Abstract
Glycogen storage disease type IX (GSD IX), the most common form of GSD, is caused by a defect in phosphorylase kinase (PhK). We describe the case of a female patient with GSD IXc harboring a homozygous mutation in PHKG2 (NM_000294.3; PHKG2 (c.280_282delATC (p. I94del)) definitively diagnosed using the GSD gene panel. She presented with hypoglycemia, hepatomegaly, and short stature and died of cirrhosis and recurrent multiple hepatocellular adenoma at the age of 69 years and 11 months.
Subject terms: Metabolic disorders, Genetics research
Glycogen storage disease type IX (GSD IX) is one of the most common forms of GSD caused by a defect in phosphorylase kinase (PhK). This disease accounts for 25% of all GSD cases, with an estimated frequency of 1 in 100,000 individuals1. PhK (EC 2.7.1.38) is a serine/threonine-specific complex protein kinase consisting of four subunits: alpha (α), beta (β), gamma (γ), and delta (δ)2. PhK activates glycogen phosphorylase (EC 2.4.1.1), which releases glucose-1-phosphate from glycogen3. Patients with GSD IX have elevated transaminases and hepatomegaly, growth retardation, hypertriglyceridemia, and hypercholesterolemia. However, compared to that in patients with other types of liver GSDs, clinical manifestation in GSD IX patients can be mild, and patients may become asymptomatic as they age4,5. Mutations in PHKA1, PHKA2, or PHKB and PHKG2 cause GSD IX. PHKG2 encodes the hepatic isoform of the γ unit of PhK. Mutation in PHKG2 causes GSD IXc (MIM: 613027), a rare form that manifests as hepatomegaly, hypotonia, and growth retardation in childhood. Although these symptoms improve with age, some patients develop hepatic fibrosis, cirrhosis, and liver dysfunction6.
A female patient was definitively diagnosed with GSD IXc at the age of 68 years using the GSD gene panel. She was diagnosed with hepatic GSD in childhood but self-discontinued clinical examination and treatment; later, she developed liver cirrhosis and hepatocellular carcinoma (HCC). Herein we present her clinical course and discuss the mechanism and treatment of HCC in GSD IXc based on her clinical course.
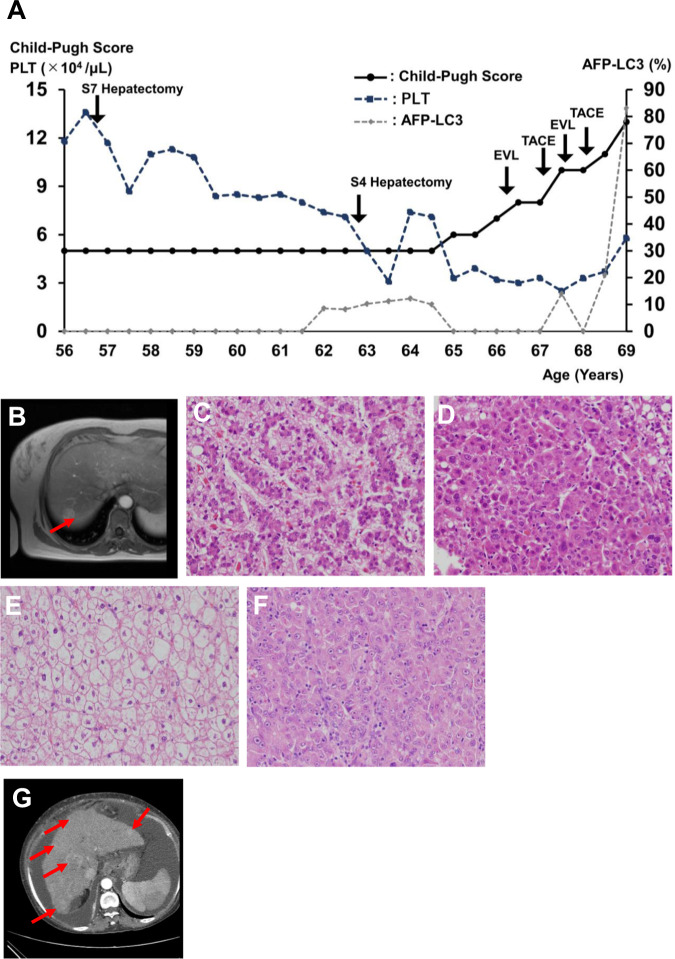
A 67-year-old female patient with suspected GSD was introduced to our institution to receive a definitive diagnosis while receiving conservative care therapy for liver cirrhosis and multiple HCC. She was the first child of healthy consanguineous parents. Her height was 141.9 cm (the average Japanese female height at 65–69 years is 153.3 ± 5.7 cm), and her weight was 54.7 kg. She presented with anasarca and ascites due to cirrhosis and HCC. She had no drinking habit and no history of hepatitis B or C infection. She also had no history of blood transfusion before receiving hepatectomy at the age of 56 years. Her intelligence was not impaired, and she could write advanced sentences. Hepatomegaly was first detected in this patient at 7 years; however, the diagnosis was not completed. At 12 years, she was diagnosed with GSD (type: unknown) at our institution, but she self-discontinued regular follow-up. At 30 years of age, she visited the Department of Internal Medicine because of frequent upper abdominal pain and fasting hypoglycemia symptoms, such as irritation and wooziness. In addition, she developed multiple adenomas and cirrhosis of the liver and received regular follow-up and medicine, including a gastrointestinal drug every month (Supplemental Data 1). She gradually lost the ability to work due to fatigue and hypoglycemia symptoms and lived a single life, receiving a pension under the social security system. At the age of 56 years, she underwent S7 partial hepatectomy because of HCC (Fig. 1A–E). At 63 years, she experienced multiple HCC relapse and underwent combination therapy of S4 partial hepatectomy and radiofrequency ablation (Fig. 1F). At the age of 66 years, she developed esophageal varix and underwent endoscopic variceal ligation (EVL), and she developed multiple recurrent HCCs and underwent transcatheter arterial chemoembolization (TACE) at the age of 67 years. In addition, she developed hepatic coma after rupture of the esophageal varix, which was rescued by EVL. At the age of 68 years, she developed recurrent multiple HCC and underwent a second TACE. After that, she could not undergo aggressive therapy for recurrent HCC because of impaired hepatic function and liver cirrhosis (Fig. 1G and Supplemental Data 1).
Fig. 1. Hepatocellular carcinoma (HCC) in a female patient with GSD IXc.
A Clinical course. Deterioration of cirrhosis and HCC progressed with age. B Liver MRI. T2-weighted imaging detected a 2-cm tumor in the liver (S7 section). C–F Pathological tissues. The S7 tumor exhibited characteristics of well-differentiated (C) and moderately differentiated (D) HCC combined with hepatocytes with a clear cell cytoplasm (E), consistent with pathology in GSD. The S4 tumor was poorly differentiated (F) and moderately differentiated HCC. G Liver CT. Multiple HCC and ascites were detected in the abdomen.
She was definitively diagnosed with GSD IXc because she harbored a homozygous c.280_282delATC (p. Ile94del) mutation in PHKG2. She died under palliative care at 69 years and 11 months.
Written informed consent was obtained from the patient’s parents. This study was approved by the Institutional Ethics Committee of the Faculty of Life Science, Kumamoto University.
We present a female patient with GSD IXc who developed liver cirrhosis and multiple HCC. She underwent aggressive surgical and medical treatment until the age of 68 years. However, she did not modify her diet, including consuming uncooked cornstarch for GSD, owing to her specific characteristics, including being stubborn, obsessive, and unwilling to accept others’ opinions. She was not aware of the need to follow a diet suitable for preventing hypoglycemia. To our knowledge, this is the first report of a patient with GSD IXc developing multiple HCC and receiving aggressive surgical and medical treatment. She carried a novel mutation in PHKG2. We have summarized the case report of GSD IXc in Table 1 (Supplemental Data 2).
Table 1.
PHKG2 mutations identified in the patients with GSD IXc.
| Patient (sex) | Ethnicity | Age (diagnosis/current) | Variants Allele 1/Allele 2 | Symptoms | Ref. |
|---|---|---|---|---|---|
| 1 (M) | Caucasian | 12 m/16 y | c.22G>T (p.E8*)/c.158_160delAGA (p.K53del) | Hepatomegaly, prominent cheeks, hypotonia, liver fibrosis, hypoglycemia, ketosis, elevated ASL/ALT, mild gross motor delay | 18 |
| 2 (M) | Chinese | 1 y 5 m/1 y 5 m | c.79_88delinsTCTGGTCG (p.K27Sfs*33)/c.166G>T (p.E56*) | Severe hepatomegaly, hypoglycemia, elevated ASL/ALT, hypertriglyceridemia, growth retardation | 21 |
| 3 (F) | Caucasian | 16 m/6 y | c.96-11G>A/c.247C>T (p.Q83*) | Hepatomegaly, liver fibrosis, hypoglycemia, ketosis, elevated ASL/ALT, normal growth | 18 |
| 4 (M) | Caucasian | 12 m/5 y | c.96-11G>A/no mutation identified | Hepatomegaly, elevated ASL/ALT, growth delayed, mild speech delay, significant mid-foot pronation, slight hind-foot valgus, slight genu valgus requiring orthoses, left hydronephrosis | 18 |
| 5 (F) | Pakistani | 1.5 y/2.5 y | c.107C>T (p.S36F) (homo) | Hypoglycemic seizure, hepatomegaly, elevated ASL/ALT, improved by dietary | 22 |
| 6 (F)a | Pakistani | 13 y/14 y | c.107C>T (p.S36F)/c.226C>T (p.R76*) | Tonic clonic seizures (since 8 m), Elevated ASL/ALT, Liver glycogenosis with mild fibrosis | 22 |
| 7 (M)a | 10 y/11 y | Tonic clonic seizures (since 15 m), elevated ASL/ALT, liver glycogenosis with mild fibrosis | |||
| 8 (F) | NA | 6 m/18 y | c.121T>C (p.C41R)/c.643G>A (p.D215N) | Hepatomegaly, elevated ASL/ALT | 16 |
| 9 (F) | Saudi Arabian | 11 m/8 y | c.130C>T (p.R44*) (homo) | Hepatomegaly, growth retardation, elevated ASL/ALT, liver cirrhosis | 6 |
| 10 (F) | Pakistani | 2 y 4 m/NA | c.144delC (p.H48Qfs*5) (homo) | Hepatomegaly, hypoglycemia, muscle weakness, fatigue, delayed puberty | 23 |
| 11 (M) | Pakistani | 6 m/13 m | c.247C>T (p.Q83*) (homo) | Hypoglycemic seizure, hepatomegaly, improved by dietary | 22 |
| 12 (M) | NA | 6 m/17 y | c.256G>A (p.G86S)/c.925C>T (p.R309W) | Hepatomegaly, elevated ASL/ALT, failure to thrive | 16 |
| 13 (F) | Norwegian | 5 m/18 y | c.265_266insC (p.H89Pfs*13) (homo) | Hepatomegaly, muscular hypotonia, growth retardation, hypoglycemia | 24 |
| 14 (F) | Norwegian | 4 m/7.5 y | c.265_266insC (p.H89Pfs*13)/c.900G>A (p.W300*) | Hepatomegaly, hypoglycemia, elevated ASL/ALT, mild liver fibrosis, markedly retarded growth | 8 |
| 15 (M) | Algerian | 8 m/NA | c.272-1G>C (homo) | Hypoglycemia, growth delay, distal amyotrophia, elevated ASL/ALT, cirrhosis, portal fibrosis | 9 |
| 16 (F) | Japanese | 15 y/26 y | c.277delC (p.L93Sfs*17) | Hepatocellular adenoma, liver cirrhosis | 6 |
| 17 (F) | Japanese | NA/died at 70 y | c.280_282delATC (p.I94del) (homo) | Hepatomegaly, elevated ASL/ALT, growth impairment, liver cirrhosis, ascites, multiple hepatocellular carcinoma | This study |
| 18 (F) | Pakistani | 15 m/NA | c.317T>G (p.V106E) (homo) | Hepatomegaly, growth retardation, severe liver fibrosis, elevated ALT and triglycerides, proliferation of bile ducts | 24 |
| 19 (F) | Turkish | 3.5 y/9.5 y | c.326+1G>A (homo) | Hepatomegaly, splenomegaly, liver fibrosis, cirrhosis | 25 |
| 20 (M) | Pakistani | 2 y/NA | c.431T>C (p.L144P) (homo) | Hepatomegaly, hypoglycemia, muscle weakness, fatigue, hyperlactic acidemia, autoimmune type 1 diabetes | 23 |
| 21 (M) | English | 7 m/3 y | c.433C>T (p.H145Y)/c.677T>G (p.L226R) | Poor growth, muscle wasting, hepatomegaly, elevated ASL/ALT and triglycerides | 4 |
| 22 (M) | Pakistani | 2.5 y/2.5 y | c.454C>T (p.R152*) (homo) | Elevated ASL/ALT, hepato-splenomegaly | 22 |
| 23 (F) | Chinese | 4 m/18 y | c.469G>A (p.E157K) (homo) | Hepatomegaly, elevated ASL/ALT, progressive splenomegaly and portal hypertension (starting from 7 y) | 8 |
| 24 (F) | Chinese | 3 y/3 y | Severe hepatomegaly, hypoglycemia, elevated ASL/ALT, hypertriglyceridemia, growth retardation | 21 | |
| 25 (F) | Chinese | 1 y 6 m/1 y 6 m | c.469G>A (p.E157K)/c.553C>T (p.R185*) | Severe hepatomegaly, hypoglycemia, elevated ASL/ALT, hypertriglyceridemia, growth retardation | |
| 26 (M) | Chinese | 1y8m/1y8m | c.469G>A (p.E157K)/c.761delC (p.E256Sfs*12) | Severe hepatomegaly, hypoglycemia, elevated ASL/ALT, hypertriglyceridemia, growth retardation | |
| 27 (M) | Chinese | 2y1m/2y1m | c.469G>A (p.E157K)/c.835C>T (p.R279C) | Severe hepatomegaly, hypoglycemia, elevated ASL/ALT, hypertriglyceridemia, growth retardation | |
| 28 (M) | NA | 5 m/NA | c.502C>T (p.R168*)/c.859C>T (p.Q287*) | Hypoglycemia, elevated ASL/ALT, cirrhosis | 9 |
| 29 (M) | Chinese | 2 y/3 y | c.553C>T (p.R185*) (homo) | Hepatomegaly, elevated ASL/ALT, high total bile acid | 26 |
| 30 (M) | Pakistani | 2 y/2.5 y | Progressive abdominal distention, hepatomegaly, elevated ASL/ALT | 22 | |
| 31 (F)a | Pakistani | 9 m/10 y | c.557-3C>G (homo) | Hypoglycemic seizure, elevated serum triglycerides, improved by dietary | 22 |
| 32 (M)a | 3 m/7 y | Hepatomegaly, improved by dietary | |||
| 33 (F) | French | 7 m/5 y | c.566G>A (p.G189E) (homo) | Hepatomegaly, growth retardation, mild muscule hypotonia, elevated ASL/ALT and triglycerides | 24 |
| 34 (F) | Norwegian | 9 m/8 y | c.643G>A (p.D215N) (homo) | Hepatomegaly, hypoglycemia, elevated ASL/ALT, gastric tube feeding (from 1 y 3 m to date (8 y)) | 8 |
| 35 (M) | Caucasian | 44 m/12 y | c.647+5G>T (homo) | Hypoglycemic seizure, hepatomegaly, liver fibrosis, elevated ASL/ALT, mild muscle weakness, mild delay in walking, normal growth but improved on therapy | 18 |
| 36 (F)a | Saudi Arabian | 9 m/>2.5 y | c.659G>A (p.G220E) (homo) | 30-week gestation, 1.6 kg birth weight, delayed developmental motor milestones, hypoglycemia, hepatomegaly, liver fibrosis, elevated ASL/ALT, cirrhosis, hypocalcemia | 17 |
| 37 (F)a | 8 m/2.5 y | Full-term normal delivery, motor delay, hypoglycemia, hepatomegaly, liver fibrosis, elevated ASL/ALT | |||
| 38 (F) | 9 m/2 y | Hypoglycemia, hepatomegaly, liver fibrosis, elevated ASL/ALT | |||
| 39 (M) | Comoran | Early childhood/>21 y | c.802_805delATCT (p.I268Pfs*12) (homo) | Hypoglycemia, cirrhosis, muscular defect with distal amyotrophia, received a liver transplantation at age 20 years | 9 |
| 40 (F) | Caucasian | 2 y/9 y | c.900G>A (p.W300*)/c.1073A>G (p.Y358C) | Full-term normal delivery, 3 kg birth weight, hepatomegaly, liver fibrosis, elevated ASL/ALT, mild muscle weakness, normal growth but improved on therapy | 18 |
| 41 (M)a | Pakistani | 3 y/11 y | c.958C>T (p.R320*) (homo) | Hypoglycemia, elevated ASL/ALT, noticed hepatomegaly at 2.5 y, but remained well by dietary | 13 |
| 42 (M)a | 3 m/7.5 y | Hepatomegaly, elevated ASL/ALT | |||
| 43 (M) | Jordonian | 14 m/>6 y | c.1034C>G (p.S345*) (homo) | Hepatomegaly, elevated ASL/ALT | 27 |
NA not available.
aSiblings.
The clinical outcome in 30 patients with GSD IXc was collected in an earlier study7. Hepatomegaly, fasting hypoglycemia, fasting ketosis, hypertriglyceridemia, hypercholesterolemia, growth delay, developmental delay, and elevated transaminase were present in 100% (30/30), 94.7% (18/19), 100% (6/6), 94.4% (17/18), 45.4% (5/11), 70.8% (17/24), 50% (10/20), and 100% (22/22) of patients with GSD IXc, respectively. Eighty percent (24/30) of patients underwent a liver biopsy. According to pathology reports, 95.8% of patients (23/24) presented with either fibrosis (mild, moderate, or severe) or cirrhosis. Three patients developed a hepatic adenoma. Only one patient developed HCC at the age of 27 years; this patient is awaiting liver transplantation.
There is no consensus on effective treatment for GSD IXc. Nasogastric tube feeding, uncooked cornstarch (0.3–2.0 g/kg body weight (BW)/day), and a high-protein diet (3.0–4.0 g/kg BW/day) are common treatments for GSD IXc4,8,9. Poor metabolic control is a risk factor for the development of long-term complications such as hepatocellular adenoma in hepatic GSD10–13. Moreover, hepatocellular adenoma may be regressed in a well-controlled metabolic state with strict dietary therapy. Nevertheless, the effectiveness of strict diet therapy is controversial because there are few adult patients with GSD IXc who receive long-term strict diet therapy.
We believe that this patient should have received a liver transplant when she was diagnosed with HCC. We had reservations in treating her HCC, which developed at the age of 58 years, particularly with regard to choosing between aggressive invasive treatment, including hepatectomy and TACE, and conservative treatment because HCC is known to recur. Overall, it is important to control the radical metabolic state in metabolic disease.
Cirrhosis is the most important risk factor for the development of HCC, regardless of etiology14. Liver fibrosis and hepatocellular carcinoma occur in patients with GSD VI15,16 caused by a defect in glycogen phosphorylase. GSD IXc with pathogenic variants in PHKG2 is associated with more severe clinical and biochemical abnormalities, including increased risk for liver fibrosis and cirrhosis15,17,18, because γ is the catalytic subunit, whereas α and β are regulatory subunits of PhK. Glycogen is a key energy store for cancer cells19, and glycogen turnover allows cancer cells to adapt and survive under adverse oxygen and nutrient conditions within the tumor microenvironment. Glycogen breakdown supports the pentose phosphate pathway, which generates the nucleotides required for proliferation and DNA repair, and NADPH, which is an important reducing agent for reactive oxygen species scavenging and for the synthesis of nucleotides, amino acids, and lipids19. Therefore, both cirrhosis and glycogen accumulation are important factors in recurrent and multiple HCC. Activated PhK β-subunit expression regulates the occurrence of HCC by inhibiting AKT/protein kinase B and signal transducer and activator of transcription 3 signaling pathway activation, independent of the glycogenolytic pathway20. In hepatic GSD, the formation and proliferation of HCC may be related to a mechanism independent of the glycogenolytic pathway, which should be investigated in the future.
The specific characteristics of our patient might be a clinical manifestation of GSD IXc. We should have realized this possibility earlier and administered appropriate counseling.
In conclusion, we present the case of a patient with GSD IXc who developed liver cirrhosis and multiple HCC. This case shows the long-term clinical course in GSD IXc. Liver cirrhosis and HCC were important complications in this long-term surviving patient with GSD IXc. However, there is no established effective therapy for GSD IXc, and it is not clear to what extent HCC should be aggressively treated with invasive treatment. Knowledge of clinical outcomes in more patients with GSD IXc is essential to design a treatment course for GSD IXc.
Supplementary information
Acknowledgements
We thank all staff in the Department of Pediatrics, Department of Gastroenterology and Hepatology, and Department of Gastroenterological Surgery, Kumamoto University Hospital for their help in clinical practice.
Author contributions
All contributors have read this manuscript and approved its submission for consideration, for publication as a Data Report in the Human Genome Variation. J. K. designed the case report and wrote the manuscript. J. K., H. M., T. W., and K. S. collected and analyzed data. J. K., H. M., and K. N. supervised this case report and clinical practice. H. S., and T. F. performed the PHKG2 gene analysis.
Funding
This work was supported in part by a Health and Labor Sciences Research Grant for Research on Rare and Intractable Diseases from the Ministry of Health, Labor and Welfare, Japan (grant number JPMH20FC1025); a Grant-in-Aid for Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (AMED; grant numbers JP19ek0109276, JP21ek0109482); and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Japan Society for the Promotion of Science [JSPS] KAKENHI: grant number JP20K08207). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
HGV database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at 10.6084/m9.figshare.hgv.3103.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41439-021-00172-8.
References
- 1.Maichele AJ, Burwinkel B, Maire I, Søvik O, Kilimann MW. Mutations in the testis/liver isoform of the phosphorylase kinase γ subunit (PHKG2) cause autosomal liver glycogenosis in the gsd rat and in humans. Nat. Genet. 1996;14:337–340. doi: 10.1038/ng1196-337. [DOI] [PubMed] [Google Scholar]
- 2.Brushia RJ. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front. Biosci. 1999;4:d618. doi: 10.2741/Brushia. [DOI] [PubMed] [Google Scholar]
- 3.Newgard CB, Hwang PK, Fletterick RJ. The family of glycogen phosphorylases: structure and function. Crit. Rev. Biochem. Mol. Biol. 1989;24:69–99. doi: 10.3109/10409238909082552. [DOI] [PubMed] [Google Scholar]
- 4.Burwinkel B, Tanner MS, Kilimann MW. Phosphorylase kinase deficient liver glycogenosis: progression to cirrhosis in infancy associated with PHKG2 mutations (H144Y and L225R) J. Med. Genet. 2000;37:376–7. doi: 10.1136/jmg.37.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morava E, et al. Biochemical characteristics and increased tetraglucoside excretion in patients with phosphorylase kinase deficiency. J. Inherit. Metab. Dis. 2005;28:703–706. doi: 10.1007/s10545-005-0095-9. [DOI] [PubMed] [Google Scholar]
- 6.Burwinkel B, Shiomi S, Al Zaben A, Kilimann MW. Liver glycogenosis due to phosphorylase kinase deficiency: PHKG2 gene structure and mutations associated with cirrhosis. Hum. Mol. Genet. 1998;7:149–154. doi: 10.1093/hmg/7.1.149. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes SA, Cooper GE, Gibson RA, Kishnani PS. Benign or not benign? Deep phenotyping of liver glycogen storage disease IX. Mol. Genet. Metab. 2020;131:299–305. doi: 10.1016/j.ymgme.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burwinkel B, Rootwelt T, Kvittingen EA, Chakraborty PK, Kilimann MW. Severe phenotype of phosphorylase kinase-deficient liver glycogenosis with mutations in the PHKG2 gene. Pediatr. Res. 2003;54:834–839. doi: 10.1203/01.PDR.0000088069.09275.10. [DOI] [PubMed] [Google Scholar]
- 9.Davit-Spraul A, et al. Liver glycogen storage diseases due to phosphorylase system deficiencies: diagnosis thanks to non invasive blood enzymatic and molecular studies. Mol. Genet. Metab. 2011;104:137–143. doi: 10.1016/j.ymgme.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Minarich LA, Kirpich A, Fiske LM, Weinstein DA. Bone mineral density in glycogen storage disease type Ia and Ib. Genet. Med. 2012;14:737–741. doi: 10.1038/gim.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melis D, et al. Impaired bone metabolism in glycogen storage disease type 1 Is associated with poor metabolic control in type 1a and with granulocyte colony-stimulating factor therapy in type 1b. Horm. Res. Paediatr. 2014;81:55–62. doi: 10.1159/000351022. [DOI] [PubMed] [Google Scholar]
- 12.Beegle RD, Brown LM, Weinstein DA. Regression of hepatocellular adenomas with strict dietary therapy in patients with glycogen storage disease type I. JIMD Rep. 2014;18:23–32. doi: 10.1007/8904_2014_344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burda P, Hochuli M. Hepatic glycogen storage disorders. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:415–421. doi: 10.1097/MCO.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 14.Balogh J, et al. Hepatocellular carcinoma: a review. J. Hepatocell. Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manzia TM, et al. Glycogen storage disease type Ia and VI associated with hepatocellular carcinoma: two case reports. Transplant. Proc. 2011;43:1181–1183. doi: 10.1016/j.transproceed.2011.01.129. [DOI] [PubMed] [Google Scholar]
- 16.Roscher A, et al. The natural history of glycogen storage disease types VI and IX: long-term outcome from the largest metabolic center in Canada. Mol. Genet. Metab. 2014;113:171–176. doi: 10.1016/j.ymgme.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Albash B, et al. Novel PHKG2 mutation causing GSD IX with prominent liver disease: report of three cases and review of literature. Eur. J. Pediatr. 2014;173:647–653. doi: 10.1007/s00431-013-2223-0. [DOI] [PubMed] [Google Scholar]
- 18.Bali DS, et al. Variability of disease spectrum in children with liver phosphorylase kinase deficiency caused by mutations in the PHKG2 gene. Mol. Genet. Metab. 2014;111:309–313. doi: 10.1016/j.ymgme.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zois CE, Favaro E, Harris AL. Glycogen metabolism in cancer. Biochem. Pharmacol. 2014;92:3–11. doi: 10.1016/j.bcp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Yang W, et al. Phosphorylase kinase β represents a novel prognostic biomarker and inhibits malignant phenotypes of liver cancer cell. Int. J. Biol. Sci. 2019;15:2596–2606. doi: 10.7150/ijbs.33278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou D, et al. Clinical features and PHKG2 gene mutation analysis of 5 Chinese patients with glycogen storage disease IXc. J. Clin. Pediatr. 2017;35:609–612. [Google Scholar]
- 22.Waheed N, et al. Variability of clinical and biochemical phenotype in liver phosphorylase kinase deficiency with variants in the phosphorylase kinase (PHKG2) gene. J. Pediatr. Endocrinol. Metab. 2020;33:1117–1123. doi: 10.1515/jpem-2019-0603. [DOI] [PubMed] [Google Scholar]
- 23.Beauchamp NJ, et al. Glycogen storage disease type IX: high variability in clinical phenotype. Mol. Genet. Metab. 2007;92:88–99. doi: 10.1016/j.ymgme.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Michele AJ, et al. Mutations in the testis/liver isoform of the phosphorylase kinase gamma subunit (PHKG2) cause autosomal liver glycogenosis in the GSD rat and in humans. Nat. Genet. 1996;14:337–340. doi: 10.1038/ng1196-337. [DOI] [PubMed] [Google Scholar]
- 25.van Beurden EACM, et al. Autosomal recessive liver phosphorylase kinase deficiency caused by a novel splice-site mutation in the gene encoding the liver gamma subunit (PHKG2) Biochem. Biophys. Res. Commun. 1997;236:544–548. doi: 10.1006/bbrc.1997.7006. [DOI] [PubMed] [Google Scholar]
- 26.Li C, et al. PHKG2 mutation spectrum in glycogen storage disease type IXc: a case report and review of the literature. J. Pediatr. Endocrinol. Metab. 2018;31:331–338. doi: 10.1515/jpem-2017-0170. [DOI] [PubMed] [Google Scholar]
- 27.Fahiminiya S, et al. Whole exome sequencing unravels disease-causing genes in consanguineous families in Qatar. Clin. Genet. 2014;86:134–141. doi: 10.1111/cge.12280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The relevant data from this Data Report are hosted at the Human Genome Variation Database at 10.6084/m9.figshare.hgv.3103.