Abstract
Objective
As clinicians and patients await consensus on intraovarian platelet-rich plasma (PRP) treatment, this project evaluated contemporary research trends in the literature.
Methods
A PubMed/NLM search aggregated all ovarian PRP-related publications (n=54) to evaluate their scope, abstract utility, submission-to-publication interval, journal selected, article processing charge (APC), free reader access to full-text manuscripts, number and nationality of authors, and inclusion of international collaborators. The NIH Clinical Trials database was also audited.
Results
Published output on intraovarian PRP has increased consistently since 2016, especially among investigators in Greece, Iran, USA, and Turkey. Between 2013 and 2021, 42 articles met the relevancy criteria, of which 40.5% reported clinical studies, small series, or case reports, 33% described experimental animal models, and 23.8% were opinion/review papers. Only two works included a placebo control group. The submission-to-publication interval (mean±standard deviation) was 130±96 days, there were 5.9±3.2 authors per project, and journals invoiced US $1,642±1,466 (range, $0–$3,860) for APCs.
Conclusion
There was no correlation between APC and time to publish (Pearson’s r=–0.01). Abstract content was inconsistent; sample size and patient age were often missing, yet free full-text “open access” was available for most publications (59.5%). The NIH Clinical Trials portal lists eight registered studies on “ovarian rejuvenation,” of which two are actively recruiting patients, while four have been terminated or have an uncertain status. Two studies have concluded, with results from one posted to the NIH website. PRP and its derivatives for ovarian treatment show early promise, but require further investigation. Research is accelerating and should be encouraged, particularly placebo-controlled randomized clinical trials.
Keywords: Fertility, Menopause, Ovary, Platelet-rich plasma, Research
Introduction
The pace of intraovarian platelet-rich plasma (PRP) research has increased since the revolutionary work by Pantos et al. in 2016 [1]. Their study was the first to describe a novel PRP application, outlining how a non-pharmacologic method can ameliorate the consequences of ovarian senescence. While hormone replacement therapy (HRT) for menopause and advanced reproductive technologies/in vitro fertilization (IVF) for infertility are well established and familiar [2], the exploration of workable alternatives is important. Successful return of menses after menopause (as a substitute for HRT) and healthy term live births for infertility patients (either with IVF or as unassisted conceptions) following intraovarian PRP have placed this procedure under intense scrutiny, as expected. While intraovarian PRP research remains developmental and nonuniform, a related challenge exists concerning how reports discussing this experimental treatment enter the literature. Against this background, our analysis addressed these open questions: (1) how have basic science research and experience with intraovarian PRP evolved in recent years, (2) what publishing options are most commonly used by those working in this specific arena, and (3) are there features of the literature that can better meet the needs of the medical authorship community?
Methods
The standard Medical Subject Heading terms “ovarian” AND “platelet rich plasma” were used to conduct a Boolean search on the U.S. NLM PubMed platform to retrieve publications for analysis. Manuscripts accessed with the following terms/key words were also queried: “ovarian,” “ovary,” “PRP,” “platelet rich plasma,” and “rejuvenation.” All results were compared (Supplementary Material 1) and the current analysis was performed on the search that returned the largest set (n=54). Papers were removed if the subject was outside the scope of reproductive biology (n=12). Next, the following information was recorded from all remaining abstracts: manuscript type (original data vs. review), veterinary versus human research, sample size, patient age data, the inclusion of a placebo group, the total number of listed authors, the nationality of the first author’s institution, and the availability of free full-text access to the publication. The interval between manuscript submission and publication was also computed from the date information for each article, if given. Article processing charges (APCs) were determined from journal homepages. Information on current clinical study activity in the “ovarian rejuvenation” space was collected from the U.S. NIH Clinical Trials internet portal (www.clinicaltrials.gov) where a similar audit was undertaken. No identifiable patient-level data were included for analysis in this study.
Results
1. Scholarly output
This study considered all NLM publications indexed as related to ovarian PRP from 1983 to 2021, inclusively. Abstracts were collated annually for each full-year sampled except 2021, for which only partial-year data were available. The eligible and excluded journals are compared in Table 1. Only two qualifying papers on ovarian PRP appeared in the NLM literature before 2017, and both involved animal research and were the only research projects to include a placebo control. During the study period, 42 articles met the relevancy criteria, of which 40.5% reported on clinical studies, small series, or case reports, 33% described experimental animal models, and 23.8% were opinion/review papers (Figure 1). Crucially, only two works included a placebo control group and none were randomized clinical trials (RCTs).
Table 1.
NLM/PubMed journals with “ovarian rejuvenation” publication activity (1983–2021) sorted by criteria, with comparisons in APC and submission-to-publish interval
| Included (2013–2021) | Excluded (1983–2019) | p-valuea) | |
|---|---|---|---|
| NLM/PubMed journal | Aging (Albany NY) | Acta Cir Bras | |
| Biol Reprodb) | Breast Cancer Res | ||
| Biosci Rep | Clin Can Resb) | ||
| Cell J | Endocrinology | ||
| Cell Transplantb) | Gynecol Endocrinol | ||
| CERM | In Vivo | ||
| Climacteric | Int J Oncol | ||
| Cureus | Int J Womens Dermatol | ||
| Diagnostics (Basel) | J Membr Biol | ||
| Exp Toxicol Pathol | Maturitas | ||
| Front Endocrinol (Lausanne) | Oncotarget | ||
| Front Vet Sci | |||
| Growth Factors | |||
| Gynecol Endocrinol | |||
| Gynecol Obstet Investb) | |||
| Heliyon | |||
| Hum Reprod | |||
| Int J Mol Cell Med | |||
| Int J Reprod Biomed | |||
| J Clin Medb) | |||
| J Hum Reprod Sci | |||
| JARGb) | |||
| Menopause | |||
| Mol Biol Rep | |||
| Neuroendocrinol Lett | |||
| Open Access Maced Med J Sci | |||
| Regen Med | |||
| Reprod Biol Endocrinol | |||
| Reprod Biomed Online | |||
| Reprod Scib) | |||
| Stem Cell Rev Rep | |||
| Syst Biol Reprod Med | |||
| Toxicol Mech Methods | |||
| Vet Sci | |||
| APC (USD) | 1,612±1,466 | 2,133±1,243 | 0.31 |
| Interval from submission to publication (day) | 130±96 | 159±54 | 0.35 |
Values are presented as mean±standard deviation.
APC, article processing charge.
By Student t-test;
Journals publishing multiple works in the designated subject area.
Figure 1.
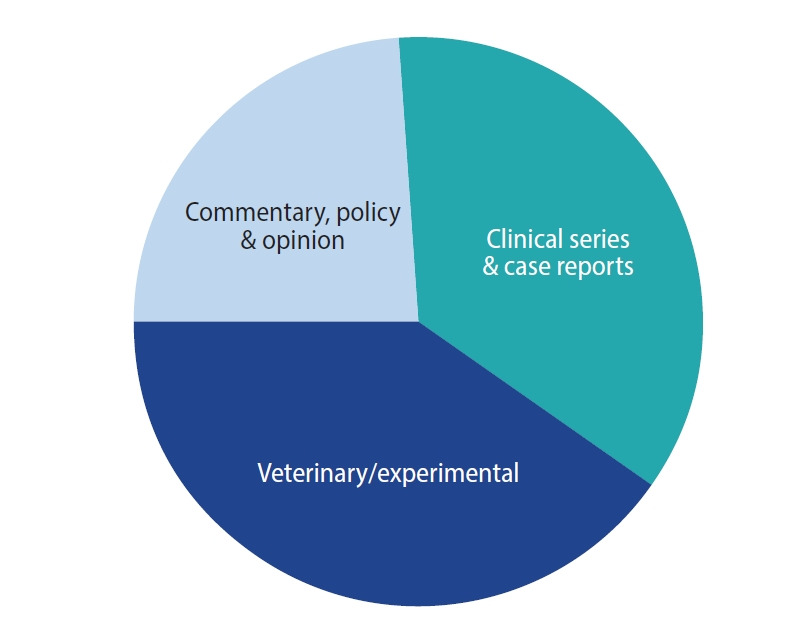
Worldwide published research activity on ovarian rejuvenation classified by manuscript type, 2013–2021. In this sample (total n=42), opinion/review papers accounted for 23.8%, while research derived from veterinary or other experimental sources comprised 33%. Most (40.5%) papers on the topic originated from clinical sources.
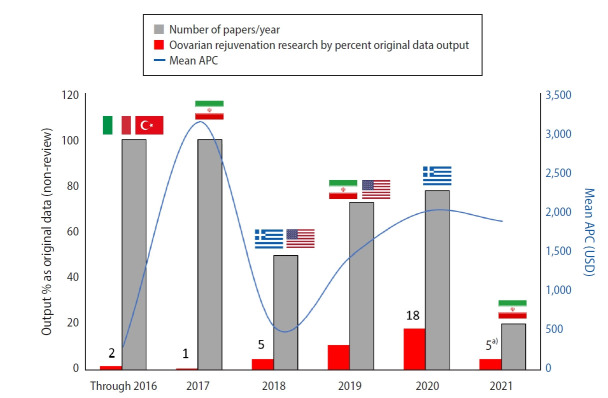
As depicted in Figure 2, the published output on intraovarian PRP has trended upward over time. In this sample, the number of authors per paper was 5.9±3.2 (range, 2–12) and when categorized by the nation of the first author’s affiliated institution, 13 nations were represented: China, Egypt, Greece, India, Iran, Italy, Macedonia, Taiwan, Turkey, United Arab Emirates, United Kingdom, Ukraine, and USA. Research deriving from international collaboration—where more than one country appeared in authorship identifiers—accounted for about 21% of published work (n=9).
Figure 2.
Summary of ovarian rejuvenation research by percent original data output, number of papers/year, and mean article processing charge (APC), through 2021. Sources of leading number of publications per year are also shown, by national origin.
a)Partial data shown for 2021.
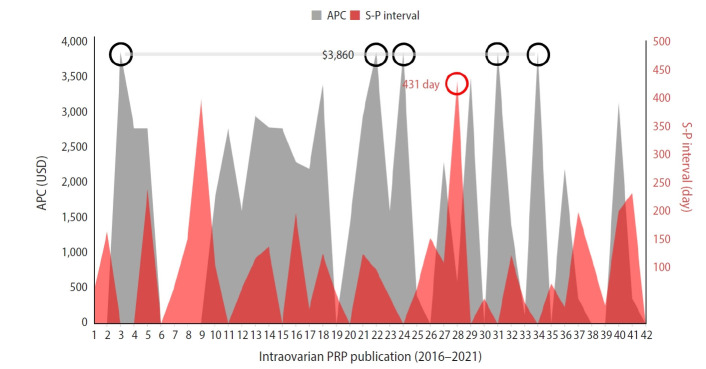
The technical aspects of intraovarian PRP publication efforts were also measured, with editorial and publication efficiency calculated from the interval between initial submission and publication. Although these details were absent in 10 of 42 articles (23.8%), where full tracking was possible, the mean±SD of submission-to-publication interval was 130±96 days (range, 27–431 days). APC data were obtained from each journal’s website to determine the cost-to-publish. In this sample, the mean±SD of APC was US $1,613±1,466 (range, $0–$3860). While most articles on intraovarian PRP (59.5%) were accessible for free full-text download, publishers of the oldest research (first published in 2013 and 2015) are still collecting access fees to view the full manuscripts. As shown in Figure 3, among the relevant papers there was no correlation between submission-to-publish and APC (Pearson’s r=–0.01).
Figure 3.
Relation between article processing charge (APC; gray) and submission-to-publish (S-P) interval (red) for published research covering ovarian platelet-rich plasma (PRP) among 42 indexed journals, with maximum values also shown (circles). USD, United States dollar.
2. Clinical trial activity
A U.S. NIH clinical trials registry search for “ovarian PRP or rejuvenation” identified eight studies, of which four were based in the USA, two in Spain, and one each from Greece and Iraq. Four studies examined treatments beyond intraovarian PRP injection, and aimed to explore intraovarian insertion (by laparoscopy) of autologous bone marrow stem cells or other complex approaches. No study design included a placebo arm. The mean±SD number of subjects enrolled or planned among all trials was 79.4±64 (range, 3–182), with the eligible patient age ranging from 18 to 60 years.
Discussion
As with any new medical application, the activity of early adopters is often balanced by appropriate mainstream skepticism. This study is the first to describe publishing patterns and measure aggregate output trends on intraovarian PRP from these “lighthouse” researchers. Here a global snapshot of research is provided, both human and veterinary, with documentation of where the research originates, who publishes it, how long it takes to become available, and other aspects. Based on the present findings, the distribution of literature among original clinical work, experimental/animal model activity, and commentary or opinion contributions seems relatively even. Most intraovarian PRP research originates from a handful of countries for now, but the full roster of experts covering this topic actually shows a wider source of global productivity. This shared international interest is also apparent on the U.S. NIH clinical trials registry, although this compilation underscores the need for more projects on ovarian rejuvenation.
Several factors identified here might help improve the current situation. Authors of descriptive studies or case reports on intraovarian PRP should include key data in the abstract content, so readers can identify specific facts regarding the population and technique when accessing the abstracts via PubMed. A checklist is now available to meet this need, and adherence to this standard will help clarify future reporting on intraovarian PRP [3]. Academic publishers also have an assignment—they should revisit their toll-collection tendencies and release full texts after 12–18 months into the public domain without charge. The commercial interface between physician-scientists and venues (journals) to disseminate their work will remain relevant given the fast-rising oligopoly of scholarly publishing [4].
As a conspectus of published scholarship on an emerging topic, this analysis has some weaknesses. Unregistered or unpublished activity on intraovarian PRP certainly exists, so the role of publication bias against negative results must be acknowledged. The U.S. NIH Clinical Trials registry also may not give an accurate forecast of research in process, and not all clinical trials are focusing exclusively on PRP. Curiously, no information is available to explain why the status for half of these investigations is either paused or unknown. Furthermore, if the current APCs are not the same as they were when a manuscript was published, this difference would escape detection using this study design. While it is encouraging that each full year’s data reveal progress with increasing activity, further monitoring will be needed to show if this trend continues.
It should be noted that even when a robust, proper RCT for intraovarian PRP does become available, mainstream clinical practice patterns might still resist meaningful change. For example, bias against intrauterine insemination and in favor of IVF as being universally superior has been spotlighted as unfounded, inappropriate, and deserving reappraisal [5]. Thus physician training, background, or experience can sometimes outweigh the persuasive gravitas of the RCT. Indeed, this laudable goal is missed by fertility subspecialists who have already admitted much into the clinical arena without RCT support. Perhaps the most conspicuous examples are IVF and intracytoplasmic sperm injection [6,7], both of which became accepted therapeutic mainstays with no RCT validation. Nonetheless, a focus on intraovarian PRP could help enlarge the therapeutic arsenal for women’s health safely, ideally with RCT data. Our analysis suggests this is already underway at numerous research units.
Footnotes
Conflict of interest
ESS and SHW have been assigned a provisional U.S. Patent for process & treatment of ovarian disorders using platelet cytokine derivatives. No other potential conflicts of interest were reported.
Author contributions
Conceptualization: ESS. Data curation: all authors. Formal analysis: all authors. Methodology: all authors. Project administration: all authors. Visualization: all authors. Writing–original draft: ESS. Writing–review & editing: all authors.
Supplementary material
Supplementary material can be found via https://doi.org/10.5653/cerm.2021.04651.
Literature reviewed, April 2021
PubMed search: “ovarian”+“platelet rich plasma”
References
- 1.Pantos K, Nitsos N, Kokkali G, Vaxevanoglou T, Markomichali C, Pantou A, et al. Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. Hum Reprod. 2016;(Suppl 1):P–401. [Google Scholar]
- 2.Sills ES. The scientific and cultural journey to ovarian rejuvenation: background, barriers, and beyond the biological clock. Medicines (Basel) 2021;8:29. doi: 10.3390/medicines8060029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Han L, Shields L, Tian J, Wang J. A PRISMA assessment of the reporting quality of systematic reviews of nursing published in the Cochrane Library and paper-based journals. Medicine (Baltimore) 2019;98:e18099. doi: 10.1097/MD.0000000000018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues RS, Abadal E, de Araújo BK. Open access publishers: the new players. PLoS One. 2020;15:e0233432. doi: 10.1371/journal.pone.0233432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahadur G, Homburg R. Growing body of evidence supports intrauterine insemination as first line treatment and rejects unfounded concerns about its efficacy, risks and cost effectiveness. JBRA Assist Reprod. 2019;23:62–7. doi: 10.5935/1518-0557.20180073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braakhekke M, Mol F, Mastenbroek S, Mol BW, van der Veen F. Equipoise and the RCT. Hum Reprod. 2017;32:257–60. doi: 10.1093/humrep/dew286. [DOI] [PubMed] [Google Scholar]
- 7.Evers JL. Do we need an RCT for everything? Hum Reprod. 2017;32:483–4. doi: 10.1093/humrep/dex003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Literature reviewed, April 2021
PubMed search: “ovarian”+“platelet rich plasma”