcome have been seen in adult populations, in which the mean diagnostic lag time decreased by more than five days between 2001–2002 and 2007–2008 [82]. Further, patients who presented to physicians with NICE qualifying symptoms had shorter diagnostic intervals than those who did not, suggesting that the guidelines may have contributed to reduced lag time through action at both the patient and physician level. Although these research findings are described in adult populations, it is suspected that a similar impact has been seen in paediatric populations. To the best of our knowledge, the impact of NICE referral guidelines has not been characterised in children and young people; however, the HeadSmart campaign [83], which works to disseminate guidelines for diagnosis of childhood brain cancer, has observed a decrease in the total diagnostic interval, from 3.3 months to 6.7 weeks between 2009 and 2013.
Differentiating lag times in HICs and LMICs
While overlapping with those in HICs, the factors determining lag time in children with cancer in LMICs occupy a larger spectrum. Those encountered in resource-poor settings have been well described during the past decade in Egypt [84], Ethiopia [85], India [86, 87], Indonesia [88], Kenya [89, 90], Nigeria [91], South Africa [92] and Uganda [90]. The published reports were characterised by small sample sizes (20–194 children). All but two reports, one limited to children with “haematological tumours” and the other to those with Burkitt lymphoma, had unselected cancers. A wide range of factors contributing to lag time were examined, although not all in every study. These included the child’s age, sex, and place of residence (rural or urban); level of parental education and income; family health insurance; use of “alternative medicines”; perception of incurability; type of cancer and initial misdiagnosis. Although median lag times were not remarkably long, some were extraordinary, such as 1826 [92] and 4055 [88] days, raising the question of recall bias. Parental delay was shorter than healthcare system delay in the majority of studies (6/9, 67%). Low socioeconomic status and absence of health insurance were commonplace as was rural residence and use of “alternative medicines”, including holy water [85]. Lag time was typically shorter for children with rapidly growing cancers, like acute lymphoblastic leukaemia [91] and germ cell tumours [84], than more slowly progressive tumours such as Hodgkin lymphoma [87, 91] and those in the central nervous system [84, 88]. The influence of age was variable; children older than 10 [85] or less than 5 [84] having longer lag times. Girls in India faced the double burden of less likelihood of diagnosis [86, 87] and longer lag times.
Two large and informative studies relate exclusively to retinoblastoma and the association between national income, age at diagnosis and lag time, and clinical outcomes. The first involved 4351 patients from 153 countries with the estimate that this was more than half of all cases globally in 2017 [93]. LMICs contributed almost 85% of cases with a median age at diagnosis of 30.5 months compared to 14.1 months in those from HICs. The corresponding rates of intraocular and metastatic disease were 49.1% and 18.9% versus 98.5% and 0.3%, respectively. The second study involved 692 patients in 10 countries on 5 continents [94]. The median lag times were 251 days in low income countries and 18 days in HICs. Not surprisingly, the mortality rates have been reported as 70% in Africa, 39% in Asia and 3–5% in Europe and North America [95]. What is clear is that addressing the factors which determine lag times in children with cancer in LMICs is an important step to improving their care and clinical outcomes [96, 97].
Conclusions
Early diagnosis of cancer is an important clinical goal for both paediatric and adult populations. Although significant differences exist between cancers in these age groups, opportunities to reduce the incidence of advanced stage disease are comparable across age groups. Diagnosis and treatment of childhood cancer which are timely, and ultimately more effective, are achievable through tailored risk-based screening, judicious early diagnosis programmes in primary care, targeted educational interventions and campaigns addressing lag times across the cancer care continuum.
Acknowledgements
We are grateful to Dr. Ligia Fu (Hospital Escuela, Tegucigalpa, Honduras), Dr. Carlos Rodriguez-Galindo (St. Jude Children’s Research Hospital, Memphis TN, United States), Dr. Kathy Pritchard-Jones (International Society of Paediatric Oncology, London, United Kingdom), Kathy Brodeur-Robb and Dr. Leah Young (C17 Children’s Cancer and Blood Disorders, Canada) and Dr. Lorna Fern (University College London, United Kingdom) for valuable suggestions and advice.
Author contributions
All authors contributed to the conceptualisation, writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
CM received an MSc. stipend from the Division of Cancer Epidemiology, McGill University. The other authors received no specific funding for this work.
Data availability
The datasets analyzed during the current study are available from the GLOBOCAN and SEER data repositories at https://gco.iarc.fr/today and https://seer.cancer.gov/csr/1975_2018/
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: Due to an error in the legend of figure 1
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/16/2021
A Correction to this paper has been published: 10.1038/s41416-021-01548-x
References
- 1.Steliarova‐Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer. Cancer. 2005;103:1457–67. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 2.Steliarova-Foucher E, Colombet M, Ries LA, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18:719–31. doi: 10.1016/S1470-2045(17)30186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart BW, Wild CP. World Cancer Report 2014. Lyon: The International Agency for Research on Cancer; 2014.
- 4.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer: a simulation-based analysis. Lancet Oncol. 2019;20:483–93. doi: 10.1016/S1470-2045(18)30909-4. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Galindo C, Friedrich P, Alcasabas P, Antillon F, Banavali S, Castillo L, et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol. 2015;33:3065. doi: 10.1200/JCO.2014.60.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Camargo B, De Andrea MLM, Franco EL. Catching up with history: treatment of Wilms’ tumor in a developing country. Med Pediatr Oncol. 1987;15:270–6. doi: 10.1002/mpo.2950150510. [DOI] [PubMed] [Google Scholar]
- 8.Bhakta N, Force LM, Allemani C, Atun R, Bray F, Coleman MP, et al. Childhood cancer burden: a review of global estimates. Lancet Oncol. 2019;20:e42–e53. doi: 10.1016/S1470-2045(18)30761-7. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Howard SC, Hunger SP, Antillon FG, Metzger ML, Israels T, et al. Treating Childhood Cancer in Low- and Middle-Income Countries. In: H Gelband, P Jha, R Sankaranarayanan, S Horton, editors. Cancer: Disease Control Priorities, 3rd edition (Volume 3). Washington (DC): The International Bank for Reconstruction and Development/The World Bank; 2015 Nov 1. Chapter 7. 2015. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343626/.
- 10.Abramson DH, Beaverson K, Sangani P, Vora RA, Lee TC, Hochberg HM, et al. Screening for retinoblastoma: presenting signs as prognosticators of patient and ocular survival. Pediatrics. 2003;112:1248–55. doi: 10.1542/peds.112.6.1248. [DOI] [PubMed] [Google Scholar]
- 11.Erwenne CM, Franco EL. Age and lateness of referral as determinants of extra-ocular retinoblastoma. Ophthalmic Paediatr Genet. 1989;10:179–84. doi: 10.3109/13816818909009874. [DOI] [PubMed] [Google Scholar]
- 12.Neal R, Tharmanathan P, France B, Din N, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92–S107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–85. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today. Accessed 21 July 2021.
- 15.Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2018. Bethesda: National Cancer Institute. https://seer.cancer.gov/csr/1975_2018/. Accessed 21 July 2021.
- 16.Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet. 2012;379:1436–46. doi: 10.1016/S0140-6736(11)61137-9. [DOI] [PubMed] [Google Scholar]
- 17.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429. [PMC free article] [PubMed] [Google Scholar]
- 18.Force LM, Abdollahpour I, Advani SM, Agius D, Ahmadian E, Alahdab F, et al. The global burden of childhood and adolescent cancer in 2017: an analysis of the Global Burden of Disease Study 2017. Lancet Oncol. 2019;20:1211–25. doi: 10.1016/S1470-2045(19)30339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benson JR, Jatoi I, Keisch M, Esteva FJ, Makris A, Jordan VC, et al. Early breast cancer. Lancet. 2009;373:1463–79. doi: 10.1016/S0140-6736(09)60316-0. [DOI] [PubMed] [Google Scholar]
- 20.Franco EL, Duarte-Franco E, Rohan TE. Evidence-based policy recommendations on cancer screening and prevention. Cancer Detect Prev. 2002;26:350–61. doi: 10.1016/s0361-090x(02)00118-6. [DOI] [PubMed] [Google Scholar]
- 21.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Pollock BH, Krischer JP, Vietti TJ. Interval between symptom onset and diagnosis of pediatric solid tumors. J Pediatr. 1991;119:725–32. doi: 10.1016/s0022-3476(05)80287-2. [DOI] [PubMed] [Google Scholar]
- 23.Stiller CA. Epidemiology and genetics of childhood cancer. Oncogene. 2004;23:6429–44. doi: 10.1038/sj.onc.1207717. [DOI] [PubMed] [Google Scholar]
- 24.Wilson JMG, Jungner G. Principles and practice of screening for disease. In: Public health papers; no. 34. Geneva: World Health Organization; 1968.
- 25.Tota JE, Isidean SD, Franco EL. Defining benchmarks for tolerable risk thresholds in cancer screening: Impact of HPV vaccination on the future of cervical cancer screening. Int J Cancer. 2020;147:3305–12. doi: 10.1002/ijc.33178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods WG, Tuchman M, Robison LL, Bernstein M, Leclerc J-M, Brisson LC, et al. A population-based study of the usefulness of screening for neuroblastoma. Lancet. 1996;348:1682–7. doi: 10.1016/S0140-6736(96)06020-5. [DOI] [PubMed] [Google Scholar]
- 27.Sawada T, Nakata T, Takasugi N, Maeda K, Hanawa Y, Shimizu K, et al. Mass screening for neuroblastoma in infants in Japan: interim report of a mass screening study group. Lancet. 1984;324:271–3. doi: 10.1016/s0140-6736(84)90311-8. [DOI] [PubMed] [Google Scholar]
- 28.Schilling FH, Spix C, Berthold F, Erttmann R, Fehse N, Hero B, et al. Neuroblastoma screening at one year of age. N Engl J Med. 2002;346:1047–53. doi: 10.1056/NEJMoa012277. [DOI] [PubMed] [Google Scholar]
- 29.Soderstrom L, Woods WG, Bernstein M, Robison LL, Tuchman M, Lemieux B, et al. Health and economic benefits of well-designed evaluations: some lessons from evaluating neuroblastoma screening. J Natl Cancer Inst. 2005;97:1118–24. doi: 10.1093/jnci/dji203. [DOI] [PubMed] [Google Scholar]
- 30.Woods WG, Gao R-N, Shuster JJ, Robison LL, Bernstein M, Weitzman S, et al. Screening of infants and mortality due to neuroblastoma. N Engl J Med. 2002;346:1041–6. doi: 10.1056/NEJMoa012387. [DOI] [PubMed] [Google Scholar]
- 31.Schilling FH, Spix C, Berthold F, Erttmann R, Sander J, Treuner J, et al. Children may not benefit from neuroblastoma screening at 1 year of age. Updated results of the population based controlled trial in Germany. Cancer Lett. 2003;197:19–28. doi: 10.1016/s0304-3835(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K, Hanada R, Kikuchi A, Ichikawa M, Aihara T, Oguma E, et al. Spontaneous regression of localized neuroblastoma detected by mass screening. J Clin Oncol. 1998;16:1265–9. doi: 10.1200/JCO.1998.16.4.1265. [DOI] [PubMed] [Google Scholar]
- 33.Brodeur G, Look A, Shimada H, Hamilton V, Maris J, Hann H, et al. Biological aspects of neuroblastomas identified by mass screening in Quebec. Med Pediatr Oncol. 2001;36:157–9. doi: 10.1002/1096-911X(20010101)36:1<157::AID-MPO1038>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 34.Barrette S, Bernstein ML, Leclerc J-M, Champagne MA, Samson Y, Brossard J, et al. Treatment complications in children diagnosed with neuroblastoma during a screening program. J Clin Oncol. 2006;24:1542–5. doi: 10.1200/JCO.2005.04.4602. [DOI] [PubMed] [Google Scholar]
- 35.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 36.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375:614–7. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 37.Tsuda T, Tokinobu A, Yamamoto E, Suzuki E. Thyroid cancer detection by ultrasound among residents ages 18 years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology. 2016;27:316. doi: 10.1097/EDE.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S, et al. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25:1127–36. doi: 10.1089/thy.2015.0116. [DOI] [PubMed] [Google Scholar]
- 39.Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract. 2015;21:686–96. doi: 10.4158/EP14466.DSCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dimaras H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL, et al. Retinoblastoma. Nat Rev Dis Prim. 2015;1:1–23. doi: 10.1038/nrdp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Committee on Practice Ambulatory Medicine. Eye examination in infants, children, and young adults by pediatricians. Pediatrics. 2003;111:902–7. [PubMed] [Google Scholar]
- 42.Skalet AH, Gombos DS, Gallie BL, Kim JW, Shields CL, Marr BP, et al. Screening children at risk for retinoblastoma: consensus report from the American Association of Ophthalmic Oncologists and Pathologists. Ophthalmology. 2018;125:453–8. doi: 10.1016/j.ophtha.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Antoneli CBG, Steinhorst F, de Cássia Braga Ribeiro K, Novaes PER, Chojniak MM, Arias V, et al. Extraocular retinoblastoma: a 13‐year experience. Cancer. 2003;98:1292–8. doi: 10.1002/cncr.11647. [DOI] [PubMed] [Google Scholar]
- 44.Leander C, Fu LC, Pena A, Howard SC, Rodriguez‐Galindo C, Wilimas JA, et al. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49:817–9. doi: 10.1002/pbc.21052. [DOI] [PubMed] [Google Scholar]
- 45.Antoneli CBG, Steinhorst F, Ribeiro KDCB, Chojniak MM, Novaes PER, Arias V, et al. The pediatrician’s ability to recognize the presenting signs and symptoms of retinoblastoma. Rev da Assocçao Médica Brasileira. 2004;50:400–2. doi: 10.1590/s0104-42302004000400030. [DOI] [PubMed] [Google Scholar]
- 46.Nathan PC, Ness KK, Mahoney MC, Li Z, Hudson MM, Ford JS, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med. 2010;153:442–51. doi: 10.1059/0003-4819-153-7-201010050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–90. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 48.Yan AP, Chen Y, Henderson TO, Oeffinger KC, Hudson MM, Gibson TM, et al. Adherence to surveillance for second malignant neoplasms and cardiac dysfunction in childhood cancer survivors: a childhood cancer survivor study. J Clin Oncol. 2020;38:1711–22. doi: 10.1200/JCO.19.01825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith R, Cokkinides V, von Eschenbach A, Levin B, Cohen C, Runowicz C. American Cancer Society Guidelines for the Early Detection of Cancer. CA Cancer J Clin. 2002;52:8–22. doi: 10.3322/canjclin.52.1.8. [DOI] [PubMed] [Google Scholar]
- 50.Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006. 10.17226/11468.
- 51.American College of Surgeons. Important information regarding CoC survivorship care plan standard. https://www.facs.org/quality-programs/cancer/news/survivorship. 2021.
- 52.Halton J, Walker E, Greenberg M, Greenberg C. Physician workforce in pediatric oncology: A pediatric oncology group of Ontario (POGO) exercise in establishing the appropriate physician ratio. Pediatr Blood Cancer. 2007;48:626–626. [Google Scholar]
- 53.Halton JM, Hand J, Byron P, Strother D, Blanchette V, C17 Council of Canadian Pediatric Hematology Oncology, T. D. et al. Establishing physician to patient ratios and predicting workforce needs for Canadian pediatric hematology‐oncology programs. Pediatr Blood Cancer. 2013;60:564–9. doi: 10.1002/pbc.24362. [DOI] [PubMed] [Google Scholar]
- 54.Centre for Surveillance and Applied Research, Public Health Agency of Canada. Cancer in Young People in Canada Data Tool. 2020 Edition. Public Health Infobase. Ottawa (ON): Public Health Agency of Canada, 2020.
- 55.Dixon SB, Bjornard KL, Alberts NM, Armstrong GT, Brinkman TM, Chemaitilly W, et al. Factors influencing risk‐based care of the childhood cancer survivor in the 21st century. CA Cancer J Clin. 2018;68:133–52. doi: 10.3322/caac.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kratz CP, Achatz MI, Brugieres L, Frebourg T, Garber JE, Greer M-LC, et al. Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin Cancer Res. 2017;23:e38–e45. doi: 10.1158/1078-0432.CCR-17-0408. [DOI] [PubMed] [Google Scholar]
- 57.Ballinger ML, Best A, Mai PL, Khincha PP, Loud JT, Peters JA, et al. Baseline surveillance in Li-Fraumeni syndrome using whole-body magnetic resonance imaging: a meta-analysis. JAMA Oncol. 2017;3:1634–9. doi: 10.1001/jamaoncol.2017.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chompret AS, Abel A, Stoppa-Lyonnet D, Brugières L, Pagès S, Feunteun J, et al. Sensitivity and predictive value of criteria for p53 germline mutation screening. J Med Genet. 2001;38:43. doi: 10.1136/jmg.38.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brodeur GM, Nichols KE, Plon SE, Schiffman JD, Malkin D. Pediatric cancer predisposition and surveillance: an overview, and a tribute to Alfred G. Knudson Jr. Clin Cancer Res. 2017;23:e1–e5. doi: 10.1158/1078-0432.CCR-17-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samuel N, Villani A, Fernandez CV, Malkin D. Management of familial cancer: sequencing, surveillance and society. Nat Rev Clin Oncol. 2014;11:723–31. doi: 10.1038/nrclinonc.2014.169. [DOI] [PubMed] [Google Scholar]
- 61.Green DM. The evolution of treatment for Wilms tumor. J Pediatr Surg. 2013;48:14–9. doi: 10.1016/j.jpedsurg.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 62.Barr RD. “Delays” in diagnosis: a misleading concept, yet providing opportunities for advancing clinical care. J Pediatr Hematol Oncol. 2014;36:169–72. doi: 10.1097/MPH.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 63.Worden JW, Weisman AD. Psychosocial components of lagtime in cancer diagnosis. J Psychosom Res. 1975;19:69–79. doi: 10.1016/0022-3999(75)90052-5. [DOI] [PubMed] [Google Scholar]
- 64.National Patient Safety Agency. Delayed diagnosis of cancer: Thematic review. London: National Reporting and Learning Service, 2010.
- 65.Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. [DOI] [PMC free article] [PubMed]
- 66.Brasme J-F, Morfouace M, Grill J, Martinot A, Amalberti R, Bons-Letouzey C, et al. Delays in diagnosis of paediatric cancers: a systematic review and comparison with expert testimony in lawsuits. Lancet Oncol. 2012;13:e445–e459. doi: 10.1016/S1470-2045(12)70361-3. [DOI] [PubMed] [Google Scholar]
- 67.Dang‐Tan T, Franco EL. Diagnosis delays in childhood cancer: a review. Cancer. 2007;110:703–13. doi: 10.1002/cncr.22849. [DOI] [PubMed] [Google Scholar]
- 68.Dobson CM, Russell AJ, Rubin GP. Patient delay in cancer diagnosis: what do we really mean and can we be more specific? BMC Health Serv Res. 2014;14:1–6. doi: 10.1186/1472-6963-14-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Purkayastha D, O’Hara C, Moran, T. Routes to diagnosis: investigating the different pathways for cancer referrals in England for teenagers and young adults. London: National Cancer Intelligence Network; 2013.
- 70.Armstrong L. It’s not about the bike: My journey back to life. Penguin; 2001.
- 71.Diorio C, Lam CG, Ladas EJ, Njuguna F, Afungchwi GM, Taromina K, et al. Global use of traditional and complementary medicine in childhood cancer: a systematic review. J Glob Oncol. 2017;3:791–800. doi: 10.1200/JGO.2016.005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fern LA, Birch R, Whelan J, Cooke M, Sutton S, Neal RD, et al. Why can’t we improve the timeliness of cancer diagnosis in children, teenagers, and young adults? BMJ. 2013;347:f6493. doi: 10.1136/bmj.f6493. [DOI] [PubMed] [Google Scholar]
- 73.Walker DA. Helping GPs to diagnose children’s cancer. Br J Gen Pract. 2021;71:151–2. doi: 10.3399/bjgp21X715241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leal‐Leal CA, Dilliz‐Nava H, Flores‐Rojo M, Robles‐Castro J. First contact physicians and retinoblastoma in Mexico. Pediatr Blood Cancer. 2011;57:1109–12. doi: 10.1002/pbc.23227. [DOI] [PubMed] [Google Scholar]
- 75.Yousef YA, AlNawaiseh T, AlJabari R, Muhsen S, Al-Nawaiseh I. Retinoblastoma awareness among first contact physicians in Jordan. Ophthalmic Genet. 2019;40:191–5. doi: 10.1080/13816810.2019.1605387. [DOI] [PubMed] [Google Scholar]
- 76.Piramal Foundation. A cancer screening program for rural Telangana. http://www.piramalswasthya.org/wp-content/uploads/2019/03/Cancer-Mobile-Unit-Launch_Swasthya.pdf. 2021.
- 77.Nair MK, Varghese C, Mathew B, Sankaranarayanan R. Prevention and early detection of oral, breast and cervical cancers: a practical approach in Indian context. J Indian Med Assoc. 1993;91:94–6. [PubMed] [Google Scholar]
- 78.Noronha V, Tsomo U, Jamshed A, Hai M, Wattegama S, Baral R, et al. A fresh look at oncology facts on south central Asia and SAARC countries. South Asian J Cancer. 2012;1:1. doi: 10.4103/2278-330X.96489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wright JG, Menaker RJ. Waiting for children’s surgery in Canada: the Canadian Paediatric Surgical Wait Times project. CMAJ. 2011;183:E559–E564. doi: 10.1503/cmaj.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Improving outcomes in children and young people with cancer. London: NICE: 2005. https://www.nice.org.uk/guidance/qs55.
- 81.Barraclough KNew. NICE guidance on referral for cancer. BMJ. 2015;351:h3640. doi: 10.1136/bmj.h3640. [DOI] [PubMed] [Google Scholar]
- 82.Neal R, Din N, Hamilton W, Ukoumunne O, Carter B, Stapley S, et al. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer. 2014;110:584–92. doi: 10.1038/bjc.2013.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shanmugavadivel D, Liu J-F, Murphy L, Wilne S, Walker D. Accelerating diagnosis for childhood brain tumours: an analysis of the HeadSmart UK population data. Arch Dis Child. 2020;105:355–62. doi: 10.1136/archdischild-2018-315962. [DOI] [PubMed] [Google Scholar]
- 84.Abdelkhalek E, Sherief L, Kamal N, Soliman R. Factors associated with delayed cancer diagnosis in egyptian children. Clin Med Insights Pediatr. 2014;8: S16413. [DOI] [PMC free article] [PubMed]
- 85.Berhane A, Hailu T, Mulugeta A. Determinants of delayed diagnosis among pediatric cancer patients from Ayder Comprehensive Specialized Hospital, Mekelle, Northern Ethiopia. BMC Pediatr. 2019;19:1–8. doi: 10.1186/s12887-019-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Venkatasai JP, Srinivasamaharaj S, Sneha LM, Scott JX, Baby AK, Rajan M. Pediatric hematological malignancy: identification of issues involved in the road to diagnosis. South Asian J Cancer. 2017;6:028–30. doi: 10.4103/2278-330X.202559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verma N, Bhattacharya S. Time to diagnosis and treatment of childhood cancer. Indian J Pediatr. 2020;87:641–3. [DOI] [PubMed]
- 88.Handayani K, Sitaresmi M, Supriyadi E, Widjajanto P, Susilawati D, Njuguna F, et al. Delays in diagnosis and treatment of childhood cancer in Indonesia. Pediatr Blood Cancer. 2016;63:2189–96. doi: 10.1002/pbc.26174. [DOI] [PubMed] [Google Scholar]
- 89.Buckle GC, Collins JP, Sumba PO, Nakalema B, Omenah D, Stiffler K, et al. Factors influencing time to diagnosis and initiation of treatment of endemic Burkitt Lymphoma among children in Uganda and western Kenya: a cross-sectional survey. Infect Agents Cancer. 2013;8:1–16. doi: 10.1186/1750-9378-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Njuguna F, Martijn H, Langat S, Musimbi J, Muliro H, Skiles J, et al. Factors influencing time to diagnosis and treatment among pediatric oncology patients in Kenya. Pediatr Hematol Oncol. 2016;33:186–99. doi: 10.3109/08880018.2016.1169566. [DOI] [PubMed] [Google Scholar]
- 91.Chukwu B, Ezenwosu O, Ikefuna A, Emodi I. Diagnostic delay in pediatric cancer in Enugu, Nigeria: a prospective study. Pediatr Hematol Oncol. 2015;32:164–71. doi: 10.3109/08880018.2014.957368. [DOI] [PubMed] [Google Scholar]
- 92.Stefan DC, Siemonsma F. Delay and causes of delay in the diagnosis of childhood cancer in Africa. Pediatr Blood Cancer. 2011;56:80–5. doi: 10.1002/pbc.22714. [DOI] [PubMed] [Google Scholar]
- 93.Fabian ID, Abdallah E, Abdullahi SU, Abdulqader RA, Boubacar SA, Ademola-Popoola DS, et al. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. 2020;6:685–95. doi: 10.1001/jamaoncol.2019.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaliki S, Ji X, Zou Y, Rashid R, Sultana S, Taju Sherief S, et al. Lag time between onset of first symptom and treatment of retinoblastoma: an international collaborative study of 692 patients from 10 countries. Cancers. 2021;13:1956. doi: 10.3390/cancers13081956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Opthalmol. 2009;93:1129–31. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 96.Rodriguez-Galindo C, Friedrich P, Morrissey L, Frazier L. Global challenges in pediatric oncology. Curr Opin Pediatr. 2013;25:3–15. doi: 10.1097/MOP.0b013e32835c1cbe. [DOI] [PubMed] [Google Scholar]
- 97.Lam CG, Howard SC, Bouffet E, Pritchard-Jones K. Science and health for all children with cancer. Science. 2019;363:1182–6. doi: 10.1126/science.aaw4892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the GLOBOCAN and SEER data repositories at https://gco.iarc.fr/today and https://seer.cancer.gov/csr/1975_2018/