Bortezomib is a backbone of induction therapies for older patients with multiple myeloma (MM), either in combination with lenalidomide-dexamethasone (VRd) or with daratumumab-melphalan-prednisone (DVMP) [1, 2]. The major limitations to the continuous administration of bortezomib [3] are the risk of developing peripheral neuropathy (PN) [4, 5] and its parenteral administration requiring patient hospitalization. Ixazomib has the advantage of the oral route of administration without the concern for PN, making it a suitable therapeutic option for all-oral combinations and continuous treatment.
Here we present the results of the UNITO-EMN10 trial assessing four ixazomib-based induction regimens followed by ixazomib maintenance in elderly, transplant-ineligible newly diagnosed (ND)MM patients.
Patients with symptomatic NDMM aged ≥65 years or younger but ineligible for autologous stem-cell transplantation (ASCT) could be enrolled. Key inclusion criteria were age ≥18 years; Eastern Cooperative Oncology Group performance status from 0 to 2; and adequate bone marrow, renal, and hepatic reserves (inclusion and exclusion criteria are listed in detail in the Supplementary Appendix). The trial was approved by the institutional review boards or ethics committees at each of the participating centers. All patients gave written informed consent before entering the trial, which was performed in accordance with the Declaration of Helsinki of 1975 (as revised in 2008) and registered on ClinicalTrials.gov as NCT02586038.
This is an open-label, multicenter, multi-arm randomized phase II clinical trial. Patients were randomized to nine 28-day induction cycles of ixazomib (I) 4 mg on days 1, 8, 15 and dexamethasone (d) 40 mg on days 1, 8, 15, 22 or to Id plus either cyclophosphamide (C) 300 mg/m2 orally on days 1, 8, 15 or thalidomide (T) 100 mg/day or bendamustine (B) 75 mg/m2 iv on days 1, 8, followed by ixazomib maintenance (4 mg on days 1, 8, 15) for up to 2 years.
The trial was designed to select the most promising regimens among the four induction treatments (Id, ICd, IBd, and ITd), conditioning the result on an external target value of 2-year progression-free survival (PFS) of at least 65% to be considered positively for further evaluations, while a 2-year PFS of 50% was considered unsatisfactory. Secondary key endpoints included PFS2 and overall survival (OS) from randomization, PFS from the start of maintenance, response rates (including a minimal residual disease [MRD] detected with a sensitivity of 10−5 by flow cytometry in all patients achieving at least a very good partial response [VGPR] at the end of induction), and safety profiles of induction regimens and ixazomib maintenance.
The times of observation were censored on 17 December, 2020. Data were analyzed using R software (version 4.0.2; see the Supplementary Appendix and Tables S1–S2 for the complete statistical details).
A total of 175 patients were enrolled between 1 October 2015 and 5 November 2018 and randomized to Id (42), ICd (61), ITd (61), and IBd (11); of these, 4 did not start treatment (Id, 1; ICd, 2; and ITd, 1) due to consent withdrawal (3) and death (1; Fig. S1). In February 2017, the protocol was amended because of a low enrollment due to the presence—among the oral, ixazomib partners in three of the four study arms—of intravenous bendamustine in the fourth arm (IBd). After enrolling 11 patients in the IBd group, this arm was closed. Furthermore, according to predefined study-stopping rules, after the first 42 patients had been enrolled, the Id arm did not reach the minimum required number of ≥VGPR (4/20 VGPR observed; ≥6/20 required) during the first 4 induction cycles and was therefore closed in March 2018. ICd and ITd arms completed their target enrollment of 61 patients.
The median age of patients enrolled was 74 years (range, 53–88). Patient and disease characteristics are listed in Table S3.
After a median follow-up of 31 months (interquartile range [IQR], 27–37), the median PFS from randomization was 10 months with Id (95% confidence interval [CI] 7–20), 19 with ICd (95% CI 13–27), 12 with Itd (95% CI 10–18), and 14 with IBd (95% CI 3 - not reached [NR]; Fig. 1A). At 2 years, the PFS was 32% in the Id (95% CI 20–50%), 41% in the ICd (95% CI 30–56%), 25% in the ITd (95% CI 16–39%), and 40% in the IBd (95% CI 19–85%) arms. The median PFS2 from randomization was 32 months with Id (95% CI 27-NR), 41 with ICd (95% CI 31-NR), 41 with ITd (95% CI 37-NR), and 41 with IBd (95% CI 21-NR; Fig. 1B). The median OS from randomization was NR in all arms (Fig. 1C).
Fig. 1. Kaplan‒Meier analyses from randomization by treatment arm.
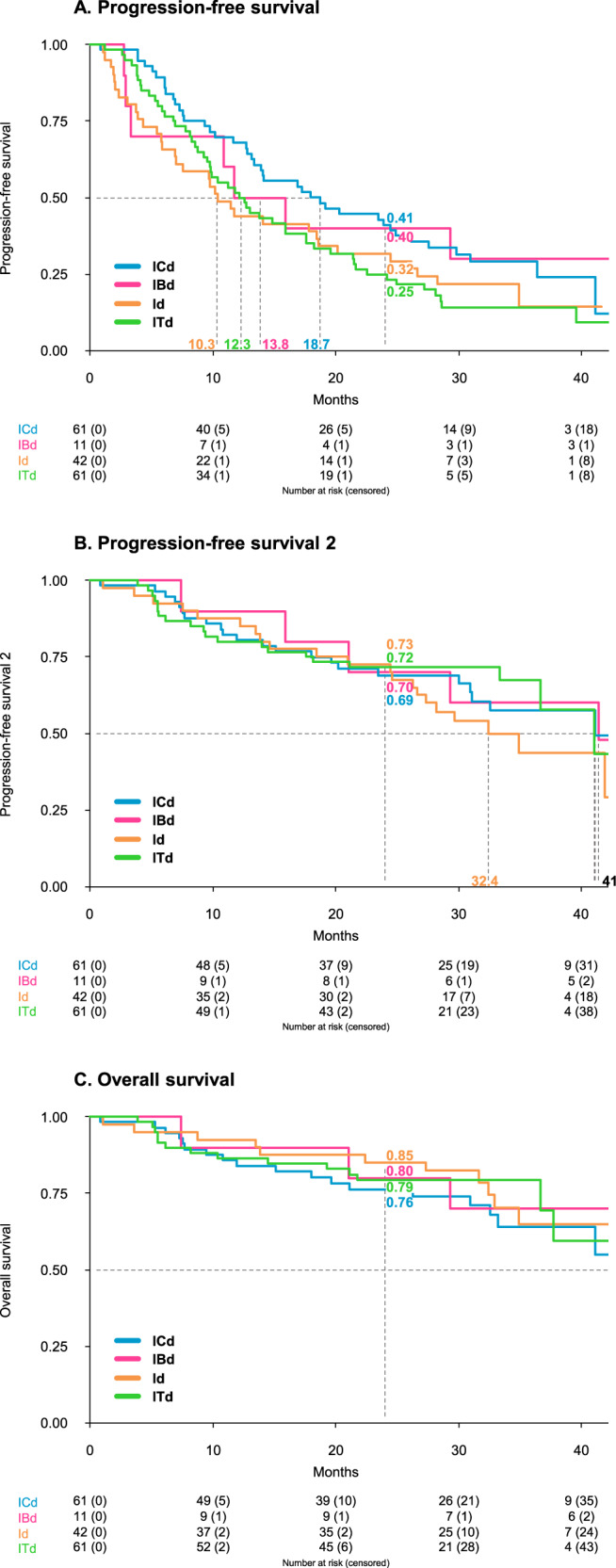
Panel A shows Kaplan‒Meier curves for progression-free survival (PFS), Panel B for PFS2, and Panel C for overall survival (OS) from randomization in patients assigned to ICd (blue line), IBd (magenta line), Id (orange line), and ITd (green line) arms. Id ixazomib-dexamethasone, ICd ixazomib-cyclophosphamide-dexamethasone, ITd ixazomib-thalidomide-dexamethasone, IBd ixazomib-bendamustine-dexamethasone.
The overall response rates (at least a partial response [PR]) after the induction phase were 57%, 75%, 84%, and 73% with Id, ICd, ITd, and IBd, respectively (Table 1). In the intention-to-treat analysis, the rates of MRD negativity at the end of the induction phase were 10%, 3%, 8%, and 9% in the Id, ICd, ITd, and IBd arms, respectively.
Table 1.
Response rates after induction and maintenance (response-evaluable population).
| All | Id | ICd | ITd | IBd | ||
|---|---|---|---|---|---|---|
| N = 175 | N = 42 | N = 61 | N = 61 | N = 11 | ||
| Induction | ||||||
| ORR | 129 (74) | 24 (57) | 46 (75) | 51 (84) | 8 (73) | |
| CR/sCR | 14 (8) | 4 (10) | 6 (10) | 3 (5) | 1 (9) | |
| VGPR | 56 (32) | 6 (14) | 22 (36) | 26 (43) | 2 (18) | |
| PR | 59 (34) | 14 (33) | 18 (30) | 22 (36) | 5 (45) | |
| SD | 35 (20) | 13 (31) | 11 (18) | 8 (13) | 3 (27) | |
| PD | 3 (2) | 2 (5) | - | 1 (2) | - | |
| NE | 8 (5) | 3 (7) | 4 (7) | 1 (2) | - | |
| MRD | NEG | 12 (7) | 4 (10) | 2 (3) | 5 (8) | 1 (9) |
| Overall | ||||||
| ORR | 131 (75) | 25 (60) | 47 (77) | 51 (84) | 8 (73) | |
| CR/sCR | 29 (17) | 9 (21) | 12 (20) | 6 (10) | 2 (18) | |
| VGPR | 46 (26) | 3 (7) | 18 (30) | 24 (39) | 1 (9) | |
| PR | 56 (32) | 13 (31) | 17 (28) | 21 (34) | 5 (45) | |
| SD | 33 (19) | 12 (29) | 10 (16) | 8 (13) | 3 (27) | |
| PD | 3 (2) | 2 (5) | - | 1 (2) | - | |
| NE | 8 (5) | 3 (7) | 4 (7) | 1 (2) | - | |
| MRD | NEG | 16 (9) | 5 (12) | 2 (3) | 7 (11) | 2 (18) |
Data are reported as numbers (percentage).
Id ixazomib-dexamethasone, ICd ixazomib-cyclophosphamide-dexamethasone, ITd ixazomib-thalidomide-dexamethasone, IBd ixazomib-bendamustine-dexamethasone, ORR overall response rate, CR complete response, sCR stringent CR, PR partial response, VGPR very good PR, SD stable disease, PD progressive disease, NE not evaluable, MRD minimal residual disease, NEG negative.
During the induction phase, ixazomib dose reductions were more common in patients receiving triplets (ICd, 24%; ITd, 20%; IBd, 18%) than in those treated with Id (2%). Treatment discontinuation due to adverse events (AEs) occurred more frequently in patients treated with ITd (17%), mostly due to PN (6%), as compared with those who received Id (10%), ICd (12%), or IBd (9%; Fig. S1).
Grade ≥3 hematologic AEs were infrequent (Id, 5%; ICd, 12%; ITd, 8%; and IBd, 18%). At least 1 grade ≥3 non-hematologic AE was reported in 17%, 19%, 48%, and 36% of patients treated with Id, ICd, ITd, and IBd, respectively, with grade ≥3 neurological and dermatologic AEs occurring more frequently in the ITd arm (17% and 13%) than in the Id (7% and 2%), ICd (7% and 2%), and IBd (9% and 0%) arms (Table S4).
Overall, 58% of enrolled patients started ixazomib maintenance (Id, 50%; ICd, 62%; ITd, 62%; and IBd, 45%). After a median follow-up of 25 months (IQR, 21–30) from the start of maintenance, the median PFS was 14.9 months (95% CI 10–19; Fig. S2).
During ixazomib maintenance, 19% of patients improved their response by at least one IMWG category (Fig. S3).
Fifteen % of patients required at least one ixazomib dose reduction. The rate of grade ≥3 hematologic and non-hematologic AEs was low (3 and 14%, respectively). Grade 1–2 PN was observed in 16% of patients, without grade ≥3 events (Table S5).
The primary objective of the trial was the selection of the most promising regimen worth further investigation, provided that a 2-year PFS of at least 65% would have been considered satisfactory. Unfortunately, with a 2-year PFS of 32% with Id, 41% with ICd, 25% with ITd, and 40% with IBd, none of the tested combinations reached the primary endpoint. The 65% target for the primary endpoint had been chosen based on the available data from the VISTA trial at the time of the study design (median time to progression, 24 months) [6, 7], expecting a PFS improvement incorporating ixazomib maintenance after the induction phase. This target may have been over-estimated, considering that the estimated percentage of patients alive and free from progression at 2 years was ~30% with VMP and ~60% with DVMP in the ALCYONE trial [2, 5] and around 40–50% with Rd [8]. Unfortunately, the lack of a control arm including a non-ixazomib-based combination does not allow to draw definitive conclusions.
Acknowledging the limitations of cross-trial comparisons, and also considering ixazomib maintenance in our trial, the combination of ixazomib with an alkylator and corticosteroids resulted in similar overall response rates (ORR, 75% vs. 74%) and median PFS (19 vs. 19 months) as compared with VMP (once-weekly, subcutaneous bortezomib) in the ALCYONE trial, although the rate of complete response obtained with ICd was lower (8%) than that reported with VMP (25%) [5].
Regarding the use of ixazomib maintenance, our results are in line with those reported in the TOURMALINE-MM4 trial, with similar median PFS from the start of maintenance (14.9 vs. 17.4 months) [9] and good tolerability, with no grade 3–4 PN events and with a rate of grade 3–4 infections (2%) lower than that associated with maintenance with continuous daratumumab (11%) [2] or lenalidomide (upper respiratory tract infections, 8%) [10].
Altogether, these results suggest that, due to its tolerability, and particularly due to the lower rate of grade 1–2 (20% vs. 34%) and grade 3–4 (2% vs. 4%) PN as compared to bortezomib, ixazomib may be more suitable as a maintenance therapy in patients in whom a deep cytoreduction has been achieved with more effective induction treatments. Moreover, it may represent an alternative to bortezomib in patients with preexisting PN or when an all-oral regimen is preferable to avoid frequent hospitalization.
Limitations of this study are the lack of a control arm that prevented the possibility to select the best performing regimen through a direct comparison with a standard treatment and the lack of a formal comparison between the investigated arms.
With these caveats, the observed results suggested that the doublet Id was associated with lower ORR (57% vs. 75%) and shorter PFS (median, 10 vs. 19 months) as compared to the triplet ICd. Furthermore, ICd was associated with similar ORR (75% vs. 84%) and ≥VGPR rates (46% vs. 48%) as compared to ITd, but with a longer median PFS (19 vs. 12 months), possibly due to a significantly lower rate of non-hematologic AEs (PN, 7% vs. 2%; dermatologic, 13% vs. 2%) and treatment discontinuation due to AEs (12% vs. 17%), as compared to ITd.
In conclusion, none of the ixazomib-based regimens tested met the primary endpoint of the trial. Among those tested, ICd may represent a viable, all-oral combination for future trials in a subset of older patients. Finally, ixazomib maintenance confirmed to be a well-tolerated approach in elderly MM patients.
Supplementary information
Acknowledgements
The authors wish to thank all the study participants and referring clinicians for their valuable contributions; the data managing staff Federica Leotta, Jessica Mastrovito, Ugo Panzani, Claudia Priola, Giorgio Schirripa, and Stefano Spada; and Elona Saraci from the laboratory staff at the Torino site. No funding was provided for the publication of this contribution.
Author contributions
RM, GC, MB, and AL conceived and designed the work that led to the submission. All authors acquired the data and interpreted the results. RM, GC, AC, PC, and AL drafted the first version of the manuscript. GC defined the statistical methods and AC performed the statistical analysis. All authors revised the manuscript and approved the final version. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability
After the publication of this article, data collected for this analysis and related documents (including the trial protocol) will be made available to others upon reasonably justified request, which needs to be written and addressed to the attention of the corresponding author Dr. Roberto Mina at the following e-mail address: roberto.mina@unito.it. The sponsor of the trial, the University of Torino (Italy), via the corresponding author Dr. Roberto Mina, is responsible to evaluate and eventually accept or refuse every request to disclose data and their related documents, in compliance with the ethical approval conditions, in compliance with applicable laws and regulations, and in conformance with the agreements in place with the involved subjects, the participating institutions, and all the other parties directly or indirectly involved in the participation, conduct, development, management, and evaluation of this analysis.
Competing interests
RM has received honoraria from Sanofi, Celgene, Takeda, and Janssen; has served on the advisory boards for Sanofi, Takeda, Bristol Myers Squibb, and Janssen; has received consultancy fees from Janssen. SBr has received honoraria from Celgene, Amgen, Oncopeptides, Janssen, and Sanofi; has served on the advisory boards for Takeda, Janssen, GlaxoSmithKline, Sanofi, Bristol Myers Squibb, and Oncopeptides; has received consultancy fees from Janssen, Takeda, Amgen, and Sanofi. AML has received research grants from Takeda, Servier, Roche, Celgene, AbbVie, Incyte, Janssen, Sanofi, Verastem, Novartis, Morphosys, GlaxoSmithKline, Oncopeptides, Karyopharm, Onconova, Archigen, Pfizer, Fibrogen, and Beigene; has received consulting fees from Incyte; has received honoraria from IQVIA, Servier, Celgene, AbbVie, Bristol Myers Squibb, and Janssen; has received travel or meeting support from Takeda, Roche, Janssen, Celgene, Bristol Myers Squibb, AbbVie, Novartis, Sanofi, IQVIA, and Verastem; has served on the advisory boards of Amgen and Servier. MTP has received honoraria from and served on the advisory boards for Celgene, Janssen-Cilag, Bristol Myers Squibb, Takeda, Amgen, Sanofi, and Karyopharm. FP has served on the advisory boards for Celgene, Janssen, Takeda, and Sanofi. MC has received advisory fees from Celgene, Janssen, and Takeda; has received research funding from Celgene. RZ has served on the advisory boards for Celgene, Takeda, Amgen, Janssen, and GlaxoSmithKline. PT has received honoraria from Amgen, Bristol Myers Squibb/Celgene, Janssen, Takeda, AbbVie, Sanofi, GlaxoSmithKline, and Oncopeptides. AB has served on the advisory boards of Janssen, Celgene, Amgen, and GlaxoSmithKline; has received consultancy fees from Takeda. LP has received honoraria from Celgene, Takeda, Amgen, Bristol Myers Squibb, and Janssen; has served on the advisory boards for Celgene, Bristol Myers Squibb, Amgen, and Janssen; has received consultancy fees from Janssen. SBa has received honoraria from and/or served on the scientific advisory boards for Celgene, Janssen, Takeda, Bristol Myers Squibb, Amgen, and Novartis. MO has received honoraria from and served on the advisory boards for Amgen, Bristol Myers Squibb, Celgene, Janssen, and Takeda. MB has received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and AbbVie; has served on the advisory boards for Janssen and GlaxoSmithKline; has received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and Mundipharma. PC has received honoraria from AbbVie, ADC Therapeutics, Amgen, Celgene, Daiichi Sankyo, Gilead/Kite, GlaxoSmithKline, Incyte, Janssen, Kyowa Kirin, Nerviano Medical Science, Novartis, Roche, Sanofi, and Takeda. AL has received honoraria from Amgen, Bristol Myers Squibb, Celgene, Janssen, and GlaxoSmithKline; has served on the advisory boards for Bristol Myers Squibb, Celgene, Janssen, and Takeda. The remaining authors declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-021-00590-5.
References
- 1.Durie BGM, Hoering A, Sexton R, Abidi MH, Epstein J, Rajkumar SV, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem. Blood Cancer J. 2020;10:53. 10.1038/s41408-020-0311-8. [DOI] [PMC free article] [PubMed]
- 2.Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395:132–41. doi: 10.1016/S0140-6736(19)32956-3. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Bringhen S, Larocca A, Rossi D, Di Raimondo F, Magarotto V, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32:634–40. doi: 10.1200/JCO.2013.52.0023. [DOI] [PubMed] [Google Scholar]
- 4.Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–27. doi: 10.1016/S0140-6736(16)31594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mateos M-V, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N. Engl J Med. 2018;378:518–28. doi: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 6.San-Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 7.Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259–66. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 8.Facon T, Dimopoulos MA, Dispenzieri A, Catalano JV, Belch A, Cavo M, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018;131:301–10. doi: 10.1182/blood-2017-07-795047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Špička I, Quach H, Oriol A, Hájek R, Garg M, et al. Ixazomib as postinduction maintenance for patients with newly diagnosed multiple myeloma not undergoing autologous stem cell transplantation: the phase III TOURMALINE-MM4 trial. J Clin Oncol. 2020;38:4030–41. doi: 10.1200/JCO.20.02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20:57–73. doi: 10.1016/S1470-2045(18)30687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
After the publication of this article, data collected for this analysis and related documents (including the trial protocol) will be made available to others upon reasonably justified request, which needs to be written and addressed to the attention of the corresponding author Dr. Roberto Mina at the following e-mail address: roberto.mina@unito.it. The sponsor of the trial, the University of Torino (Italy), via the corresponding author Dr. Roberto Mina, is responsible to evaluate and eventually accept or refuse every request to disclose data and their related documents, in compliance with the ethical approval conditions, in compliance with applicable laws and regulations, and in conformance with the agreements in place with the involved subjects, the participating institutions, and all the other parties directly or indirectly involved in the participation, conduct, development, management, and evaluation of this analysis.


