Abstract
Theca lutein cysts are rare, benign lesions responsible for gross cystic enlargement of both ovaries during pregnancy. This condition is also termed hyperreactio luteinalis. Elevated human chorionic gonadotropin (hCG) levels or states of hCG hypersensitivity seem to promote these changes, which in up to 30% of patients produce clinical signs of hyperandrogenism. Given the self-limiting course of theca lutein cysts, which are subject to spontaneous postpartum resolution, conservative treatment is the mainstay of patient management. Described herein is a rare case of theca lutein cysts with maternal virilization that failed to regress by 9 months after childbirth. Surgical intervention was eventually undertaken, necessitated by adnexal torsion.
Keywords: Hyperandrogenism, Hyperreactio luteinalis, Ovarian torsion, Theca lutein cyst, Virilism
Introduction
Theca lutein cysts are rare, benign, and typically bilateral cystic lesions producing grossly enlarged ovaries during pregnancy. In the realm of pathology, the term hyperreactio luteinalis is applied [1,2]. Although the pathogenesis is unclear, elevated levels of human chorionic gonadotropin (hCG) or hypersensitivity to prolonged hCG exposure may provoke exaggerated ovarian responses and cyst formation, triggering hyperandrogenism [3]. These derangements generally resolve spontaneously within months after delivery, regardless of ovarian size. In singleton pregnancies, pertinent publications consist chiefly of case reports and accounts detailing spontaneous resolution between 6 and 12 weeks postpartum [4-9]. Herein, we present a rare case of theca lutein cyst with virilization that failed to regress by 9 months after childbirth. Emergency laparoscopic surgery was eventually required due to adnexal torsion.
Case report
This study was approved by the Institutional Review Board at Severance Hospital (IRB No. 4-2019-0268) and adhered to the principles stipulated by the Declaration of Helsinki. The board members waived patient consent, provided there was no disclosure of identifiable personal information.
A 33-year-old woman (primigravida) was referred to our tertiary hospital for bilateral multilocular ovarian cysts (right: 17.0×8.9×16.2 cm; left: 11.0×5.8×14.4 cm) that developed at week 12 of gestation (Figure 1A). Prior to week 10, ultrasound studies of both ovaries were normal. The patient claimed to have regular menstrual cycles and denied use of ovulation-inducing drugs. Deepening of the voice by week 21 was the sole indicator of virilization. At week 38 (+4 days), she delivered a male infant (weight, 3,230 g; Apgar score, 7–8) via cesarean section. Both cysts remained unchanged at childbirth, conferring no apparent fetal virilization.
Figure 1.
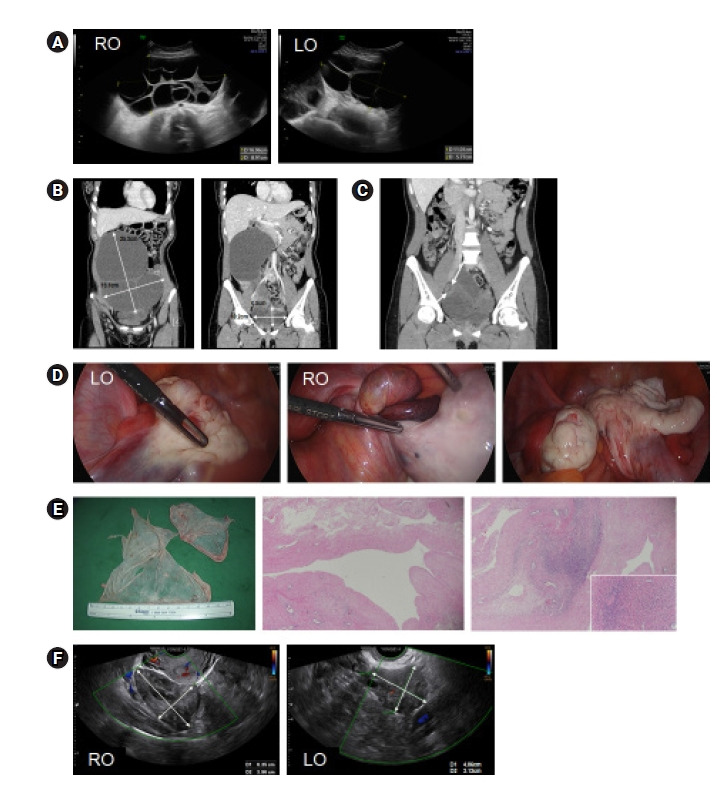
(A) Sonograms of multilocular ovarian cysts (right and left) discovered early in pregnancy (12 weeks). (B) Computed tomography (CT) views of the same cysts, 10 weeks postpartum. (C) CT image showing a smaller left ovary (LO) and enlarged right ovary (RO) with twisted pedicle (arrows), 9 months postpartum. (D) Operative photos of the shrunken left ovary, the enlarged right ovary with a twisted pedicle, and the right ovary after torsion release and cyst enucleation. (E) Gross findings of the right ovary (left), with a benign denuded wall and mildly edematous stroma (H&E sections; original magnification, ×40; inset ×200). (F) Sonograms of normally appearing ovaries 10 weeks after surgery.
At 10 weeks postpartum, the ovarian dimensions were still sizable (right: 25.3×19.1 cm; left: 10.0×6.1 cm) on computed tomography (CT) imaging (Figure 1B). Her voice continued to deepen, and excessive hair growth had begun in a male-pattern distribution. There were marked elevations of serum total testosterone (7.542 ng/mL), free testosterone (13.38 pg/mL), and 17–hydroxyprogesterone (35.5 ng/mL); and the free androgen index (FAI) was high (32.1). However, serum dehydroepiandrosterone sulfate was within the normal range. The serum total hCG level was still elevated (23 mlU/mL), with suppressed levels of luteinizing hormone (LH; <0.1 mIU/mL) and follicle-stimulating hormone (0.6 mIU/mL). The estradiol concentration was 94.9 pg/mL (Table 1). Various tumor markers, including carcinoembryonic antigen, α-fetoprotein, cancer antigen (CA) 125, and CA 19-9, were within the respective normal ranges.
Table 1.
Postpartum chronology of shifting hormonal levels and ovarian cyst regression
| Variable | 2 mo | 4 moa) | 6 mob) | 9 mo | 13 mo/2 mo PO | Normal range |
|---|---|---|---|---|---|---|
| Total T (ng/mL) | 7.542 | 3.140 | 0.942 | 0.084 | 0.084–0.481 | |
| SHBG (nmol/L) | 80.4 | 210.1 | 114.0 | 82.3 | 6–152 | |
| FAI | 32.6 | 5.2 | 2.9 | 0.4 | ||
| Free T (pg/mL) | 13.38 | 3.62 | 2.31 | 0.89 | 1.33 | 0.00–3.09 |
| 17OHP (ng/mL) | 35.5 | 11.67 | 5.16 | 2.16 | 0.78 | 0.11–1.08 (F) |
| 0.95–5.0 (L) | ||||||
| ThCG (mIU/mL) | 23 | 6 | 2 | <0.2 | <1 | |
| DHEAS (ug/dL) | 226.7 | 25.9–460.2 | ||||
| LH (IU/L) | <0.1 | 3.3 | 1.9–12.5 (F) | |||
| FSH (IU/L) | 0.6 | 9.0 | 2.5–10.2 (F) | |||
| E2 (pg/mL) | 94.9 | 39.3 | 19.5–144.2 (F) | |||
| AMH (ng/mL) | 2.03 | 1.37 | 0.58–8.13 | |||
| Ovarian size (cm) | ||||||
| Right | 25.3×19.1 | 16.6×7.5 | 11.2×10.7 | 6.4×4.0 | ||
| Left | 10.0×6.1 | 8.2×3.4 | 4.1×4.1 | 4.1×3.1 |
Normal hormonal ranges (assay specifications) pertain to healthy nonpregnant women.
PO, postoperative; T, testosterone; SHBG, sex hormone-binding globulin; FAI, free androgen index; 17OHP, 17-hydroxyprogesterone; F, follicular phase; L, luteal phase; ThCG, total human chorionic gonadotropin; DHEAS, dehydroepiandrosterone sulfate; LH, luteinizing hormone; FSH, follicle-stimulating hormone; E2, estradiol; AMH, anti-Müllerian hormone.
1 month after medical intervention;
3 months after medical intervention.
To lower androgen levels and slow progression of virilization, oral contraceptives (ethinyl estradiol [20 μg] and drospirenone [3 mg]), were administered, along with spironolactone (50 mg/day). After 3 months of treatment, serum androgen levels had declined but remained elevated: testosterone, 3.14 ng/mL; free testosterone, 3.62 pg/mL; 17-hydroxyprogesterone, 11.67 ng/mL; and FAI, 5.2. Serum total hCG was also much lower (6 mlU/mL) (Table 1), with unchanged ovarian dimensions on ultrasound. Having curbed the virilization and reduced androgen levels, we discontinued the anti-androgenic regimen.
Six months after delivery, serum testosterone, free testosterone, and 17-hydroxy-progesterone levels further declined to 0.942 ng/mL, 2.31 pg/mL, and 5.16 ng/mL, respectively. The serum total hCG level was lower as well (2 mlU/mL) (Table 1). Virilization did not progress after medication withdrawal, both ovaries again appearing unchanged. Her menstrual cycle was restored at 6 months after delivery.
Nine months after delivery, serum testosterone, free testosterone, and 17-hydroxyprogesterone levels had normalized (0.084 ng/mL, 0.89 pg/mL, and 2.16 ng/mL, respectively), and the serum total hCG level was <0.2 mlU/mL. The cystic ovaries had finally regressed (right: 11.2×10.7 cm; left: 4.1×4.1 cm), albeit more so on the left (Table 1).
Seventeen days later, the patient presented to the emergency room with intermittent, severe abdominal pain. Torsion of the enlarged right ovary was evident on CT (Figure 1C), calling for emergency laparoscopic surgery. During the procedure, we encountered a sizeable cyst (up to 10.0 cm) of the twisted right ovary (Figure 1D). We released the torsion and enucleated the cyst, which later proved benign. Its denuded lining and mildly edematous stroma are visible in Figure 1E. Eight weeks after surgery, theca lutein cysts of both ovaries were undetectable by ultrasound (Figure 1F), and all signs of virilization (e.g., deepening of the voice, excessive hair growth) had noticeably improved.
Discussion
Theca lutein cysts are common causes of maternal hyperandrogenism [10]. Because LH and hCG are almost structurally identical, exaggerated follicular responses to the LH-like effects of hCG promote hypertrophy and luteinization of theca cells, inducing hyperandrogenism [11]. Consequently, up to 30% of patients will experience temporal balding, deepening of the voice, clitoromegaly, hirsutism, or acne [10]. However, fetal masculinization or virilization seldom occurs, as in patients with luteoma or androgen-producing ovarian tumors [1]. Our patient delivered a male fetus with no untoward effects of high androgen exposure in utero. Although there have been few case reports of virilized female fetuses [12,13], female fetuses born in mothers with virilization could show the effects of high androgen exposure including labioscrotal fusion and clitoromegaly. However, exposure to high androgen levels after 12 weeks of gestation generally does not produce labial fusion [12,13]. Besides the effect of the timing of androgen exposure, the lack of androgen-related fetal manifestations is also likely due to several protective mechanisms, including a rise in maternal sex hormone-binding globulin concentration, progesterone competition for androgen receptors or androgen activation in target tissues, and placental androgen aromatization [1].
This patient experienced some virilization during pregnancy (deepened voice, week 21) that worsened after childbirth (hirsutism, 10 weeks postpartum). Theca lutein cysts usually regress following delivery, but these persisted, causing marked and increasingly problematic androgen elevations. We thus prescribed oral contraceptives and spironolactone to lower androgen levels and slow the progressive effects. Androgen levels declined after 3 months of this regimen, despite unchanged ovarian status. Three months later, they had normalized spontaneously, along with gradual cystic regression. Unfortunately, surgical intervention was necessary to release right-sided adnexal torsion. It is likely that conservative treatment would have otherwise sufficed. Five months after laparoscopic enucleation of a right ovarian cyst, both ovaries appeared normal on ultrasound.
Theca lutein cysts are diagnosable clinically based on the characteristic spoke-wheel sonographic sign and physiological flow of color Doppler velocimetry [14]. Biochemical markers and magnetic resonance imaging may be helpful in differentiating theca lutein cysts from other disorders, especially malignancies [8]. Histologically, luteinized theca cells line the cyst walls, and the luteinized stromal cells appear edematous [3,4]. In most cases, such cysts are asymptomatic incidental findings discovered through routine sonography or cesarean section. Large ovarian cysts may cause abdominal discomfort, dyspnea, or abdominal pain due to torsion; peritonitis related to hemorrhage/rupture or mass effects; or overt virilization [9]. Past studies have reported natural regression of theca lutein cysts at 6–12 weeks postpartum [4-9]. Compared to previous examples, in the present case, the theca lutein cyst regressed gradually, but persisted until 9 months after delivery. To the best of our knowledge, this is the case of virilizing theca lutein cyst that persisted for the longest time after delivery.
Both of the patient’s ovaries showed spoke-wheel signs on ultrasound studies performed early in pregnancy (12 weeks). Having no history of ovulation-inducing drugs, theca lutein cystic change was favored rather than ovarian hyperstimulation syndrome as a diagnostic possibility. The inordinate persistence of cysts 2 months after delivery compelled us to consider other virilizing tumorous conditions of pregnancy, particularly Krukenberg, mucinous cystic, Brenner, and endodermal sinus tumors; serous cystadenoma; and dermoid cyst [15]. CT and tumor marker studies ruled out these disorders, with the denuded epithelium rendering cystadenoma indistinguishable on histologic grounds. Nonetheless, the left ovarian cyst spontaneously regressed, and there was similar shrinkage on the right, entirely compatible with theca lutein cysts.
Given their relation to hCG, theca lutein cysts tend to arise in conjunction with multiple pregnancies, gestational trophoblastic disease, or choriocarcinoma [2]. This case involved a singleton pregnancy and lacked any basis for such disorders. Levels of hCG also peak during early pregnancy (~100,000 mIU/mL), declining substantially thereafter (to ~30,000 mIU/mL) and persisting until term. Following delivery, they fall rapidly and reach normal levels for nonpregnant women by 3 weeks postpartum [16]. In our patient, hCG remained elevated (23 mIU/mL) at 2 months postpartum, failing to normalize until 9 months after delivery. This curious regressive delay may be attributable to LH/hCG receptor-mediated mechanisms that prolong hCG elevations.
Considering that breastfed infants appear not to be adversely affected by maternal testosterone therapy [17], maternal hyperandrogenism is not a contraindication of lactation. However, due to the inhibitory effect of androgen on milk production, whereas lactogenesis in normal pregnancies begins between 30 and 40 hours postpartum, high maternal levels of testosterone and hCG can delay lactogenesis for as long as 31 days. It has also been reported that testosterone levels of approximately 3 ng/mL or less are required for successful milk production [1]. In this case, however, the patient breastfed until 2 months after delivery, even though her total serum testosterone was over 7 ng/mL.
The majority of theca lutein cysts have been successfully managed with a conservative approach; nevertheless, prior research found that 36.2% of patients underwent surgery, of whom 23.8% had an acute complication (e.g., ovarian torsion, pain, or hemoperitoneum) and the remaining 76.2% were suspected of having a malignancy [9]. Although there will always be a role for surgical exploration in atypical cases or cases complicated by torsion or rupture-associated hemorrhage, management of theca lutein cysts must be based on a conservative approach, mitigating undue surgical and reproductive morbidity [1]. In the present case, the virilization symptoms gradually improved with the decrease of androgen levels and subsided without surgery around 9 months after delivery. In addition, since the theca lutein cysts gradually regressed before surgery, they could be successfully managed with a conservative approach if adnexal torsion did not occur.
Herein, we presented a rare case of virilizing theca lutein cysts that did not readily regress after childbirth. Ultimately, adnexal torsion necessitated emergency laparoscopic surgery. Even if the resolution is delayed (i.e., >9 months postpartum), it appears that theca lutein cysts may be managed conservatively and surgical intervention could be reserved for acute complications.
Acknowledgments
The Korea Health R&D Project of the Korea Health Industry Development Institute, funded by the Ministry o Technology f Health & Welfare, Republic of Korea (HI18C2047).
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SK, IL, YSC. Data curation: SK, IL,EP, YJR, KK, AIA. Funding acquisition: SC, YSC. Formal analysis: SK, JHP, JHL, BHY, SKS, SC, YSC, BSL. Project administration: SK, IL, YSC. Visualization: EP, KK. Writing–original draft: SK, IL, YSC. Writing–review & editing: SK, IL, YSC.
References
- 1.Malinowski AK, Sen J, Sermer M. Hyperreactio luteinalis: maternal and fetal effects. J Obstet Gynaecol Can. 2015;37:715–23. doi: 10.1016/S1701-2163(15)30176-6. [DOI] [PubMed] [Google Scholar]
- 2.Montz FJ, Schlaerth JB, Morrow CP. The natural history of theca lutein cysts. Obstet Gynecol. 1988;72:247–51. [PubMed] [Google Scholar]
- 3.al-Harbi O, al-Saleem A, al-Tayeb O, Giangreco AB. Recurrent bilateral theca lutein cysts in association with normal pregnancy. Ultrasound Obstet Gynecol. 1998;11:222–4. doi: 10.1046/j.1469-0705.1998.11030222.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Aleem H, Al-Hussaini T, Karoush S. Hyperreactio luteinalis associated with normal singleton pregnancy. J Obstet Gynaecol. 2000;20:315. doi: 10.1080/01443610050009746. [DOI] [PubMed] [Google Scholar]
- 5.Teoh SH, Teoh LS, Teng WC. Conservative management of recurrent bilateral ovarian cysts in pregnancy: a case report. Singapore Med J. 2003;44:536–8. [PubMed] [Google Scholar]
- 6.Baxi LV, Grossman LC, Abellar R. Hyperreactio luteinalis in pregnancy and hyperandrogenism: a case report. J Reprod Med. 2014;59:509–11. [PubMed] [Google Scholar]
- 7.Edell H, Shearkhani O, Rahmani MR, Kung RC. Incidentally found hyperreactio luteinalis in pregnancy. Radiol Case Rep. 2018;13:1220–3. doi: 10.1016/j.radcr.2018.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amoah C, Yassin A, Cockayne E, Bird A. Hyperreactio luteinalis in pregnancy. Fertil Steril. 2011;95:2429. doi: 10.1016/j.fertnstert.2011.03.060. e1-3. [DOI] [PubMed] [Google Scholar]
- 9.Cavoretto P, Giorgione V, Sigismondi C, Mangili G, Serafini A, Dallagiovanna C, et al. Hyperreactio luteinalis: timely diagnosis minimizes the risk of oophorectomy and alerts clinicians to the associated risk of placental insufficiency. Eur J Obstet Gynecol Reprod Biol. 2014;176:10–6. doi: 10.1016/j.ejogrb.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Kanova N, Bicikova M. Hyperandrogenic states in pregnancy. Physiol Res. 2011;60:243–52. doi: 10.33549/physiolres.932078. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Tohya T, Yoshimura T, Matsui K, Fujisaki S, Okamura H. Theca lutein cysts with maternal virilization and elevated serum testosterone in pregnancy. Acta Obstet Gynecol Scand. 1987;66:565–6. doi: 10.3109/00016348709015737. [DOI] [PubMed] [Google Scholar]
- 12.Lambers DS, Rosenn B. Hyperreactio luteinalis complicating a normal singleton pregnancy. Am J Perinatol. 1996;13:491–4. doi: 10.1055/s-2007-994434. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S. Comparison between spontaneous ovarian hyperstimulation syndrome and hyperreactio luteinalis. Arch Gynecol Obstet. 2004;269:227–9. doi: 10.1007/s00404-003-0570-1. [DOI] [PubMed] [Google Scholar]
- 14.Van Holsbeke C, Amant F, Veldman J, De Boodt A, Moerman P, Timmerman D. Hyperreactio luteinalis in a spontaneously conceived singleton pregnancy. Ultrasound Obstet Gynecol. 2009;33:371–3. doi: 10.1002/uog.6325. [DOI] [PubMed] [Google Scholar]
- 15.Bolat F, Parlakgumus A, Canpolat T, Tuncer I. Benign mucinous cystadenoma with stromal luteinization responsible for maternal virilization and fetal intrauterine growth restriction. J Obstet Gynaecol Res. 2011;37:893–6. doi: 10.1111/j.1447-0756.2010.01406.x. [DOI] [PubMed] [Google Scholar]
- 16.Haenel AF, Hugentobler W, Brunner S. The postpartum course of the HCG titer of maternal blood and its clinical relevance. Z Geburtshilfe Perinatol. 1986;190:275–8. [PubMed] [Google Scholar]
- 17.Glaser RL, Newman M, Parsons M, Zava D, Glaser-Garbrick D. Safety of maternal testosterone therapy during breast feeding. Int J Pharm Compd. 2009;13:314–7. [PubMed] [Google Scholar]


