Abstract
During the winter months of 2020/2021 a wave of multisystem inflammatory syndrome in children (MIS-C) emerged in Poland. We present the results of a nationwide register aiming to capture and characterise MIS-C with a focus on severity determinants. The first MIS-C wave in Poland was notably high, hence our analysis involved 274 children. The group was 62.8% boys, with a median age of 8.8 years. Besides one Asian, all were White. Overall, the disease course was not as severe as in previous reports, however. Pediatric intensive care treatment was required for merely 23 (8.4%) of children, who were older and exhibited a distinguished clinical picture at hospital admission. We have also identified sex-dependent differences; teenage boys more often had cardiac involvement (decreased ejection fraction in 25.9% vs. 14.7%) and fulfilled macrophage activation syndrome definition (31.0% vs. 15.2%). Among all boys, those hospitalized in pediatric intensive care unit were significantly older (median 11.2 vs. 9.1 years). Henceforth, while ethnicity and sex may affect MIS-C phenotype, management protocols might be not universally applicable, and should rather be adjusted to the specific population.
Subject terms: Infectious diseases, Inflammation, Epidemiology, Immunological disorders, Infectious diseases
Introduction
Pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or multisystem inflammatory syndrome in children (herein referred to as MIS-C) is a new entity which has emerged in countries particularly hit by coronavirus disease 2019 (COVID-19) pandemic1–9. Despite similarities to other inflammatory conditions, e.g. Kawasaki disease (KD), macrophage activation syndrome (MAS) or toxic shock syndrome, MIS-C has its distinct features8,9. MIS-C is characterized by a sudden onset of rapidly progressing multisystem inflammation which particularly affects the cardiovascular system, resulting in cardiac dysfunction and shock. Milder forms of fever and inflammation were also described, however in the largest published cohorts majority of patients necessitated treatment in a pediatric intensive care unit (PICU)4–11.
Following COVID-19 second wave in Autumn 2020 (first significant wave in Poland), a rise in MIS-C prevalence has emerged. Here we report the results of a national register of inflammatory disorders in children (MultiOrgan Inflammatory Syndromes COVID-19 Related Study, MOIS-CoR) revealing a consistent picture of MIS-C in our population, yet with some unique local characteristics.
Results
Study group
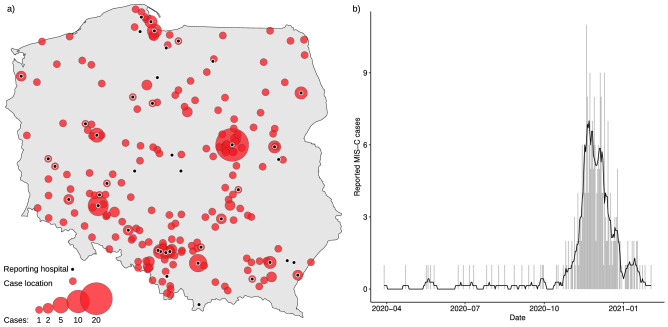
Children aged 0–18 years old with inflammatory conditions were voluntarily reported by treating clinicians. The surveillance was launched on 25th May 2020, and, as of 20th February 2021, 399 children have been reported from 42 cities all over the country (Fig. 1). 342 cases fulfilled the inclusion criteria and 274 fulfilled MIS-C diagnostic criteria (Table 1, Supplementary Table 3). The following results apply only to the cohort of 274 children, aged from 7 months to 17.6 years, with MIS-C. Precise demographic characteristics of the MIS-C cohort are presented in Table 1. All but one Asian child were of White ethnicity, 172 (62.8%) were male. A minority of children had any comorbidities, with obesity (6.7%), asthma (4.1%) and neurological disorders (2.3%) being the most prevalent.
Figure 1.
Geospatial and temporal distribution of analyzed cases. (a) Shows locations of analyzed cases and reporting sentinel sites. (b) Shows the counts MIS-C hospitalisations in respective days in the analyzed period, between 4th March 2020 and 20th February 2021. Bars indicate daily counts, while the black line presents the 7-day moving average.
Table 1.
Demographic characteristic and SARS-CoV-2 status of a studied group.
| All (399) | MIS-C (274) | |
|---|---|---|
| Male sex | 250 (63%) | 171 (62%) |
| Age (years) | 7.8 (3.9–11.5) | 8.8 (5.2–12.1) |
| Ethnicity | ||
| White | 397 (99%) | 273 (100%) |
| Asian | 2 (1%) | 1 (0%) |
| Comorbidity | 58 (19%) | 38 (18%) |
| Asthma | 13 (3%) | 11 (4%) |
| Obesity | 29 (8%) | 16 (7%) |
| Neurological disorder | 7 (2%) | 6 (2%) |
| Immunosuppression | 3 (1%) | 1 (0%) |
| BMI Z-score | 0.0 (− 0.8 to 0.9) | 0.1 (− 0.8 to 0.9) |
| SARS-CoV-2 tests and anamnesis | ||
| Contact with a confirmed COVID-19 case | 144 (45%) | 120 (52%) |
| Confirmed preceding SARS-CoV-2 infection | 30 (9%) | 24 (10%) |
| Positive SARS-CoV RT-PCR result | 41 (12%) | 29 (13%) |
| Positive antibodies against SARS-CoV-2 at admission | 262 (80%) | 241 (95%) |
Binary data given as count (per-cent), and numerical data as median (interquartile range).
BMI body mass index, COVID-19 coronavirus disease 2019, RT-PCR real-time transcription polymerase chain reaction, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Clinical presentation
The median time between first symptoms and hospital admission was 5 days, and the median fever length was 7 days. The vital signs at admission and at their respective peaks are presented in Table 2 and Supplementary Table 5. Complete clinical characteristics of children with MIS-C are presented in Table 3 and Supplemental Table 4.
Table 2.
Vital signs and laboratory results of MIS-C cohort at admission and at respective peaks.
| All (274) | PICU (23) | Non-PICU (251) | P | P|Age | P|Sex | |
|---|---|---|---|---|---|---|
| CRT > 2 s adm | 30 (13%) | 8 (40%) | 22 (11%) | < 0.01 | < 0.01 | < 0.01 |
| CRT max > 2 s | 32 (15%) | 10 (56%) | 22 (11%) | < 0.01 | < 0.01 | < 0.01 |
| Saturation adm [%] | 98.0 (96.0–99.0) | 97.0 (95.0–98.0) | 98.0 (96.0–99.0) | 0.09 | 0.08 | 0.09 |
| Saturation min [%] | 96.0 (92.0–98.0) | 92.0 (89.8–97.0) | 96.0 (93.0–98.0) | 0.02 | 0.04 | 0.02 |
| Respiratory rate adm [1/min] | 20.0 (18.0–25.0) | 30.0 (20.0–45.0) | 20.0 (18.0–25.0) | 0.01 | < 0.01 | < 0.01 |
| Respiratory rate max [1/min] | 25.0 (20.0–30.0) | 40.0 (30.0–49.5) | 24.0 (20.0–30.0) | < 0.01 | < 0.01 | < 0.01 |
| Heart rate adm [1/min] | 120.0 (100.0–133.0) | 127.0 (110.0–140.0) | 120.0 (100.0–133.0) | 0.3 | 0.08 | 0.2 |
| Heart rate max [1/min] | 132.5 (118.0–150.0) | 135.0 (120.0–160.0) | 132.0 (118.0–146.5) | 0.2 | 0.06 | 0.2 |
| SBP adm [mmHg] | 100.0 (90.0–110.0) | 89.0 (78.8–99.5) | 100.0 (91.0–110.0) | < 0.01 | < 0.01 | < 0.01 |
| SBP min [mmHg] | 88.0 (78.0–96.0) | 74.0 (62.5–82.5) | 89.0 (80.0–96.8) | < 0.01 | < 0.01 | < 0.01 |
| Non-alert AVPU adm | 17 (6%) | 5 (22%) | 12 (5%) | < 0.01 | < 0.01 | < 0.01 |
| Non-alert AVPU min | 38 (15%) | 8 (44%) | 30 (13%) | < 0.01 | < 0.01 | < 0.01 |
| WBC adm [/l] | 9.6 (6.6–13.3) | 11.6 (7.1-19.1) | 9.6 (6.6-12.8) | 0.1 | 0.1 | 0.1 |
| WBC min [/l] | 6.7 (5.0–9.0) | 6.0 (5.3–9.2) | 6.7 (5.0–9.0) | 0.9 | 0.8 | 0.9 |
| WBC max [/l] | 14.5 (10.9–19.7) | 19.8 (13.1–28.4) | 14.3 (10.5–19.1) | < 0.01 | < 0.01 | < 0.01 |
| Lymphocytes adm [/l] | 1.0 (0.7–1.8) | 0.8 (0.6–1.0) | 1.1 (0.7–1.8) | 0.05 | 0.3 | 0.05 |
| Lymphocytes min [/l] | 1.0 (0.6–1.8) | 0.6 (0.5–0.9) | 1.0 (0.6–1.8) | < 0.01 | 0.07 | < 0.01 |
| Hemoglobin adm [g/dl] | 11.7 (10.7–12.7) | 11.1 (10.3–12.1) | 11.8 (10.7–12.7) | 0.1 | 0.02 | 0.1 |
| Hemoglobin min [g/dl] | 10.3 (9.4–11.2) | 9.7 (8.8–10.7) | 10.3 (9.4–11.2) | 0.06 | < 0.01 | 0.05 |
| Platelets adm [/l] | 176.0 (127.0–248.0) | 153.0 (121.0–187.5) | 178.0 (128.5–255.8) | 0.08 | 0.1 | 0.07 |
| Platelets min [/l] | 160.0 (109.8–230.8) | 135.5 (93.8–182.5) | 163.5 (111.0–243.2) | 0.08 | 0.09 | 0.06 |
| CRP adm [mg/l] | 140.0 (83.7–194.9) | 242.0 (123.3–289.0) | 133.4 (78.8–187.2) | < 0.01 | < 0.01 | < 0.01 |
| CRP max [mg/l] | 166.3 (94.4—242.1) | 264.6 (206.7–309.4) | 161.4 (93.3–226.2) | < 0.01 | < 0.01 | < 0.01 |
| Procalcitonin adm [ng/ml] | 2.5 (1.0–6.9) | 13.2 (2.1–51.2) | 2.3 (0.9–6.2) | < 0.01 | < 0.01 | < 0.01 |
| Procalcitonin max [ng/ml] | 4.3 (1.3–12.9) | 17.0 (10.2–30.7) | 3.5 (1.2–10.0) | < 0.01 | < 0.01 | < 0.01 |
| ESR adm [mm] | 44.0 (31.0–66.0) | 78.5 (73.0–81.2) | 44.0 (30.0–65.0) | 0.03 | 0.02 | 0.02 |
| ESR max [mm] | 57.0 (37.8–80.0) | 77.0 (52.5–82.5) | 55.0 (37.0–78.0) | 0.4 | 0.4 | 0.4 |
| Ferritin adm [ng/ml] | 331.0 (197.9–622.4) | 671.0 (475.9–1052.8) | 317.1 (186.2–533.8) | < 0.01 | < 0.01 | < 0.01 |
| Ferritin max [ng/ml] | 402.2 (217.9–672.2) | 671.0 (559.2–1113.4) | 367.9 (207.3–616.5) | < 0.01 | < 0.01 | < 0.01 |
| Triglycerides adm [mg/dl] | 147.0 (123.0–218.9) | 181.0 (148.5–266.5) | 145.0 (119.0–213.0) | 0.05 | 0.04 | 0.05 |
| Triglycerides max [mg/dl] | 172.5 (129.0–256.2) | 194.0 (169.0–359.0) | 167.0 (125.0–246.0) | 0.07 | 0.05 | 0.07 |
| D-dimers adm [mg/l] | 2.6 (1.5–4.6) | 3.9 (2.5–5.7) | 2.5 (1.4–4.4) | 0.01 | < 0.01 | < 0.01 |
| D-dimers max [mg/l] | 3.8 (2.0–6.3) | 5.7 (3.6–8.2) | 3.7 (2.0–6.2) | 0.03 | 0.05 | 0.02 |
| AlAT adm [U/l] | 24.0 (16.0–40.1) | 35.0 (16.0–92.0) | 23.0 (15.5–40.0) | 0.05 | 0.06 | 0.05 |
| AlAT max [U/l] | 33.3 (21.0–65.8) | 55.0 (32.0–122.0) | 33.0 (20.0–59.2) | < 0.01 | 0.03 | < 0.01 |
| AST adm [U/l] | 32.1 (25.0–51.5) | 37.0 (25.0–59.0) | 32.0 (24.5–51.0) | 0.3 | 0.3 | 0.3 |
| AST max [U/l] | 43.0 (30.0–65.0) | 57.0 (44.0–108.0) | 42.0 (30.0–63.5) | < 0.01 | 0.01 | < 0.01 |
| CK adm [U/l] | 51.0 (37.0–92.0) | 22.4 (13.2–30.9) | 52.0 (38.0–93.0) | 0.01 | < 0.01 | 0.02 |
| CK max [U/l] | 51.0 (37.0–85.0) | 27.9 (24.0–38.0) | 56.5 (37.2–92.8) | 0.01 | < 0.01 | 0.01 |
| Sodium adm [mmol/l] | 135.0 (132.0–137.0) | 134.0 (132.0–137.8) | 135.0 (132.0–137.0) | 1 | 0.9 | 0.8 |
| Sodium min [mmol/l] | 133.6 (131.0–135.9) | 133.0 (130.0–135.0) | 133.8 (131.0–135.9) | 0.5 | 0.6 | 0.6 |
| Albumins adm [g/dl] | 3.3 (2.8–3.7) | 2.8 (2.4–3.4) | 3.3 (2.8–3.7) | 0.02 | < 0.01 | 0.01 |
| Albumins min [g/dl] | 2.8 (2.5–3.3) | 2.5 (2.3–2.7) | 2.8 (2.5–3.3) | < 0.01 | < 0.01 | < 0.01 |
| Troponin elevated adm | 62 (28%) | 17 (77%) | 45 (22%) | < 0.01 | < 0.01 | < 0.01 |
| Troponin elevated max | 92 (51%) | 18 (86%) | 74 (47%) | < 0.01 | < 0.01 | < 0.01 |
| BNP/NT-proBNP elevated adm | 171 (86%) | 16 (89%) | 155 (85%) | 0.7 | 0.6 | 0.7 |
| BNP/NT-proBNP elevated max | 204 (91%) | 20 (100%) | 184 (90%) | 0.1 | 0.2 | 0.1 |
| eGFR adm [ml/min/1.73 m] | 110.1 (86.2–134.3) | 77.1 (50.8–89.5) | 113.1 (90.5–134.8) | < 0.01 | < 0.01 | < 0.01 |
| eGFR min [ml/min/1.73 m] | 104.9 (82.0–126.6) | 73.0 (45.4–88.0) | 107.4 (85.4–128.8) | < 0.01 | < 0.01 | < 0.01 |
Binary data given as count (per-cent), and numerical data as median (interquartile range). Values at admission are marked with adm, lowest obtained with min, while highest with max. Troponin is considered elevated at > 50 ng/l, while BNP/NT-proBNP at > 150 ng/ml.
AlAT, alanine transaminase, AST, aspartate transaminase, AVPU AVPU scale, BNP/NT-proBNP brain natriuretic peptide or N-terminal-pro-BNP, CK creatinine kinase, CRT capillary refill time, CRP, C-reactive protein, eGFR estimated glomerular filtration rate, ESR, erythrocyte sedimentation rate, MIS-C multisystem inflammatory syndrome in children, NT-proBNP N-terminal prohormone of brain natriuretic peptide, PICU, pediatric intensive care unit, SBP systolic blood pressure, WBC white blood cell count, P|Age age-adjusted p-value, P|Sex sex-adjusted p-value.
Table 3.
Demographic and clinical characteristics of MIS-C cohort respective of age.
| All (274) | 0–5 y.o. (64) | 5–12 y.o. (140) | 12–18 y.o. (70) | P | P|Sex | |
|---|---|---|---|---|---|---|
| Male sex | 171 (62%) | 30 (47%) | 91 (65%) | 50 (71%) | < 0.01 | – |
| Symptoms and signs | ||||||
| Fever length | 7.0 (6.0–9.0) | 7.0 (6.0–8.0) | 7.5 (6.0–9.0) | 7.0 (6.0–9.0) | 0.3 | 0.3 |
| Mucocutaneous and lymph nodes | 262 (97%) | 59 (95%) | 138 (99%) | 65 (94%) | 0.2 | 0.2 |
| Rash | 218 (83%) | 53 (84%) | 118 (87%) | 47 (71%) | 0.02 | 0.03 |
| Conjunctivitis | 207 (78%) | 47 (76%) | 109 (81%) | 51 (75%) | 0.6 | 0.5 |
| Hands/feet swelling or erythema | 142 (55%) | 39 (64%) | 75 (56%) | 28 (45%) | 0.1 | 0.2 |
| Oral inflammation | 173 (66%) | 43 (68%) | 96 (72%) | 34 (52%) | 0.02 | 0.02 |
| Cervical lymphadenopathy | 98 (38%) | 30 (48%) | 46 (36%) | 22 (32%) | 0.1 | 0.06 |
| Gastrointestinal | 250 (93%) | 56 (90%) | 132 (95%) | 62 (90%) | 0.3 | 0.3 |
| Abdominal pain | 222 (85%) | 42 (76%) | 120 (88%) | 60 (87%) | 0.1 | 0.2 |
| Nausea | 162 (62%) | 28 (47%) | 100 (75%) | 34 (51%) | < 0.01 | < 0.01 |
| Diarrhea | 164 (62%) | 36 (59%) | 84 (61%) | 44 (65%) | 0.8 | 1 |
| Lower respiratory | 128 (50%) | 23 (40%) | 64 (48%) | 41 (63%) | 0.04 | 0.04 |
| Chest pain | 48 (19%) | 3 (6%) | 19 (14%) | 26 (39%) | < 0.01 | < 0.01 |
| Cough | 74 (28%) | 17 (28%) | 33 (25%) | 24 (35%) | 0.3 | 0.2 |
| Breathing effort | 63 (24%) | 8 (13%) | 36 (27%) | 19 (28%) | 0.08 | 0.1 |
| Neurological | 212 (82%) | 52 (84%) | 109 (81%) | 51 (80%) | 0.8 | 0.9 |
| Lethargy | 151 (59%) | 36 (61%) | 88 (66%) | 27 (44%) | 0.02 | 0.01 |
| Irritability | 108 (42%) | 40 (65%) | 52 (39%) | 16 (25%) | < 0.01 | < 0.01 |
| Headache | 112 (46%) | 16 (32%) | 67 (51%) | 29 (47%) | 0.07 | 0.04 |
| Meningeal signs | 27 (10%) | 6 (10%) | 15 (11%) | 6 (9%) | 0.9 | 0.8 |
| Cardiovascular | 139 (58%) | 16 (33%) | 79 (63%) | 44 (69%) | < 0.01 | < 0.01 |
| Hypotension | 99 (41%) | 7 (14%) | 57 (46%) | 35 (51%) | < 0.01 | < 0.01 |
| Coronary dilation or aneurysm | 21 (8%) | 6 (11%) | 10 (7%) | 5 (8%) | 0.8 | 0.5 |
| EF < 55% | 58 (23%) | 3 (5%) | 31 (23%) | 24 (39%) | < 0.01 | < 0.01 |
| Musculo-osteoarticular | 111 (44%) | 17 (32%) | 58 (44%) | 36 (55%) | 0.04 | 0.03 |
| Muscle pain | 103 (41%) | 15 (29%) | 53 (40%) | 35 (54%) | 0.02 | 0.01 |
| Arthralgia | 50 (19%) | 6 (10%) | 29 (21%) | 15 (24%) | 0.1 | 0.08 |
| Arthritis | 12 (5%) | 1 (2%) | 6 (4%) | 5 (8%) | 0.2 | 0.2 |
| Clinical presentation | ||||||
| KD or aKD | 170 (67%) | 43 (70%) | 94 (71%) | 33 (54%) | 0.06 | 0.08 |
| KD | 105 (42%) | 29 (48%) | 57 (44%) | 19 (31%) | 0.1 | 0.2 |
| MAS | 59 (22%) | 4 (6%) | 30 (22%) | 25 (36%) | < 0.01 | < 0.01 |
| DIC | 42 (17%) | 5 (9%) | 22 (17%) | 15 (22%) | 0.1 | 0.06 |
Binary data given as count (per-cent), and numerical data as median (interquartile range).
BMI body mass index, DIC disseminated intravascular coagulation, EF ejection fraction, KD Kawasaki disease, MAS macrophage activation syndrome, MIS-C multisystem inflammatory syndrome in children, y.o. years old, P|Sex sex-adjusted p-value. Hypotension defined as minimal SBP in mmHg less than age in years or 90 for children over 10 years old.
Mucocutaneous and lymph node involvement was observed in 95.6% of children, and 66.7% fulfilled American Heart Association (AHA) KD or atypical KD (aKD) diagnostic criteria, irrespective of age. Rash was less common in children >12 years of age (71.2% vs. 86.4%, p < 0.01), whereas conjunctival injection, distal extremity changes and cervical lymphadenopathy were equally prevalent across age groups. The second most common group of associating symptoms was gastrointestinal (92.6%), with no significant differences across age groups, except for nausea and vomiting, which were more prevalent in children 5–12 years of age (74.6% vs. 48.8%, p < 0.01). Ten children underwent abdominal surgery due to acute abdominal symptoms.
Hypotension was present in 30/213 (14.1%) of patients at admission, while 99/243 (40.7%) developed it at some point during hospitalization. At least one echocardiogram was reported for 255 children, of whom 85 (33.3%) had any of the following abnormalities: decreased ejection fraction (EF), coronary artery abnormality (CAA) or pericardial effusion. EF < 55% was reported in 58/255 (22.7%) of children (four had EF < 35%), and the age of children with heart dysfunction overall was significantly higher (median [IQR] from 7.9 [4.9–11.3] to 11.0 years [8.8–13.6], p < 0.01). CAA developed in 21/255 (8.2%) of patients irrespective of age or KD or aKD phenotype. In particular, eight children developed coronary artery aneurysms; three of them resolved in the follow up before discharge. Pericardial effusion was present in 24/255 (9.4%) of reports.
Neurological symptoms included lethargy (59.4%), irritability (41.7%), headache (46.1%) and photophobia (11.0%). Children >12 years old less frequently presented lethargy (44.3% vs. 64.2%, p < 0.01) and irritability (25.0% vs. 47.2%, p < 0.01). Meningeal signs were observed in 27/260 (10.4%) of cases, irrespective of age, and six patients had aseptic meningitis.
Respiratory symptoms included sore throat (34.0%), cough (28.1%) and dyspnoea (24.1%), and they were uniformly prevalent across age groups. Patients >12 years old more often complained of chest pain (39.4% vs. 11.8%, p < 0.01) and muscle pain (53.8% vs. 36.6%, p = 0.01).
Dysuria was reported in 40/257 (15.6%) of children, while sterile leukocyturia in 48/247 (19.4%).
Laboratory results
Laboratory parameters at admission and at respective peaks are summarized in Table 2. Lymphopenia was present in 195/269 (72.5%) of children, and 148/269 (55.0%) developed lymphopenia of below /l. Anemia was present in 254/273 (93.0%) of patients. Thrombocytopenia occurred in 131/273 (48.0%) of children, and 31/273 (11.4%) had a platelet count below l.
Following MIS-C case definition, all children had increased inflammatory markers: 158/266 (59.4%) had procalcitonin >2.5 ng/ml and 157/273 (57.5%) had C-reactive protein (CRP) >150 mg/l. Hypoalbuminemia was present in 219/260 (84.2%) of patients, and in 71/260 (27.3%) albumin level fell under 2.5 g/dl. Hyponatremia was found in 203/272 (74.6%) of patients, and in 9/272 (3.3%) it was < 125 mmol/l.
Finally, 62/222 (27.9%) of patients had elevated troponin at admission, and this ratio increased to 51.4% when assessed later in course of the disease. Children with elevated troponin levels at admission were older (median [IQR] from 7.9 [4.9–10.7] to 11.4 years [9.0–13.5], p < 0.01), and the same observation held for troponin level at its peak (median [IQR] from 8.5 [5.0–11.9] to 11.2 years [8.7–13.4], p < 0.01).
Sex-dependent clinical and laboratory characteristics
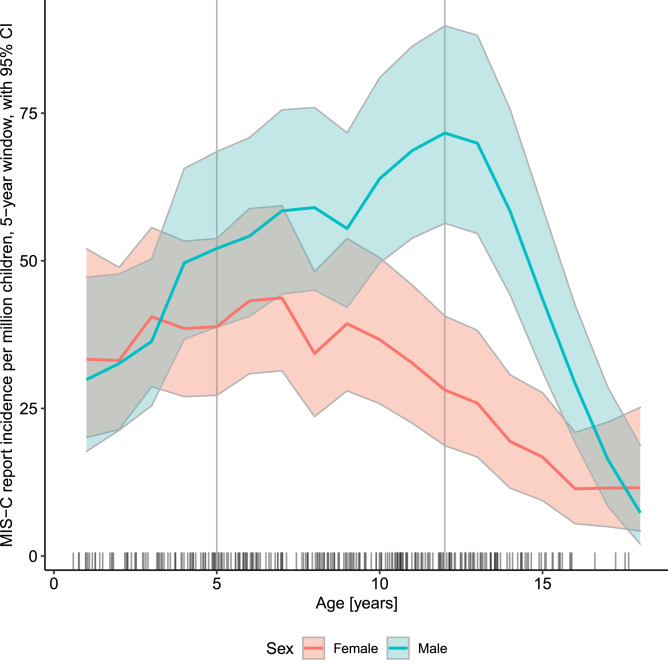
Male patients were diagnozed with MIS-C more often than expected from demographic structure, but only in the older age bracket (Fig. 2). We have identified some characteristics which corresponded with this discrepancy (Fig. 3, Table 3).
Figure 2.
Incidence of reported MIS-C cases within the Polish population of children aged 0–18 years, according to age and sex.
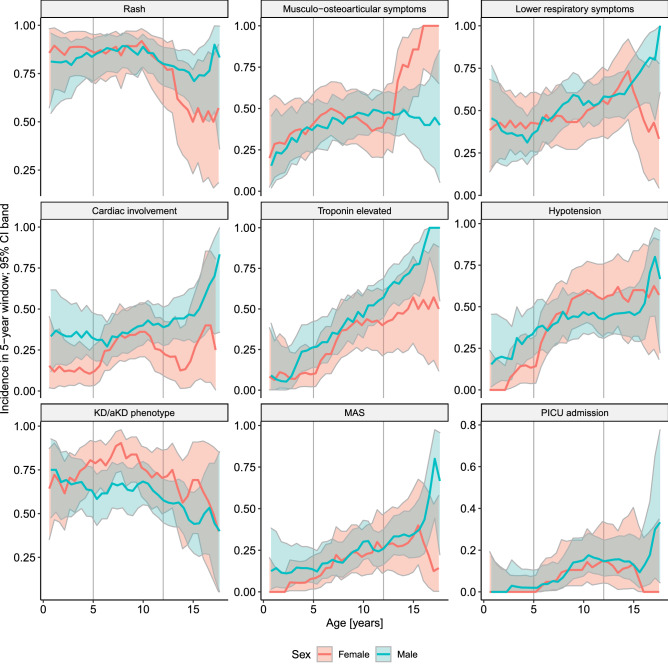
Figure 3.
Incidence of the selected events within the study group, according to age and sex. KD/aKD, Kawasaki disease/atypical Kawasaki disease; MAS, macrophage activation syndrome. Musculo-osteoarticular symptoms encompassed: muscle pain, arthralgia, or arthritis. Lower respiratory symptoms encompassed: chest pain, cough, or dyspnea. Cardiac involvement encompassed left ventricular ejection fraction < 55%, or coronary artery abnormalities (dilation or aneurysm), or pericardial effusion.
Teenage boys over 12 years old more prevalently had cardiac involvement (25.9% vs. 14.7%, p = 0.03) and fulfilled MAS diagnostic criteria more often (31.0% vs. 15.2%, p < 0.01). Among all boys, those hospitalised in PICU were significantly older (median [IQR] from 9.1 [5.7–12.3] to 11.2 years [10.3–12.6], p=0.02), while there was no such trend for girls.
On the other hand, teenage girls more frequently presented osteoarticular and muscular symptoms (12.6% vs. 2.9%, p < 0.01), but less frequently rash (5.0% vs. 15.2%, p=0.01). KD/aKD phenotype prevalence did not differ between reported girls and boys of any age.
Pediatric intensive care treatment
Statistics regarding PICU treatment are presented in Tables 4 and 5. PICU treatment was required in 23/274 (8.4%) of children, ten of whom were mechanically ventilated. There were no children treated with extracorporeal membrane oxygenation (ECMO), neither with renal replacement therapy. Two deaths were reported: one in a severely immunocompromised child, and one in previously healthy teenager with fulminant multiorgan dysfunction, both with positive real-time polymerase chain reaction (RT-PCR) test result for SARS-CoV-2. In either case, it was impossible to determine whether the cause of death was a cytokine storm due to COVID-19 or MIS-C; they fulfilled the MIS-C criteria though, and hence were included in this analysis.
Table 4.
Demographic and clinical characteristics of MIS-C cohort respective of pediatric intensive care involvement.
| All (274) | PICU (23) | Non-PICU (251) | P | P|Age | P|Sex | |
|---|---|---|---|---|---|---|
| Male sex | 171 (62%) | 17 (74%) | 154 (61%) | 0.2 | 0.4 | 1 |
| Age (years) | 8.8 (5.2–12.1) | 11.2 (10.1–12.6) | 8.4 (5.0–11.9) | < 0.01 | 0.03 | < 0.01 |
| BMI Z-score | 0.1 (− 0.8 to 0.9) | 0.7 (− 0.3 to 0.9) | 0.0 (− 0.8 to 0.9) | 0.2 | 0.3 | 0.2 |
| Any comorbidity | 38 (18%) | 4 (21%) | 34 (18%) | 0.7 | 0.8 | 0.7 |
| Symptoms and signs | ||||||
| Fever length | 7.0 (6.0–9.0) | 8.0 (7.0–10.0) | 7.0 (6.0–9.0) | 0.03 | 0.06 | 0.04 |
| Mucocutaneous and lymph nodes | 262 (97%) | 22 (96%) | 240 (97%) | 0.8 | 0.8 | 0.7 |
| Rash | 218 (83%) | 15 (71%) | 203 (84%) | 0.2 | 0.2 | 0.1 |
| Conjunctivitis | 207 (78%) | 14 (67%) | 193 (79%) | 0.2 | 0.2 | 0.2 |
| Hands/feet swelling or erythema | 142 (55%) | 15 (68%) | 127 (54%) | 0.2 | 0.1 | 0.1 |
| Oral inflammation | 173 (66%) | 14 (67%) | 159 (66%) | 0.9 | 0.8 | 0.9 |
| Cervical lymphadenopathy | 98 (38%) | 7 (33%) | 91 (38%) | 0.7 | 0.9 | 0.6 |
| Gastrointestinal | 250 (93%) | 21 (91%) | 229 (93%) | 0.8 | 0.8 | 0.6 |
| Abdominal pain | 222 (85%) | 21 (91%) | 201 (85%) | 0.4 | 0.5 | 0.5 |
| Nausea | 162 (62%) | 16 (70%) | 146 (61%) | 0.4 | 0.5 | 0.5 |
| Diarrhea | 164 (62%) | 18 (78%) | 146 (60%) | 0.09 | 0.1 | 0.1 |
| Lower respiratory | 128 (50%) | 18 (86%) | 110 (47%) | < 0.01 | < 0.01 | < 0.01 |
| Chest pain | 48 (19%) | 5 (25%) | 43 (18%) | 0.5 | 1 | 0.5 |
| Cough | 74 (28%) | 7 (32%) | 67 (28%) | 0.7 | 0.8 | 0.7 |
| Breathing effort | 63 (24%) | 14 (67%) | 49 (20%) | < 0.01 | < 0.01 | < 0.01 |
| Neurological | 212 (82%) | 20 (95%) | 192 (80%) | 0.09 | 0.08 | 0.08 |
| Lethargy | 151 (59%) | 19 (90%) | 132 (57%) | < 0.01 | < 0.01 | < 0.01 |
| Irritability | 108 (42%) | 6 (27%) | 102 (43%) | 0.2 | 0.4 | 0.2 |
| Headache | 112 (46%) | 8 (38%) | 104 (47%) | 0.4 | 0.3 | 0.5 |
| Meningeal signs | 27 (10%) | 2 (9%) | 25 (11%) | 0.8 | 0.8 | 0.9 |
| Cardiovascular | 139 (58%) | 22 (100%) | 117 (54%) | < 0.01 | < 0.01 | < 0.01 |
| Hypotension | 99 (41%) | 18 (86%) | 81 (36%) | < 0.01 | < 0.01 | < 0.01 |
| Coronary dilation or aneurysm | 21 (8%) | 3 (14%) | 18 (8%) | 0.3 | 0.3 | 0.4 |
| EF < 55% | 58 (23%) | 14 (64%) | 44 (19%) | < 0.01 | < 0.01 | < 0.01 |
| Musculo-osteoarticular | 111 (44%) | 8 (42%) | 103 (44%) | 0.8 | 0.6 | 0.9 |
| Muscle pain | 103 (41%) | 8 (42%) | 95 (41%) | 0.9 | 0.8 | 0.9 |
| Arthralgia | 50 (19%) | 4 (20%) | 46 (19%) | 0.9 | 0.8 | 0.9 |
| Arthritis | 12 (5%) | 1 (5%) | 11 (5%) | 1 | 0.8 | 1 |
| Clinical presentation | ||||||
| KD or aKD | 170 (67%) | 18 (86%) | 152 (65%) | 0.05 | 0.03 | 0.04 |
| KD | 105 (42%) | 10 (50%) | 95 (41%) | 0.4 | 0.3 | 0.3 |
| MAS | 59 (22%) | 9 (43%) | 50 (20%) | 0.02 | 0.08 | 0.02 |
| DIC | 42 (17%) | 5 (22%) | 37 (16%) | 0.5 | 0.7 | 0.4 |
Binary data given as count (per-cent), and numerical data as median (interquartile range).
BMI body mass index, DIC disseminated intravascular coagulation, EF ejection fraction, KD, Kawasaki disease, MAS macrophage activation syndrome, MIS-C multisystem inflammatory syndrome in children, PICU pediatric intensive care unit, y.o. years old; P|Age age-adjusted p-value, P|Sex, sex-adjusted p-value. Hypotension defined as minimal SBP in mmHg less than age in years or 90 for children over 10 years old.
Table 5.
Therapy and outcome of MIS-C cohort respective of age.
| All (274) | 0–5 y.o. (64) | 5–12 y.o. (140) | 12–18 y.o. (70) | P | P|Sex | |
|---|---|---|---|---|---|---|
| Admission since onset (days) | 5.0 (4.0–6.0) | 5.0 (4.0–5.0) | 5.0 (4.0–6.0) | 5.0 (4.0–6.5) | 0.5 | 0.6 |
| Intensive care | 23 (8%) | 1 (2%) | 13 (9%) | 9 (13%) | 0.05 | 0.07 |
| Mechanical ventilation | 10 (4%) | – | 7 (5%) | 3 (5%) | 0.2 | 0.2 |
| Oxygen supplementation | 61 (23%) | 5 (8%) | 41 (31%) | 15 (23%) | < 0.01 | < 0.01 |
| No immunomodulatory agent | 8 (3%) | 3 (5%) | 3 (2%) | 2 (3%) | 0.6 | 0.4 |
| IVIG | 238 (93%) | 55 (90%) | 122 (95%) | 61 (91%) | 0.3 | 0.3 |
| GCS | 143 (67%) | 27 (56%) | 74 (69%) | 42 (71%) | 0.2 | 0.2 |
| IVIG and GCS | 133 (62%) | 24 (50%) | 69 (65%) | 40 (67%) | 0.1 | 0.2 |
| Any other immunomodulator | ||||||
| Tocilizumab | 1 (0%) | 1 (2%) | – | – | 0.2 | 0.1 |
| Cyclosporin A | 2 (1%) | – | 2 (1%) | – | 0.4 | 0.3 |
| ASA | 226 (85%) | 51 (81%) | 119 (88%) | 56 (84%) | 0.4 | 0.4 |
| Heparin | 91 (38%) | 14 (25%) | 45 (38%) | 32 (49%) | 0.02 | 0.02 |
| Warfarin | 1 (0%) | 1 (2%) | – | – | 0.2 | 0.1 |
| Complete recovery at discharge | 218 (93%) | 55 (95%) | 115 (94%) | 48 (87%) | 0.2 | 0.2 |
Binary data given as count (per-cent), and numerical data as median (interquartile range).
ASA acetylsalicylic acid, GCS glucocorticoids, IVIG intravenous immunoglobulin, y.o. years old, P|Sex sex-adjusted p-value.
*All signs and symptoms resolved at discharge.
Children who were hospitalised in PICU were significantly older (median [IQR] from 8.4 [5.0–11.9] to 11.2 years [10.1–12.6], p < 0.01). The PICU admission rate did not significantly differ for patients with comorbidities, including obesity (Table 4, Supplementary Table 4). The median time of hospital admission since the first symptoms was 5 days and it was not significantly different in children admitted to PICU versus the others. At admission, chil×dren who necessitated intensive care, had higher respiratory rate (from median [IQR] 20.0 [18.0–25.0] to 30.0/min [20.0–45.0], p = 0.01) and lower systolic blood pressure (SBP) (from median [IQR] 89.0 [78.8–99.5] to 100.0 mmHg [91.0–110.0], p < 0.01), despite their higher age. They were also more likely to have prolonged capillary refill time (CRT) (40.0% vs. 10.8%, p < 0.01) and AVPU scale score below A (21.7% vs. 5.0%, p < 0.01). PICU-hospitalized children had also significantly higher CRP (from median [IQR] 133.4 [78.8–187.2] to 242.0 mg/dl [123.3–289.0], p < 0.01), procalcitonin (from median [IQR] 2.3 [0.9–6.2] to 13.2 ng/ml [2.1–51.2], p < 0.01), ferritin (from median [IQR] 317.1 [186.2–533.8] to 671.0 g/l [475.9–1052.8], p < 0.01), D-dimers (from median [IQR] 2.5 [1.4–4.4] to 3.9 g/ml [2.5–5.7], p = 0.01), lower estimated glomerular filtration rate (eGFR) (from median [IQR] 77.1 [50.8–89.5] to 113.1 [90.5-134.8], p < 0.01) and more commonly elevated troponin (77.3% vs. 22.5%, p < 0.01). In the later course, children in PICU more commonly had lymphopenia (81.0% vs. 47.0%, p < 0.01), and hypoalbuminemia (100.0% vs. 81.0%, p=0.04).
Discussion
Prevalence of MIS-C in Poland
Our study supports current knowledge about MIS-C. Notably, this single-country observation is also one of the most numerous European MIS-C cohorts. The number of 274 MIS-C cases captured in Poland with a 7.31 million children population and 2060 cases reported at the same time in the United States of America (USA) with 74 million children1,12 suggest that MIS-C prevalence could have reached a comparable level in both countries. The number of reported COVID-19 cases per 100,000 inhabitants in the USA was at that time approximately twice as high as in Poland13.
Moreover, Payne estimated the incidence of MIS-C as approximately 9-fold higher among Black and Hispanic or Latino Americans than among White Americans in general population and this trend sustained (with slightly lower rates) among SARS-CoV-2 infected children14. In this context, the high number of cases captured in Poland is even more surprising, as all but one child were White.
Age and sex-related differences in MIS-C presentation
The mucocutaneous, gastrointestinal or respiratory manifestations and laboratory picture in our cohort were more uniform across age groups than in others4–6,8–10,15,16. Cardiovascular involvement significantly increased with age however, which is in line with Dufort and Belay findings10,17. In opposition to Payne14 we found sex-related differences in MIS-C prevalence—higher for boys, but only from pubertal age. Teenage boys also more commonly had cardiac involvement, fulfilled MAS definition and required PICU hospitalisation. More severe course of COVID-19 in adult males is well established12. While some authors postulate that it is linked to genetic and immunological background18, others suggest that sex hormones play a role19,20. COVID-19 and MIS-C are separate entities, but share some similarities being hyperinflammatory conditions. In our cohort, the sex-related differences appeared from pubertal age, which might support the hormonal theory.
Kawasaki-like disease as the most common presentation of MIS-C
The exact pre-pandemic incidence of KD in Poland was not known (there had been no national reporting effort before the pandemic), though it was not likely to substantially differ from other European countries21. Similarly as in other countries7,22,23, we have observed a sharp increase in KD cases following COVID-19 wave. The data limited to two reporting sites supporting these observations are presented in Supplementary Table 6. Despite being initially reported as Kawasaki-like disease, MIS-C appeared to be a distinct entity soon after8,9,23–25. Children in our cohort, more frequently than in others, fulfilled KD/aKD diagnostic criteria but concomitantly presented unique features typical for MIS-C7,15–17,26,27.
More prevalent KD/aKD phenotype in our cohort could be explained in several ways. Firstly, we have included both: typical and atypical KD presentations. In previous studies which included both clinical variants, KD prevalence was two times more common than in those in which only typical KD definition was used15–17,27. Moreover, the comparison of patients with KD from pre-pandemic and pandemic periods in Spain revealed more frequent atypical presentation (71%) among patients with documented SARS-CoV-2 history23. Secondly, KD/aKD phenotype could have been over-represented due to our inclusion criteria and the fact, that the cases were identified and reported by pediatricians, who were more familiar with recognising KD than either TSS, MAS or new inflammatory syndrome. Thirdly, once in the database, the fulfillment of the KD/aKD criteria was verified by the dedicated software, not by clinicians. Thus, we captured cases of atypical presentation that might had been overlooked if identified by clinician’s diagnosis only (as in other reports10,11,15–17). Fourthly, some distinct clinical features of our cohort e.g., more prevalent KD phenotype, could be due to specific, homogeneous ethnic background, differing from all other cohorts10,11,16,26,27. Clinical phenotype of MIS-C has not been analyzed in relation to ethnicity/race thus far and needs to be further studied.
Milder course of MIS-C in Poland
In Western Europe and the USA more than half of children with MIS-C required intensive care10,11,15–17,27, contributing to a multifold increase in the PICU admissions number7. Approximately 1.4–3% of children with MIS-C died7,10,16,28. In our study, the course of the disease appeared substantially milder—only 8.4% of patients were hospitalised in PICU and two deaths were reported. Treatment used in our cohort did not differ substantially from other reports—most children received IVIG and a large proportion also got steroids. Fewer children required more than two different immunomodulatory agents4–9,15–17,27,29. Moreover, the median day of hospital admission since the first symptoms was similar to other reports6,17. Hence, the therapeutic approach is an unlikely factor responsible for more favourable outcome among Polish children with MIS-C.
The data about cardiovascular complications from previous reports are inconsistent. This is partially due to different (sometimes unspecified) definitions used by authors4,6,17,30, and various inclusion criteria—either broader than World Health Organization (WHO) MIS-C case definition30, or narrowed only to the most severe cases7. Decreased SBP, occasionally defined as a shock, was reported in 36–86% of patients with MIS-C, whereas heart failure—in 20–45%4–8,10,30. Our findings place Polish children with MIS-C within the “milder end” of the acute cardiovascular complications spectrum described above. Similarly, the prevalence of coronary artery involvement in MIS-C is debatable. Undoubtedly aneurysms may appear, cases of giant aneurysms have been described30. The true prevalence of CAA is unknown though and may be over-estimated, as febrile condition or myocarditis can cause transient coronary dilation too31. Both coronary artery aneurysms and dilations were less prevalent in our group than in other reports4–6,8–10,30.
It is not established whether race/ethnicity is associated with the severity of the disease4,6,32. Non-Hispanic White children comprised only 13–30% of cases in the most numerous MIS-C cohorts and systematic reviews4–6,8–10,14. Predominance of White children in our cohort could be considered as a possible explanation of milder clinical presentation with more favourable outcome. However, due to the lack of a control group of other ethnicities in our study, this conclusion should be treated with caution and requires further analysis.
Another distinguishing feature of our cohort was the small proportion of obese children as compared to reports from other countries (6.7% vs. 18–26%)4,6,9. This could have possibly resulted from lower obesity prevalence in Polish children (up to 13%)33. It is unknown whether obesity is a risk factor for developing MIS-C nor if it is connected to its severity. In our study, we found no association of body mass index (BMI) Z-score or obesity with severity of the disease.
Severity predictors
We aimed to identify clinical and laboratory features specific to patients who required intensive care. Older age (in line with other reports4,6) and male sex were the only demographic characteristics associated with PICU admission. The median time from the first symptoms to hospital admission did not differ significantly for PICU patients. They could be distinguished already at admission by their vital signs: decreased level of consciousness, longer CRT, higher respiratory rate and lower SBP and by the laboratory results. Apart from previously established high inflammatory markers6,8, high D-dimers, low eGFR, and presence of the heart injury markers turned out to be severity predictors.
Limitations
We have noted the following limitations of the presented work. The study relied on voluntary participation, hence a number of MIS-C cases might have been missed or biased by non-random sampling. Some patients meeting the MIS-C criteria may have been misclassified, e.g. due to unequal access to SARS-CoV-2 testing or missing data. Outliers in our data were verified at source whenever possible. Because of the broad MIS-C case definition and highly prevalent COVID-19 in the society, children with alternative diagnoses and coincidental positive SARS-CoV-2 results could be included in our cohort.
Precise epidemiological data on COVID-19 prevalence among age groups in Poland is lacking. We also assumed the risk of contracting the virus in the juvenile population to be homogeneous.
Conclusion
The severity of MIS-C is not as uniform as it seemed based on previous reports. In particular, race/ethnicity, age, and sex may affect MIS-C phenotype. Consequently, management protocols might not be universally applicable, and should rather be adjusted to the specific population. Patients with altered vital signs and higher inflammatory markers, lower eGFR and markers of heart injury at admission or lower lymphocyte count and albumin concentration during hospitalisation have greater risk of deterioration.
Methods
Data sources
The inclusion criteria are presented in Supplementary Table 1. Ethical approval was obtained from the Bioethics Committee at Wroclaw Medical University, Poland (CWN UMW BW: 313/2020). All research was performed in accordance with relevant guidelines and regulations. The Bioethics Committee at Wroclaw Medical University granted waiver of informed consent, as only de-identified data were transmitted and analyzed.
Anonymised patients’ data (demographic, clinical characteristics, laboratory parameters, cardiovascular evaluation results, treatment and outcome data) were extracted from health records and collected through an online questionnaire developed for that purpose. Vital signs and laboratory parameters were obtained at admission and at their respective peaks.
Here we retrospectively report and analyze data covering the period from 4th March 2020 (when the first COVID-19 case was confirmed in Poland) to 20th February 2021. Nine of the presented cases were included in our previous, cursory report34.
MIS-C case definition
We adopted WHO MIS-C case definition3, which requires all of the following:
children 0–18 years old with fever lasting at least 3 days;
- at least two of the following:
- rash or bilateral conjunctivitis, or mucocutaneous inflammation signs;
- hypotension defined by a minimal SBP below age (in years) mmHg or below 90 mmHg for children older than 10 years35;
- features of myocardial dysfunction, pericarditis, or CAA, based on echocardiographic findings or elevated B-type natriuretic peptide (BNP)/N-terminal-pro-BNP (NT-proBNP), or troponin;
- evidence of coagulopathy (by international normalised ratio (INR) > 1.1, activated partial thromboplastin time > 40 s or D-dimer > 500 g/ml);
- acute gastrointestinal problems.
elevated inflammatory markers: erythrocyte sedimentation rate (ESR) 40 mm/h, CRP 30 mg/l, or procalcitonin 0.5 ng/ml;
no other apparent microbial cause;
evidence of COVID-19 (positive RT-PCR, antigen test, or serology), or personal history of COVID-19 or contact with a proven COVID-19 case.
Standardised study definitions
All standardised study definitions and measures, including laboratory and echocardiographic abnormalities, consciousness level and nutritional status are presented in Supplementary Table 2. They were automatically evaluated by a dedicated software.
Laboratory abnormalities
We defined lymphopenia as lymphocyte count /l, anemia according to age-related norms, thrombocytopenia as platelets /l, elevated alanine transaminase as 40 U/l, hyponatremia as serum sodium < 135 mmol/l, hypoalbuminemia as serum albumin < 3.5 g/dl, elevated BNP/NT-proBNP as > 150 ng/ml. A threshold of 50 ng/l defined elevated both T and I troponin. Renal dysfunction was defined by estimated glomerular filtration rate (eGFR) < 90 ml/min/1.73 m, calculated using the revised Schwartz formula.
Echocardiographic abnormalities
Echocardiography results were categorised based on left ventricular EF and coronary artery measurements whenever available. The worst available EF and the largest coronary Z-scores were included. The echocardiography results were assessed by two independent cardiologists. Dilation was defined as a Z-score between 2 and less than 2.5, while aneurysm as Z-score 2.536. Hypotension was defined by a minimal SBP below age (in years) mmHg or below 90 mmHg for children over 10 years old35.
Clinical definitions
Diagnostic criteria of KD in its typical and atypical form were adapted from AHA guidelines36. MAS was defined based on Paediatric Rheumatology International Trials Organization criteria (Supplementary Table 1)37. DIC was diagnosed using modified DIC score38.
Statistical methods
We described variables in relation to the sum of cases for which the variable was recorded. We assumed missing values to be distributed randomly and independently from the data, and propagated them through clinical definitions according to Łukasiewicz’s logic. For the assessment of univariate relationships, either direct or divided by sex or age group, and disease severity, we used Cochran–Mantel–Haenszel’s test for two categorical variables and Kruskal–Wallis’s test for categorical-continuous variable pairs. Confidence intervals for incidence values were estimated according to Clopper–Pearson’s method. Significance level of 0.05 and two-sided testing was employed. All statistical analyses were performed in R, version 4.0.3 (R Foundation for Statistical Computing), using coin and GenBinomApps packages. MIS-C incidence was estimated based on the demographic data published by Statistics Poland39.
Due to an exploratory nature of this study, we have not adjusted p-values for multiple comparisons.
Supplementary Information
Acknowledgements
We thank all the pediatricians who contributed to our work by reporting patients and to Aleksandra Tracewska (WSB University in Wrocław, ul. Fabryczna 29-31, 53-609 Wrocław, Poland) for proofreading.
Author contributions
Conceptualization: K.M.L., M.O.N., C.S., M.B.K., E.K. and L.S.; data curation: K.M.L., M.O.N., N.D., P.T., K.P.P., M.B., C.S., M.B.K., E.K. and L.S.; formal analysis: K.P.P. and M.B.K.; funding acquisition: A.A., D.C., C.S., M.B.K. and E.K.; investigation: K.M.L., M.O.N., N.D., P.T., J.K., M.B., E.K. and L.S.; methodology: K.M.L., M.O.N. and M.B.K.; project administration: K.M.L., M.O.N., E.K. and L.S.; resources: K.M.L., M.O.N., N.D., P.T., M.B., B.W., M.B.K., E.L. and L.S.; software: K.M.L., M.O.N., K.P.P. and M.B.K.; supervision: J.K., B.W., T.J., E.K. and L.S.; validation: K.M.L., M.O.N., N.D., P.T., J.K., A.A., C.S. and M.B.K.; visualisation: N.D., K.P.P. and M.B.K.; original writing: K.M.L., M.O.N., N.D., P.T., M.B., A.A., D.C., C.S. and M.B.K.; review writing: K.M.L., M.O.N., C.S., M.B.K., B.W., T.J., E.K. and L.S. All the authors approved the final version to be published. K.M.L. and M.O.N. contributed equally to this paper as first authors; E.K. and L.S. contributed equally to this paper as supervising authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kamila Maria Ludwikowska, Magdalena Okarska-Napierała, Ernest Kuchar and Leszek Szenborn.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Miron Bartosz Kursa, Email: M.Kursa@icm.edu.pl.
MOIS CoR Study Group:
Marta Barszcz, Elżbieta Berdej-Szczot, Sebastian Brzuszkiewicz, Piotr Buda, Alicja Czajka, Agnieszka Czech, Ewa Czerwińska, Magdalena Figlerowicz, Małgorzata Firek-Pędras, Aneta Gawlik, Ewelina Gowin, Olga Izdebska, Danuta Januszkiewicz-Lewandowska, Justyna Kiepuszka, Agnieszka Koczwara, Danuta Koszałko, Magdalena Kośmider-Żurawska, Janusz Książyk, Beata Kucińska, Martyna Kukawska, Anita Lackowska, Katarzyna Łapacz, Agnieszka Maliszak, Anna Mania, Joanna Mańdziuk, Artur Mazur, Katarzyna Mazur-Melewska, Cezary Niszczota, Paulina Opalińska-Zielonka, Ilona Pałyga-Bysiecka, Katarzyna Rojewska, Anna Rożnowska-Wójtowicz, Bartosz Siewert, Paulina Sobiczewska, Lidia Stopyra, Agnieszka Stroba-Żelek, Joanna Stryczyńska-Kazubska, Tomasz Szatkowski, Barbara Szczepańska, Maciej Szczukocki, Robert Szylo, Filip Tyc, Katarzyna Wielgos, Ewa Wołowska, Jacek Wysocki, Anna Zacharzewska, Marcin Zaniew, Marzena Zielińska, and Katarzyna Zięba-Glonek
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02669-2.
References
- 1.Centers for Disease Control and Prevention . Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 2.Centers for Disease Control and Prevention . Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 3.World Health Organisation . Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. World Health Organisation; 2021. [Google Scholar]
- 4.Abrams JY, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child Adolesc. Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed M, et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bautista-Rodriguez C, et al. Multisystem inflammatory syndrome in children: An international survey. Pediatrics. 2021;147:e2020024554. doi: 10.1542/peds.2020-024554. [DOI] [PubMed] [Google Scholar]
- 7.Davies P, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with sars-cov-2 (pims-ts) in the UK: A multicentre observational study. Lancet Child Adolesc. Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoste L, Paemel RV, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021;180:2019. doi: 10.1007/s00431-021-03993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasuhara J, Watanabe K, Takagi H, Sumitomo N, Kuno T. COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatr. Pulmonol. 2021;56:837–848. doi: 10.1002/ppul.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belay ED, et al. Trends in geographic and temporal distribution of us children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175:837. doi: 10.1001/jamapediatrics.2021.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flood J, et al. Paediatric multisystem inflammatory syndrome temporally associated with sars-cov-2 (pims-ts): Prospective, national surveillance, United Kingdom and Ireland, 2020. Lancet Reg. Health Eur. 2021;3:100075. doi: 10.1016/j.lanepe.2021.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peckham H, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and itu admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie, H. et al.Coronavirus Pandemic (COVID-19) (2020). (Accessed 4 July 2021); https://ourworldindata.org/coronavirus.
- 14.Payne AB, et al. Incidence of multisystem inflammatory syndrome in children among us persons infected with sars-cov-2. JAMA Netw. Open. 2021;4:e2116420. doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldstein LR, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfred-Cato S, et al. COVID-19-associated multisystem inflammatory syndrome in children—United States, March–July 2020. Morb. Mortal. Wkly. Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dufort EM, et al. Multisystem inflammatory syndrome in children in New York state. N. Engl. J. Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nature. 2021;27:28. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarty D, et al. Sex differences in sars-cov-2 infection rates and the potential link to prostate cancer. Commun. Biol. 2020;3:374. doi: 10.1038/s42003-020-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding T, et al. A multi-hospital study in Wuhan, China: Protective effects of non-menopause and female hormones on sars-cov-2 infection. MedRxiv. 2020 doi: 10.1101/2020.03.26.20043943. [DOI] [Google Scholar]
- 21.Piram M. Epidemiology of Kawasaki disease in Europe. Front. Pediatr. 2021;9:469. doi: 10.3389/fped.2021.673554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. The Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Cooke E, et al. Prevalence and clinical characteristics of sars-cov-2 confirmed and negative Kawasaki disease patients during the pandemic in Spain. Front. Pediatr. 2021;8:617039. doi: 10.3389/fped.2020.617039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Smith JJ, et al. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: A cohort study. Lancet Rheumatol. 2021;3:e574. doi: 10.1016/S2665-9913(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toubiana J, et al. Distinctive features of Kawasaki disease following sars-cov-2 infection: A controlled study in Paris, France. J. Clin. Immunol. 2021;41:526–535. doi: 10.1007/s10875-020-00941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belot A, et al. Sars-cov-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Eurosurveillance. 2020;25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whittaker E, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with sars-cov-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldstein LR, et al. Characteristics and outcomes of us children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArdle AJ, et al. Treatment of multisystem inflammatory syndrome in children. N. Engl. J. Med. 2021;385:11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valverde I, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 31.Muniz JCG, et al. Coronary artery dimensions in febrile children without Kawasaki disease. Circ. Cardiovasc. Imaging. 2013;6:239–244. doi: 10.1161/CIRCIMAGING.112.000159. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes DM, et al. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J. Pediatr. 2021;230:23–31. doi: 10.1016/j.jpeds.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulaga Z, et al. The prevalence of overweight and obesity among polish school- aged children and adolescents. Przegl. Epidemiol. 2016;70:641–651. [PubMed] [Google Scholar]
- 34.Okarska-Napierała M, et al. Pediatric inflammatory multisystem syndrome (PIMS) did occur in Poland during months with low COVID-19 prevalence, preliminary results of a nationwide register. J. Clin. Med. 2020;9:3386. doi: 10.3390/jcm9113386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleinman ME, et al. Pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S876. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 36.McCrindle BW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 37.Minoia F, et al. Development and initial validation of the ms score for diagnosis of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 2019;78:1357–1362. doi: 10.1136/annrheumdis-2019-215211. [DOI] [PubMed] [Google Scholar]
- 38.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br. J. Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 39.Statistics Poland, Demographic Surveys Department. Population, Size and Structure and Vital Statistics in Poland by Territorial Division in 2020, as of 30th June (2020). (Accessed 4 July 2021); https://stat.gov.pl/obszary-tematyczne/ludnosc/ludnosc/ludnosc-stan-i-struktura-ludnosci-oraz-ruch-naturalny-w-przekroju-terytorialnym-stan-w-dniu-30-06-2020,6,28.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.