Abstract
Background
This study examined the associations between metabolic syndrome(MetS), obesity, their combination as a metabolic obesity phenotype, and the risk of breast cancer in East Asian postmenopausal women.
Methods
A total of 3,095,336 postmenopausal cancer-free women aged 40–79 years who underwent the National Health Insurance Service health examination between 2009 and 2010 were included. The incidence of invasive breast cancer was followed up until 2018. The presence of obesity (body mass index[BMI] ≥25 kg/m2), MetS, and each component of MetS was investigated.
Results
Obesity and MetS were associated with breast cancer risk, but when the effects of obesity and MetS were mutually adjusted, the associations were attenuated, especially for MetS. Only elevated fasting blood glucose levels and waist circumference increased the risk of breast cancer after adjusting for BMI. Compared to metabolically healthy normal-weight women, metabolically unhealthy normal-weight women, metabolically healthy obese, and metabolically unhealthy obese women had an increased risk of breast cancer.
Conclusions
Obesity and MetS were independently associated with an increased risk of breast cancer in postmenopausal women, despite the relationship between MetS and breast cancer appearing to result from a partial association with BMI. Postmenopausal women should be encouraged to control their weight and metabolic health.
Subject terms: Risk factors, Weight management
Background
Obesity, a major public health problem worldwide, is a major risk factor for chronic diseases, including cardiovascular disease (CVD) and certain types of cancer. Epidemiological studies have suggested that obesity increases the risk of breast cancer in postmenopausal women, but it has an inverse association in premenopausal women [1, 2]. However, recent studies have indicated a heterogeneous association in Asian women, such as an increased risk of premenopausal breast cancer in women with high adiposity [3–5], suggesting a complex relationship between obesity and breast cancer risk [3, 6]. Similar to obesity in general, metabolic syndrome (MetS) is associated with an increased risk of breast cancer in postmenopausal women, but not in premenopausal women [7, 8]. Obesity and MetS are caused by common mechanisms, such as insulin resistance, inflammatory response, sex hormone metabolism, and energy metabolism [9, 10]. Hence, obesity and MetS may have comparable associations with cancer risk.
Despite the close relationship between MetS and obesity, some individuals with obesity have no other features or components of MetS and are considered to have a metabolically healthy obese (MHO) status. Despite the higher risk of MetS in individuals with MHO status [11, 12], studies have suggested that these individuals are at a lower risk of CVD and mortality than those of metabolically unhealthy obese individuals because of favourable insulin sensitivity, adipose tissue function, and adipokine function [13, 14]. A previous study has shown that normal-weight individuals with impaired metabolic characteristics (metabolically unhealthy normal weight [MUNW]) have an increased risk of CVD and CVD mortality [14]. However, few studies have investigated whether MHO status increases the risk of cancer or cancer mortality. A recent study by the UK Biobank found an increased risk of 5 of 22 site-specific cancers in MHO individuals [15]. Previous studies have shown that obesity increases the risk of breast cancer in postmenopausal women, irrespective of their metabolic health [15–17]. However, an increased risk of breast cancer has been observed in postmenopausal women with MUNW in a few studies [17]. Most of these studies evaluating the associations between MetS, obesity, and the risk of breast cancer were conducted in Western countries, and limited information is available regarding these associations in Asian women.
The incidence of breast cancer has been increasing, and the MHO status is more common in East Asian postmenopausal women [11]. In addition, different associations between obesity and breast cancer risk according to ethnicity need to be considered [3–5]. Thus, in the present study, we investigated the potential associations between MetS, obesity, and their combination as a metabolic obesity phenotype in terms of metabolically healthy normal weight (MHNW), MUNW, MHO, and metabolically unhealthy obesity (MUO), and the risk of breast cancer in East Asian postmenopausal women using data from a nationwide representative cohort.
Methods
Study population
The National Health Insurance Service (NHIS) is a single mandatory healthcare insurance system covering the entire population of South Korea. The NHIS provides biennial health examinations and screening for breast cancer in women aged ≥40 years. The NHIS health examination includes a self-reported questionnaire about lifestyle factors, family history of chronic diseases and cancer, reproductive factors, anthropometric measurements, and laboratory measurements. Before the health examination, consent for the transfer of results to the national health screening database was obtained from each participant. The rate of participation in the NHIS health examination was approximately 43% in 2002, which increased to 75% in 2017. The details of the NHIS database have been described elsewhere [18]. After review and approval of the study proposal by the National Health Insurance Sharing Service, the NHIS database was made available for research. The Institutional Review Board (IRB) of Hanyang University College of Medicine approved the study protocol (IRB no. HYI-18-175-1), and we obtained national health screening data from the NHIS based on IRB approval.
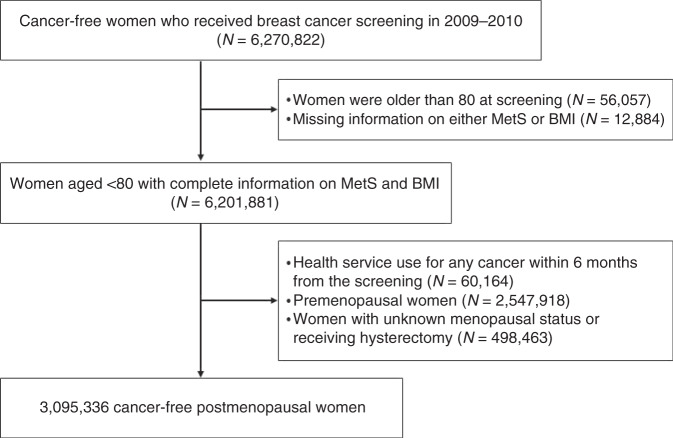
In this study, women who underwent national breast cancer screening and health examinations between 2009 and 2010 were initially considered because of the biennial cycle of health examinations. Of the 6,270,822 cancer-free women initially considered, those aged >80 years (N = 56,057), those with missing information on either MetS or body mass index (BMI) (N = 12,884), and those who had availed healthcare services for any type of cancer or catastrophic illness before or within 6 months from the date of health examination (N = 60,164) were excluded. In addition, premenopausal women (N = 2,547,918), those who reported an unknown menopausal status, or those who had undergone hysterectomy (N = 498,463) were excluded. Thus, 3,095,336 postmenopausal women were included in the study (Fig. 1).
Fig. 1.
Flow chart of the selection of study participants.
Definition of baseline MetS and obesity
The presence or absence of MetS was evaluated according to the results of laboratory investigations. The modified National Cholesterol Rationale Education Program Adult Treatment Program III (NECP-ATP III) defines MetS as the presence of ≥3 of the following components: (1) waist circumference (WC) ≥80 cm; (2) elevated fasting blood glucose (FBG) levels, defined as fasting plasma glucose levels ≥100 mg/dL; (3) triglyceride (TG) levels ≥150 mg/dL; (4) high-density lipoprotein cholesterol (HDL) levels <50 mg/dL for women; and (5) elevated blood pressure (BP) (systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg) [19]. The absence of MetS was defined as the presence of <3 of the above five components. BMI was calculated using anthropometric measurements; obesity was defined as a BMI ≥ 25.0 kg/m2 and normal weight was defined as a BMI < 25.0 kg/m2 according to the BMI criteria for Asians [20]. Based on the presence of MetS, presence of each component of MetS, and obesity status, participants were classified as having an MHNW (BMI < 25.0 kg/m2 and no MetS), MUNW (BMI < 25.0 kg/m2 and presence of MetS), MHO (BMI ≥ 25.0 kg/m2 and no MetS), or MUO (BMI ≥ 25.0 kg/m2 and presence of MetS) status. In addition, for the combined associations between each component of MetS and obesity, MHNW, MUNW, MHO, and MUO were defined according to the presence of each component of MetS (no or yes) and BMI status (BMI < 25.0 kg/m2 or BMI ≥ 25.0 kg/m2).
Follow-up and identification of breast cancer incidence
The development of invasive breast cancer among participants was identified using the linkage between the National Health Screening Database and the NHIS healthcare utilisation database until December 31, 2018. The incidence of cancer was defined according to a combination of the International Classification of Disease, 10th version, codes for malignant neoplasms of the breast (C50), and catastrophic illness codes for cancer in the NHIS healthcare utilisation database. The catastrophic illness code is related to the reduced coinsurance rate (from 20% to 5%) for patients with diseases that have a great financial burden in South Korea; thus, it requires relevant clinical information. Hence, the estimation of cancer incidence based on the NHIS database is considered to be reliable [21]. Participants were considered to be censored if they had not developed cancer, had died from any cause, or developed cancer other than breast cancer until December 31, 2018. If a participant had more than two types of cancer, the first cancer was considered. The period from the date of the health examination between 2009 and 2010 until December 31, 2018, date of death, or date of first record of cancer, whichever occurred first, was considered the follow-up period.
Covariates assessment
The adjusted variables in the analysis were age, reproductive factors, lifestyle factors, and family history of any cancer in their first-degree relatives. Age was estimated using the birth year and year of the health examination and treated as a continuous variable. Reproductive factors included age at menarche (<15, 15–16, or ≥17 years), age at menopause (<45, 45–52, or ≥53 years), hormone replacement therapy use after menopause (never used, <5 years, or ≥5 years), delivery (nullipara, 1 child, or ≥2 children), duration of breastfeeding (none, <1 year, or ≥1 year), and oral contraceptive use (ever or never used). Lifestyle factors included drinking frequency per week during the last 1 year (zero, 1 day/week, or ≥2 days/week), smoking (never or ever), vigorous physical activity per week (never, 1–2 days/week, or ≥3 days/week), moderate physical activity per week (never, 1–2 days/week, or ≥3 days/week), and walking for more than 30 min per week (never, 1–2 days/week, or ≥3 days/week). Data on reproductive factors, lifestyle factors, and family history of cancer were collected using a self-reported questionnaire.
Statistical analysis
The basic characteristics of the study participants are presented as numbers and percentages for categorical variables and as mean and standard deviation for continuous variables across the combination of MetS and obesity. The associations between obesity, MetS, number of components of MetS, each component of MetS, and the risk of breast cancer were estimated using a Cox proportional hazards regression model. All analyses were adjusted for age, age at menarche, age at menopause, hormone replacement therapy use after menopause, delivery, duration of breastfeeding, oral contraceptive use, family history of any cancer, drinking frequency per week during the last 1 year, smoking, and physical activity including vigorous physical activity, moderate physical activity, and walking per week. The proportional hazard assumption was tested using Kaplan–Meier curves, and the survival distribution function showed parallel lines. To independently identify the associations between MetS and the risk of breast cancer and between obesity and the risk of breast cancer, we mutually adjusted for each of them. To estimate the risk of breast cancer according to obesity and MetS simultaneously, the hazard ratios (HRs) and 95% confidence intervals (CIs) of the MUNW, MHO, and MUO groups with MHNW as a reference group were calculated. The analysis of the simultaneous associations between each component of MetS and obesity, the HRs of only obese women (MHO), women with each component (MUNW), and women with both obesity and each component (MUO) were analysed using women without each component of MetS and obesity as a reference group (MUNW). The association between the number of components of MetS (per increment) and breast cancer was analysed according to obesity status. In addition, the numbers of MetS components were grouped into the following intervals: 0, 1–2, and ≥3. The combination of the group number for MetS components and obesity status, and their association with breast cancer, were analysed. Finally, the associations between the combination of elevated FBG levels, obesity, abdominal obesity (WC ≥ 80 cm), and the risk of breast cancer were assessed against women with none of these conditions as a reference. A sensitivity analysis and repeated analysis were conducted after excluding incident breast cancer cases within the first 2 years of follow-up to minimise possible reverse causation. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
Results
Table 1 shows the baseline characteristics of the study participants according to BMI categories and the presence of MetS at baseline. Of the 3,095,336 postmenopausal women, 1,159,536 (37.5%) were obese and 1,036,970 (33.5%) had MetS. Women with MHO and MUO status comprised 17.9% and 19.6% of the total participants and 47.7% and 52.3% of obese women, respectively.
Table 1.
Basic characteristics of study participants by body mass index categories and metabolic syndrome status.
| <25 kg/m2 | ≥25 kg/m2 | |||||||
|---|---|---|---|---|---|---|---|---|
| MetS: No | MetS: Yes | MetS: No | MetS: Yes | |||||
| N | % | N | % | N | % | N | % | |
| Age | ||||||||
| Mean (SD) | 59.5 | (8.2) | 64.0 | (8.2) | 60.6 | (7.6) | 63.1 | (7.8) |
| Age at menarche | ||||||||
| <15 years | 229,683 | (15.3) | 46,485 | (10.8) | 78,476 | (14.2) | 74,817 | (12.3) |
| 15–16 years | 594,328 | (39.5) | 155,914 | (36.3) | 213,783 | (38.7) | 224,133 | (36.9) |
| ≥17 years | 650,813 | (43.2) | 219,018 | (50.9) | 249,090 | (45.1) | 295,573 | (48.7) |
| Missing | 30,930 | (2.1) | 8629 | (2.0) | 11,263 | (2.0) | 12,401 | (2.0) |
| Age at menopause | ||||||||
| <45 years | 97,331 | (6.5) | 33,784 | (7.9) | 36,259 | (6.6) | 46,656 | (7.7) |
| 45–52 years | 985,542 | (65.5) | 275,496 | (64.1) | 344,114 | (62.3) | 374,685 | (61.7) |
| ≥ 53 years | 312,569 | (20.8) | 99,245 | (23.1) | 136,158 | (24.6) | 153,153 | (25.2) |
| Missing | 110,312 | (7.3) | 21,521 | (5.0) | 36,081 | (6.5) | 32,430 | (5.3) |
| Hormone replacement therapy use after menopause | ||||||||
| Never use | 1,168,767 | (77.6) | 357,901 | (83.2) | 445,628 | (80.6) | 511,163 | (84.2) |
| <5 years | 224,672 | (14.9) | 43,914 | (10.2) | 67,859 | (12.3) | 56,801 | (9.4) |
| ≥5 years | 50,340 | (3.3) | 9483 | (2.2) | 15,302 | (2.8) | 11,810 | (1.9) |
| Missing | 61,975 | (4.1) | 18,748 | (4.4) | 23,823 | (4.3) | 27,150 | (4.5) |
| Delivery | ||||||||
| Nullipara | 50,246 | (3.3) | 10,117 | (2.4) | 14,254 | (2.6) | 14,428 | (2.4) |
| 1 child | 117,965 | (7.8) | 21,131 | (4.9) | 31,349 | (5.7) | 27,977 | (4.6) |
| ≥2 children | 1,333,804 | (88.6) | 397,934 | (92.5) | 505,479 | (91.5) | 563,138 | (92.8) |
| Missing | 3739 | (0.2) | 864 | (0.2) | 1350 | (0.2) | 1381 | (0.2) |
| Duration of breastfeeding | ||||||||
| Never | 132,234 | (8.8) | 24,088 | (5.6) | 34,800 | (6.3) | 32,426 | (5.3) |
| <1 year | 431,137 | (28.6) | 86,213 | (20.0) | 125,071 | (22.6) | 114,203 | (18.8) |
| ≥1 year | 933,256 | (62.0) | 317,406 | (73.8) | 389,205 | (70.4) | 456,761 | (75.3) |
| Missing | 9117 | (0.6) | 2339 | (0.5) | 3536 | (0.6) | 3534 | (0.6) |
| Oral contraceptive use | ||||||||
| Never | 1,206,810 | (80.2) | 341,639 | (79.4) | 432,208 | (78.2) | 473,633 | (78.0) |
| Ever | 215,041 | (14.3) | 63,310 | (14.7) | 88,096 | (15.9) | 97,740 | (16.1) |
| Missing | 83,903 | (5.6) | 25,097 | (5.8) | 32308 | (5.8) | 35,551 | (5.9) |
| Family history of any cancer | ||||||||
| No | 1,210,452 | (80.4) | 362,866 | (84.4) | 450,829 | (81.6) | 509,760 | (84.0) |
| Yes | 295,302 | (19.6) | 67,180 | (15.6) | 101783 | (18.4) | 97,164 | (16.0) |
| Drinking frequency during the last 1 year | ||||||||
| No | 1,280,493 | (85.0) | 382,158 | (88.9) | 471,562 | (85.3) | 534,210 | (88.0) |
| 1 day/week | 129,248 | (8.6) | 26,005 | (6.1) | 46,718 | (8.5) | 40,816 | (6.7) |
| ≥2 day/week | 83,814 | (5.6) | 18,546 | (4.3) | 29,965 | (5.4) | 27,355 | (4.5) |
| Missing | 12,199 | (0.8) | 3337 | (0.8) | 4367 | (0.8) | 4543 | (0.8) |
| Smoking | ||||||||
| Never | 1439103 | (95.6) | 410,656 | (95.5) | 533,131 | (96.5) | 582,242 | (95.9) |
| Ever | 59859 | (4.0) | 17,678 | (4.1) | 16,878 | (3.1) | 22,141 | (3.6) |
| Missing | 6792 | (0.4) | 1712 | (0.4) | 2603 | (0.5) | 2541 | (0.4) |
| Vigorous physical activity | ||||||||
| No | 1049681 | (69.7) | 322,050 | (74.9) | 394,915 | (71.5) | 458,338 | (75.5) |
| 1–2 day/week | 220008 | (14.6) | 52,253 | (12.2) | 73,543 | (13.3) | 70,568 | (11.6) |
| ≥3 day/week | 227229 | (15.1) | 53,471 | (12.4) | 81,139 | (14.7) | 74,896 | (12.3) |
| Missing | 8836 | (0.6) | 2272 | (0.5) | 3015 | (0.6) | 3122 | (0.5) |
| Moderate physical activity | ||||||||
| No | 939474 | (62.4) | 290,625 | (67.6) | 356,405 | (64.5) | 414,701 | (68.3) |
| 1–2 day/week | 243488 | (16.2) | 59,783 | (13.9) | 82,780 | (15.0) | 82,400 | (13.6) |
| ≥3 day/week | 311141 | (20.7) | 76,065 | (17.7) | 109,071 | (19.7) | 104,774 | (17.3) |
| Missing | 11651 | (0.8) | 3573 | (0.8) | 4356 | (0.8) | 5049 | (0.8) |
| Walking | ||||||||
| No | 523887 | (34.8) | 162,895 | (37.9) | 205,264 | (37.1) | 238,072 | (39.2) |
| 1–3 days/week | 453691 | (30.1) | 122,496 | (28.5) | 159,851 | (28.9) | 172,106 | (28.4) |
| 4–6 days/week | 311160 | (20.7) | 79,162 | (18.4) | 106,325 | (19.2) | 106,941 | (17.6) |
| 7 days/week | 208162 | (13.8) | 62,798 | (14.6) | 77,747 | (14.1) | 85,956 | (14.2) |
| Missing | 8854 | (0.6) | 2695 | (0.6) | 3425 | (0.6) | 3849 | (0.6) |
MetS metabolic syndrome, SD standard deviation.
Both obesity and MetS were associated with the risk of breast cancer, with an HR of 1.30 (95% CI = 1.26–1.33) and 1.16 (95% CI = 1.13–1.19), respectively (Table 2). When the effects of obesity and MetS were adjusted mutually, the associations were attenuated, especially for MetS; the HR for obesity was 1.26 (95% CI = 1.22–1.29) and that for MetS was 1.06 (95% CI = 1.03–1.09). Upon increasing the number of MetS components, the HR for the risk of breast cancer was 1.06 (95% CI = 1.05–1.07) before adjustment for obesity and 1.03 (95% CI = 1.02–1.04) after adjustment. All components of MetS significantly increased the risk of breast cancer by 3–21% (range of HR, 1.03–1.21). However, after adjusting for BMI, only FBG levels and WC showed an association with breast cancer (HR for elevated FBS levels: 1.08 [95% CI = 1.05–1.11]; HR for high WC: 1.06 [95% CI = 1.03–1.10]), while the other three components did not show a significant association.
Table 2.
Associations between obesity, metabolic syndrome, and the risk of breast cancer in postmenopausal women who underwent a national Korean breast cancer screening program from 2009 to 2010.
| BMI, metabolic phenotype | No. participants | Person-years | No. cases | Adjusted HR (95% CI)a | P-value | Adjusted HR (95% CI)b | P-value |
|---|---|---|---|---|---|---|---|
| BMI | |||||||
| <25 kg/m2 | 1,935,800 | 16,868,556.0 | 13,749 | 1.00 | 1 | ||
| ≥25 kg/m2 | 1,159,536 | 10,083,792.0 | 9835 | 1.30 (1.26–1.33) | <0.001 | 1.26 (1.22–1.29) | <0.001 |
| MetS | |||||||
| No | 2,058,366 | 17,931,890.4 | 15,705 | 1.00 | 1 | ||
| Yes | 1,036,970 | 9,020,457.7 | 7879 | 1.16 (1.13–1.19) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| N of component of MetS | |||||||
| Per 1 increment | – | – | – | 1.06 (1.05–1.07) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Component of MetS | |||||||
| Elevated BP | |||||||
| No | 1,697,774 | 14,787,997.2 | 13,324 | 1.00 | 1.00 | ||
| Yes | 1,397,562 | 12,164,350.9 | 10,260 | 1.06 (1.03–1.09) | <0.001 | 1.01 (0.99–1.04) | 0.405 |
| Elevated FBG | |||||||
| No | 2,017,573 | 17,589,624.6 | 15,120 | 1.00 | 1.00 | ||
| Yes | 1,077,763 | 9,362,723.5 | 8464 | 1.13 (1.10–1.16) | <0.001 | 1.08 (1.05–1.11) | <0.001 |
| High WC | |||||||
| No | 1,512,909 | 13,185,877.7 | 11,264 | 1.00 | 1.00 | ||
| Yes | 1,582,427 | 13,766,470.4 | 12,320 | 1.21 (1.18–1.24) | <0.001 | 1.06 (1.03–1.10) | 0.001 |
| Elevated TG | |||||||
| No | 2,176,753 | 18,948,154.5 | 16.737 | 1.00 | 1.00 | ||
| Yes | 918,583 | 8,004,193.5 | 6847 | 1.06 (1.03–1.09) | <0.001 | 1.01 (0.98–1.04) | 0.596 |
| Reduced HDL | |||||||
| No | 2,042,142 | 17,775,976.8 | 15,748 | 1.00 | 1.00 | ||
| Yes | 1,053,194 | 9,176,371.3 | 7,836 | 1.03 (1.00–1.06) | 0.024 | 1.02 (0.99–1.05) | 0.203 |
MetS metabolic syndrome, BP blood pressure, FBG fasting blood glucose, WC waist circumference, TG triglyceride, HDL high-density lipoprotein, HR hazard ratio, CI confidence interval.
aAdjusted for age, age at menarche, age at menopause, hormone replacement therapy use after menopause, delivery, duration of breastfeeding, oral contraceptive use, family history of any cancer, drinking frequency per week during the last 1 year, smoking, and physical activity including vigorous physical activity, moderate physical activity, and walking per week.
bAdjusted for the variables mentioned abovea and additionally mutually adjusted for BMI (<25 kg/m2, ≥ 25 kg/m2) and MetS (no, yes).
The associations between metabolic obesity phenotypes (as a combination of MetS status, each component of MetS, and obesity) and breast cancer risk in postmenopausal women are presented in Table 3. With regard to MetS, compared with women with MHNW status, women with MUNW, MHO, or MUO status had an increased risk of breast cancer, especially women with MUO status (HR = 1.05, 95% CI = 1.01–1.10 for women with MUNW status; HR = 1.25, 95% CI = 1.21–1.30 for women with MHO status; HR = 1.37, 95% CI = 1.32–1.42 for women with MUO). Regarding the presence of each component of MetS and obesity, compared with women with MHNW status, women with MHO or MUO status had an increased risk of breast cancer, with the highest risk occurring in women with MUO status. Regarding elevated BP, elevated TG, and reduced HDL, the risk of breast cancer in women with MUNW status was unchanged compared with that in women with MHNW status. However, regarding elevated FBG and elevated WC, compared with women with MHNW status, those with MUNW had a slightly higher risk of breast cancer (HR 1.08, 95% CI = 1.04–1.12 for FBG; HR 1.06, 95% CI = 1.02–1.10 for WC).
Table 3.
Hazard ratio and 95% confidence interval of the combined effects of metabolic syndrome and obesity status on the risk of breast cancer in postmenopausal women who underwent a national Korean breast cancer screening program from 2009 to 2010.
| Metabolic phenotype | No. participants | Person-years | No. cases | Adjusted HRa (95% CI) | P |
|---|---|---|---|---|---|
| MeS | |||||
| MHNW | 1,505,754 | 13,122,024.1 | 10,996 | 1.00 | |
| MHO | 552,612 | 4,809,866.3 | 4709 | 1.25 (1.21–1.30) | <0.001 |
| MUNW | 430,046 | 3,746,531.9 | 2753 | 1.05 (1.01–1.10) | 0.019 |
| MUO | 606,924 | 5,273,925.8 | 5126 | 1.37 (1.32–1.42) | <0.001 |
| Elevated BP | |||||
| MHNW | 1,175,139 | 10,239,426.2 | 8825 | 1.00 | |
| MHO | 522,635 | 4,548,571.0 | 4499 | 1.25 (1.21–1.30) | <0.001 |
| MUNW | 760,661 | 6,629,129.8 | 4924 | 1.00 (0.96–1.03) | 0.808 |
| MUO | 636,901 | 5,535,221.1 | 5336 | 1.33 (1.29–1.38) | <0.001 |
| Elevated FBG | |||||
| MHNW | 1,346,303 | 11,740,805.9 | 9559 | 1.00 | |
| MHO | 671,270 | 5,848,818.7 | 5561 | 1.27 (1.23–1.32) | <0.001 |
| MUNW | 589,497 | 5,127,750.1 | 4190 | 1.08 (1.04–1.12) | <0.001 |
| MUO | 488,266 | 4,234,973.3 | 4274 | 1.41 (1.36–1.46) | <0.001 |
| High WC | |||||
| MHNW | 1,359,005 | 11,843,719.9 | 9944 | 1.00 | |
| MHO | 153,904 | 1,342,157.8 | 1320 | 1.19 (1.12–1.26) | <0.001 |
| MUNW | 576,795 | 5,024,836.1 | 3805 | 1.06 (1.02–1.10) | <0.001 |
| MUO | 1,005,632 | 8,741,634.3 | 8515 | 1.34 (1.30–1.38) | <0.001 |
| Elevated TG | |||||
| MHNW | 1,447,899 | 12,613,075.6 | 10,447 | 1.00 | |
| MHO | 728,854 | 6,335,078.9 | 6290 | 1.31 (1.26–1.35) | <0.001 |
| MUNW | 487,901 | 4,255,480.4 | 3302 | 1.04 (1.00–1.08) | 0.067 |
| MUO | 430,682 | 3,748,713.2 | 3545 | 1.31 (1.26–1.36) | <0.001 |
| Reduced HDL | |||||
| MHNW | 1,331,239 | 11,595,263.0 | 9697 | 1.00 | |
| MHO | 710,903 | 6,180,713.9 | 6051 | 1.28 (1.24–1.32) | <0.001 |
| MUNW | 604,561 | 5,273,293.1 | 4052 | 1.00 (0.96–1.03) | 0.871 |
| MUO | 448,633 | 3,903,078.1 | 3784 | 1.32 (1.27–1.37) | <0.001 |
MetS metabolic syndrome, BP blood pressure, FBG fasting blood glucose, WC waist circumference, TG triglyceride, HDL high-density lipoprotein, HR hazard ratio, CI confidence interval; MHNW (BMI < 25.0 kg/m2 and no MetS or each component of MetS), MUNW (BMI < 25.0 kg/m2 and presence of MetS or each component of MetS), MHO (BMI ≥ 25.0 kg/m2 and no MetS) or each component of MetS, and MUO (BMI ≥ 25.0 kg/m2 and presence of MetS or each component of MetS).
aAdjusted for age, age at menarche, age at menopause, hormone replacement therapy use after menopause, delivery, duration of breastfeeding, oral contraceptive use, family history of any cancer, drinking frequency per week during the last 1 year, smoking, and physical activity including vigorous physical activity, moderate physical activity, and walking per week.
The HR for each increment in MetS components was similar in obese and non-obese women (HR 1.04, 95% CI = 1.02–1.05 in both groups, Table 4). Compared with non-obese women without any component of MetS, non-obese women with 1–2 components of MetS did not show increased risk of breast cancer, but non-obese women with ≥3 components of MetS showed a slightly increased risk of breast cancer (HR 1.08, 95% CI = 1.03–1.14). The HR of obese women without any MetS component was 1.17 (95% CI = 1.04–1.32), which was higher than that of non-obese women with ≥3 MetS components. The HRs of obese women with 1–2 components and ≥3 components of MetS were 1.29 (95% CI = 1.23–1.35) and 1.41 (95% CI = 1.34–1.47), respectively.
Table 4.
Hazard ratio and 95% confidence interval of the combined effects of the number of components of metabolic syndrome and obesity status on the risk of breast cancer in postmenopausal women who underwent a national Korean breast cancer screening program from 2009 to 2010.
| BMI (kg/m2) | No. participants | Person-years | No. cases | Adjusted HR* (95% CI) | P | |
|---|---|---|---|---|---|---|
| Per 1 increment | <25 | – | – | – | 1.04 (1.02–1.05) | <0.001 |
| ≥25 | – | – | – | 1.04 (1.02–1.05) | <0.001 | |
| 0 | <25 | 433,565 | 3,776,535.3 | 3,384 | 1.00 | |
| ≥25 | 34,061 | 297,067.3 | 299 | 1.17 (1.04–1.32) | 0.010 | |
| 1–2 | <25 | 1,072,189 | 9,345,488.8 | 7,612 | 1.04 (0.99–1.08) | 0.083 |
| ≥25 | 518,551 | 4,512,799.0 | 4,410 | 1.29 (1.23–1.35) | <0.001 | |
| > = 3 | <25 | 430,046 | 3,746,531.9 | 2,753 | 1.08 (1.03–1.14) | 0.003 |
| ≥25 | 606,924 | 5,273,925.8 | 5,126 | 1.41 (1.34–1.47) | <0.001 |
BMI body mass index; HR hazard ratio; CI confidence interval.
*Adjusted for age, age at menarche, age at menopause, hormone replacement therapy use after menopause, delivery, duration of breastfeeding, oral contraceptive use, family history of any cancer, drinking frequency per week during the last 1 year, smoking, and physical activity including vigorous physical activity, moderate physical activity, and walking per week.
Based on the independent association between elevated FBG levels and high WC after adjusting for obesity (Table 2), we assessed the association between the combination of obesity, FBG, WC, and the risk of breast cancer (Table 5). Compared with non-obese women with normal FBG levels and WC, obese women with elevated FBG levels and WC had a higher risk of breast cancer (HR = 1.44, 95% CI = 1.38–1.50). The HR of women with obesity only was similar to that of non-obese women with elevated FBS levels and WC.
Table 5.
Hazard ratios and 95% confidence intervals for the combined effects of elevated fasting blood glucose levels, obesity, and central obesity on the risk of breast cancer in postmenopausal women who underwent a national Korean breast cancer screening program from 2009 to 2010.
| Elevated FBG | BMI (kg/m2) | High WC | No. participants | Person-years | No. cases | Adjusted HRa (95% CI) | P |
|---|---|---|---|---|---|---|---|
| No | <25 | No | 986,593 | 8,602,975.4 | 7203 | 1.00 | |
| Yes | 359,710 | 3,137,830.6 | 2356 | 1.05 (1.00–1.10) | 0.040 | ||
| ≥25 | No | 104,324 | 911,094.3 | 862 | 1.15 (1.08–1.24) | <0.001 | |
| Yes | 566,946 | 4,937,724.4 | 4699 | 1.32 (1.27–1.37) | <0.001 | ||
| Yes | <25 | No | 372,412 | 3,240,744.5 | 2741 | 1.08 (1.03–1.13) | 0.001 |
| Yes | 217,085 | 1,887,005.6 | 1449 | 1.13 (1.07–1.20) | <0.001 | ||
| ≥25 | No | 49,580 | 431,063.5 | 458 | 1.34 (1.22–1.48) | <0.001 | |
| Yes | 438,686 | 3,803,909.9 | 3816 | 1.44 (1.38–1.50) | <0.001 |
FBG fasting blood glucose, WC waist circumference.
aAdjusted for age, age at menarche, age at menopause, hormone replacement therapy use after menopause, delivery, duration of breastfeeding, oral contraceptive use, family history of any cancer, drinking frequency per week during the last 1 year, smoking, and physical activity including vigorous physical activity, moderate physical activity, and walking per week.
In sensitivity analysis excluding incident cases within the first two years of follow-up, none of the associations were changed, allowing for the exclusion of possible reverse causation (Supplementary Tables S1–S4).
Discussion
In this large study, despite the association of both obesity and MetS with breast cancer risk in postmenopausal women, mutually adjusted results showed that the association with obesity persisted, but that the associations with MetS, number of MetS, and MetS components were attenuated. This suggests that the relationship between MetS and breast cancer appears to result from a partial association with BMI. However, the significantly increased HR of MetS after adjustment for BMI, the increased risk of breast cancer in postmenopausal women with MUNW, MHO, or MUO status compared with that in postmenopausal women with MHNW, and the highest risk in women with MUO may suggest independent contributions of both obesity and MetS to breast cancer development. Among MetS components, only elevated FBG levels and high WC were independently associated with the risk of breast cancer after adjusting for BMI. The combination of obesity status, FBG levels, and WC showed that women with all three components had a higher breast cancer risk, suggesting independent and combined effects. To the best of our knowledge, this is the first study to identify an independent association between metabolic obesity phenotype, in which the combined effect of obesity and MetS was considered, and the risk of breast cancer in Asian women.
The increased risk of breast cancer in postmenopausal women with MUO compared to that in women with MHNW status has been consistently observed in previous studies [15–17, 22, 23]. However, the observed risks of breast cancer in women with MHO and MUNW were inconsistent, followed by various conclusions regarding the association between metabolic obesity phenotypes, considered as a combination of MetS status and obesity, and breast cancer [24]. Several studies have shown an increased risk of breast cancer in postmenopausal women with MHO status, but not in postmenopausal women with MUNW, and have proposed the contribution of metabolic health to the risk of breast cancer in only overweight/obese women [15, 16, 23]. A previous study showed an elevated risk of breast cancer in postmenopausal women with MUNW but not in postmenopausal women with MHO status, suggesting that metabolic health would be more useful than adiposity for risk stratification [22]. Another study showed an increased risk of breast cancer in women with both MHO and MUNW status compared with that in women with MHNW status, suggesting an independent role of obesity and metabolic dysregulation [17]. In the present study, the independent association between MetS and obesity, and the increased risk in women with MUNW or MHO status compared with that in women with MHNW, support the result [17].
In previous studies, various definitions of metabolic health have been applied. Some studies have applied the definition of MetS using NECP-ATP III [15, 16] or the objective measurement of FBG [23]. Park et al. [17] defined metabolic abnormalities for type 2 diabetes or dyslipidemia based on self-reported medical history. In addition, being metabolically unhealthy was defined as having at least one of four factors, whereas the definition of NECP-ATP III criteria applies ≥3 factors. Gunter et al. [22] defined metabolic health using a homoeostasis model assessment of insulin resistance (HOMA-IR) and fasting insulin. Various definitions of metabolic health may be attributed to these inconsistent results.
Regarding each component of MetS, despite the possibility that the association between WC and postmenopausal breast cancer is a result of the correlation with BMI [25], a recent meta-analysis suggested that WC could be a predictor for breast cancer independent of BMI [6], which is consistent with our results. The increased risk of breast cancer in women with type 2 diabetes has been well established in a previous meta-analysis [26]. The association between hypertension and the risk of breast cancer has been inconsistent. A meta-analysis did not find a significant association between hypertension and postmenopausal breast cancer in five studies; however, when only two studies were considered for multiple adjustments, increased risk was observed and remained controversial [27]. Regarding dyslipidemia, the association between elevated TG and a decreased risk of total breast cancer and between elevated HDL and decreased risk of postmenopausal breast cancer was reported in a recent meta-analysis [28]. However, this study assessed the relative risk of the highest vs. lowest risk category and did not consider certain thresholds, such as NECP-ATP or WHO [28]. The five components of MetS are largely and mutually affected by general obesity (BMI), but the consideration of BMI in previous studies varied. In this study, all MetS components increased postmenopausal risk of breast cancer, but when BMI was additionally adjusted, the associations were attenuated and statistical significance was maintained only for elevated FBG and WC, suggesting that the association could be at least partially attributed to BMI. Zhao et al. reported that central obesity and diabetes are essential components of MetS related to the risk of breast cancer [8], which supports our results after adjusting for BMI.
Despite the suggestion that central obesity is critical for the relationship between metabolic phenotype and breast cancer [17], among the independently associated metabolic obesity phenotypes, WC showed a smaller effect than that of both BMI and FBS in this study. A lower cut-off for abdominal obesity may explain the effect of WC. However, when we applied a WC cut-off of ≥85 cm, the results were comparable with those with a WC cut-off of ≥80 cm (results not shown). General obesity (based on the BMI) could affect postmenopausal breast cancer in two ways: overlapping pathogenesis with MetS [29] and increased oestrogen from the aromatisation of androgen in adipose tissue, thus showing a greater effect. Insulin resistance and central obesity are suggested to be the main factors in the pathogenesis of MetS [30, 31]. These findings may explain the independent and combined effects of BMI, FBS, and WC and their importance.
This study has several limitations. First, to define MetS, we did not consider treatment for hypertension, hyperglycaemia, or hyperlipidemia due to a lack of information on medication. Thus, medication-induced normal BP, FBG, HDL, or TG levels could be mixed in the normal group. Second, we did not consider changes in the MetS status and BMI during the follow-up period. As seen in the Reasons for Geographic and Racial Differences in Stroke cohort [32], we expected nondifferential changes in MetS status and BMI related to baseline estimates. Third, women who underwent health examinations were included in this study, and their characteristics might differ from those who did not undergo the examination. However, the participation rate in the NHIS health examination was ~70%, and we included all female examinees. Thus, the effect of selection bias on the associations observed in this study is expected to be minimal. Fourth, despite the various associations between obesity, MetS, and breast cancer subtypes, such as hormone receptor status [3], these factors were not considered in this study because the NHIS data did not have information on tumour characteristics. Fifth, some confounders such as diet could not be adjusted due to a lack of information, leaving possible residual confounding. Sixth, a follow-up period of 9 years may be considered a short timeframe for investigating the causal association between obesity, metabolic health, and breast cancer risk. Nevertheless, the follow-up period of this study is comparable to or longer than that in previous prospective studies [15, 17, 33].
In conclusion, obesity and MetS were independently associated with an increased risk of breast cancer in postmenopausal women. However, the contribution of MetS, independent of obesity, on breast cancer risk was observed despite the fact that MetS and breast cancer appear to result from a partial association with BMI. Based on the increased postmenopausal risk of breast cancer in women with MUNW, MHO, and MUO, postmenopausal obese women should be encouraged to control their weight and metabolic health, especially FBG and WC.
Supplementary information
Author contributions
Conceptualisation, BP and MSC; Methodology, BP and CC; Formal analysis, BP and SK; Data curation, SK and HK; Writing—original draft preparation, BP and CC; Writing—review & editing, BP. and MSC; Project administration, SK and HK.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant number 2021R1A2C1011958).
Data availability
The data that support the findings of this study are available on the website of the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/), and can be accessed by submitting a study protocol, document of IRB approval, and request form after being reviewed by the relevant committee.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Before the health examination, consent for the transfer of results to the national health screening database was obtained from each participant. The Institutional Review Board (IRB) of Hanyang University College of Medicine approved the study protocol (IRB no. HYI-18-175-1). Before the health examination, consent for the transfer of results to the national health screening database was obtained from each participant.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01540-5.
References
- 1.Xia X, Chen W, Li J, Chen X, Rui R, Liu C, et al. Body mass index and risk of breast cancer: a nonlinear dose-response meta-analysis of prospective studies. Sci Rep. 2014;4:7480. doi: 10.1038/srep07480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14:665–78. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- 3.Agurs-Collins T, Ross SA, Dunn BK. The many faces of obesity and its influence on breast cancer risk. Front Oncol. 2019;9:765. doi: 10.3389/fonc.2019.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KR, Hwang IC, Han KD, Jung J, Seo MH. Waist circumference and risk of breast cancer in Korean women: a nationwide cohort study. Int J Cancer. 2018;142:1554–9. doi: 10.1002/ijc.31180. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Liu L, Cui S, Tian F, Fan Z, Geng C, et al. Distinct effects of body mass index and waist/hip ratio on risk of breast cancer by joint estrogen and progestogen receptor status: results from a case-control study in Northern and Eastern China and implications for chemoprevention. Oncologist. 2017;22:1431–43. doi: 10.1634/theoncologist.2017-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen GC, Chen SJ, Zhang R, Hidayat K, Qin JB, Zhang YS, et al. Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev. 2016;17:1167–77. doi: 10.1111/obr.12443. [DOI] [PubMed] [Google Scholar]
- 7.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer. A systematic review and meta-analysis. Diabetes Care. 2012;35:2402–11. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao P, Xia N, Zhang H, Deng T. The metabolic syndrome is a risk factor for breast cancer: a systematic review and meta-analysis. Obes Facts. 2020;13:384–96. doi: 10.1159/000507554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Investig. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellastella G, Scappaticcio L, Esposito K, Giugliano D, Maiorino MI. Metabolic syndrome and cancer: “The common soil hypothesis”. Diabetes Res Clin Pract. 2018;143:389–97. doi: 10.1016/j.diabres.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Investig. 2019;129:3978–89. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echouffo-Tcheugui JB, Short MI, Xanthakis V, Field P, Sponholtz TR, Larson MG, et al. Natural history of obesity subphenotypes: dynamic changes over two decades and prognosis in the Framingham heart study. J Clin Endocrinol Metab. 2018;104:738–52. doi: 10.1210/jc.2018-01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–62. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 14.Stefan N. Metabolically healthy and unhealthy normal weight and obesity. Endocrinol Metab. 2020;35:487–93. doi: 10.3803/EnM.2020.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Z, Zheng X, Yang H, Li S, Xu F, Yang X, et al. Association of obesity status and metabolic syndrome with site-specific cancers: a population-based cohort study. Br J Cancer. 2020;123:1336–44. doi: 10.1038/s41416-020-1012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabat GC, Kim MY, Lee JS, Ho GY, Going SB, Beebe-Dimmer J, et al. Metabolic obesity phenotypes and risk of breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2017;26:1730-5. [DOI] [PMC free article] [PubMed]
- 17.Park YM, White AJ, Nichols HB, O’Brien KM, Weinberg CR, Sandler DP. The association between metabolic health, obesity phenotype and the risk of breast cancer. Int J cancer. 2017;140:2657–66. doi: 10.1002/ijc.30684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–7. doi: 10.1097/01.crd.0000380842.14048.7e. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Regional Office for the Western P. The Asia-Pacific perspective: redefining obesity and its treatment: Sydney: Health Communications Australia; 2000.
- 21.Seo H, Oh I-H, Yoon S-J. A Comparison of the cancer incidence rates between the National Cancer Registry and Insurance Claims Data in Korea. Asian Pac J Cancer Prev. 2012;13:6163–8. doi: 10.7314/APJCP.2012.13.12.6163. [DOI] [PubMed] [Google Scholar]
- 22.Gunter MJ, Xie X, Xue X, Kabat GC, Rohan TE, Wassertheil-Smoller S, et al. Breast cancer risk in metabolically healthy but overweight postmenopausal women. Cancer Res. 2015;75:270-4. [DOI] [PMC free article] [PubMed]
- 23.Moore LL, Chadid S, Singer MR, Kreger BE, Denis GV. Metabolic health reduces risk of obesity-related cancer in Framingham study adults. Cancer Epidemiol Biomarkers Prev. 2014;23:2057-65. [DOI] [PMC free article] [PubMed]
- 24.Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1:36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev. 2003;4:157–73. doi: 10.1046/j.1467-789X.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 26.Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107:1608–17. doi: 10.1038/bjc.2012.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seretis A, Cividini S, Markozannes G, Tseretopoulou X, Lopez DS, Ntzani EE, et al. Association between blood pressure and risk of cancer development: a systematic review and meta-analysis of observational studies. Sci Rep. 2019;9:8565. doi: 10.1038/s41598-019-45014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni H, Liu H, Gao R. Serum lipids and breast cancer risk: a meta-analysis of prospective cohort studies. PLoS ONE. 2015;10:e0142669. doi: 10.1371/journal.pone.0142669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, Gude F, Cadarso-Suarez C, García F, et al. Insulin resistance index (HOMA-IR) levels in a general adult population: curves percentile by gender and age. The EPIRCE study. Diabetes Res Clin Pract. 2011;94:146–55. doi: 10.1016/j.diabres.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Kabat GC, Kim MY, Lane DS, Zaslavsky O, Ho GYF, Luo J, et al. Serum glucose and insulin and risk of cancers of the breast, endometrium, and ovary in postmenopausal women. Eur J Cancer Prev. 2018;27:261-8. [DOI] [PubMed]
- 31.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269-75. [DOI] [PubMed]
- 32.Akinyemiju T, Moore JX, Pisu M, Judd SE, Goodman M, Shikany JM, et al. A prospective study of obesity, metabolic health, and cancer mortality. Obes (Silver Spring, Md) 2018;26:193–201. doi: 10.1002/oby.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo M, Liu T, Li P, Wang T, Zeng C, Yang M, et al. Association between metabolic syndrome and breast cancer risk: an updated meta-analysis of follow-up studies. Front Oncol. 2019;9:1290. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on the website of the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/), and can be accessed by submitting a study protocol, document of IRB approval, and request form after being reviewed by the relevant committee.