Highlights
-
•
DWI has potential as a non-invasive biomarker of early treatment response in patients with cervical cancer.
-
•
A change in ADC seen early in a therapy regime significantly correlated with eventual response.
-
•
Further studies are required to determine an ideal cut-off or range of ADC values that can be used to predict response.
Keywords: Cervical cancer, Diffusion MRI, Chemoradiation, Response
Abstract
Objective
Diffusion-weighted magnetic resonance imaging (DWI) has shown promise in predicting response to therapy in several malignancies. This systematic review and meta-analysis aimed to evaluate DWI in the prediction of response to treatment in patients with cervical cancer.
Methods
A systematic search was conducted on PubMed, Web of Science, Cochrane and Google Scholar databases Studies that evaluated DWI and apparent diffusion coefficient (ADC) for response evaluation before, during and after treatment with a correlation to conventional response criteria were included. The primary endpoint was the mean ADC values of cervical cancer at these timepoints. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) was used to assess the quality of the studies.
Results
Nine studies, comprising 270 patients, were included. Pre-treatment ADC values showed no correlation with eventual response. However, in our meta-analysis, there was a significant correlation with early treatment ADC values obtained within the first 3 weeks of therapy and response, as well as a significant correlation with the percentage change in ADC (ΔADC) and response. In addition, the pooled mean ΔADC percentage was also significantly higher in responders than in non-responders (49.7% vs 19.7%, respectively, p = 0.016).
Conclusion
DWI shows potential as a biomarker of early treatment response in patients with cervical carcinoma. Use of the change in ADC particularly within the first 3 weeks of therapy seems to be predictive of response and may serve as a suitable marker in the determination of early response.
1. Introduction
In recent years, DWI has been proposed as a potential biomarker of treatment response in various tumors (Harry et al., 2008, Dzik-Jurasz et al., 2002). Although initially used in the imaging of cerebral stroke (Šaňák et al., 2006, Oppenheim, 2006), this technique has the ability to integrate morphological and functional changes that offer great promise as predictors or early indicators of therapy response in malignancies.
A challenge in cancer management has always been the absence of a reliable and early marker of treatment response. This would have substantial clinical value as persisting with ineffective therapy is associated with poor outcome, increased toxicity and morbidity, undue expense and ultimately, delay in administering potentially more effective treatment.
Conventional imaging techniques have historically relied on changes in morphology and size of tumor, but these are limited since changes in gross tumor dimension may lag behind the biological alterations that occur earlier in responders (Hayes et al., 2002, Padhani, 2002, Pickles et al., 2006).
DWI is a functional imaging technique that is sensitive to the microscopic motion of water molecules (Chenevert et al., 2002, Chenevert, 2000, Hamstra et al., 2007), and can be applied to a conventional magnetic resonance sequence (Stejskal and Tanner, 1965). It therefore allows for non-invasive characterization of biological tissues based on their water-diffusion properties and can be quantified as the ADC which is an indicator of tumor microstructure.
In this way, DWI (and ADC) has been proposed as a surrogate marker of tumor cellularity by observing the movement of water within tumors (Hamstra et al., 2007, Le Bihan et al., 1988, Ross, 1994, Ross, 2003, Zhao et al., 1996) and has the ability to detect the changes in cell density due to the necrosis and apoptosis that occur with various forms of anticancer therapy. In addition, these changes occur before macroscopic indicators of response such as tumor size and volume are observed (Pickles et al., 2006, Lee et al., 2007).
The standard treatment for locally advanced cervical cancer is concurrent chemoradiation. To date, there are no accepted imaging modalities that have can reliably predict the outcome of therapy and many of these women must contend with post-treatment scans in order to determine their eventual response. It would therefore be clinically relevant to identify an early biomarker of response that can be used well before the end of therapy thus allowing for modifications of the regime.
Members of this team produced one of the earliest studies that evaluated DWI as a biomarker of early response in cervical cancer (Harry et al., 2008). Since then, DWI has been increasingly explored in the prediction and early assessment of treatment response in cervical carcinomas (Chen et al., 2010, Dashottar et al., 2019, Hameeduddin and Sahdev, 2015, Kuang et al., 2014, Lee et al., 2014, McVeigh et al., 2008, Yang et al., 2018, Zheng et al., 2020). Using the DW images, ADC maps have been generated and allow for the quantification of ADC before and during treatment, potentially offering a more individualized approach in treating these patients while reducing unnecessary toxicity.
A recent review found that ADC values detected pre-therapy in cervical cancers could not be used to predict therapy response (MEYER et al., 2021), but our team felt that the more appropriate marker would be the change in ADC with treatment as DWI is sensitive to the dynamic water movement within tumors that occurs with therapy, more so than just absolute values documented before the start of treatment. This theory has been borne out in other solid tumors as well (Amodeo et al., 2018, Vollenbrock et al., 2020). Hence, it is entirely more appropriate to perform a comprehensive review on ADC changes with treatment.
Therefore, the aim of this study was to systematically review the evidence for use of DWI and ADC, particularly the change in ADC, in locally advanced cervical cancer for prediction and early response assessment of treatment.
2. Methods
This systematic review followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009).
2.1. Search strategy
A systematic search was performed in the PubMed, Web of Science, Google Scholar and Cochrane library electronic databases from January 2005 to April 2021, as the first article on this technique was published in 2005 (Naganawa et al., 2005). The following search terms were used: “diffusion-weighted MRI” OR “diffusion-weighted imaging” OR “DWI”; “apparent diffusion coefficient” OR “ADC” AND “cervical cancer” OR “cervical carcinoma” AND “response’ OR “predict” OR “prognosis”. References of articles included in the final section were reviewed.
2.2. Eligibility criteria
Studies were included if they met the following criteria: biopsy-confirmed cervical cancer, treatment by concurrent chemoradiation or neoadjuvant chemotherapy followed by surgery or radiotherapy, DWI performed before therapy and again early in the treatment regime; within the first 3 weeks of treatment, ADC values given at these time points and the reference standard for tumor response to therapy as measured by the Response Evaluation Criteria in Solid Tumors (RECIST) on follow-up scanning in patients (Duffaud and Therasse, 2000, Eisenhauer et al., 2009).
The search strategy was limited to human clinical studies and those published in English or any language for which English translation was readily available. Studies that were excluded were case reports, review articles, conference presentations, studies without ADC values and xenograft or animal model experimental studies. Trial registries were not searched and any unpublished studies that were identified during this review were not included.
2.3. Study selection
The literature search and study selection were independently performed by two authors, VH and SP. Title and abstract screening were carried out and publications for full text review were included if they reported the use of DWI in cervical cancers. Any discrepancies were resolved through a consensus discussion with all authors afterwards.
2.4. Data extraction and quality assessment
The primary endpoint was defined as the ADC values in patients (both responders and non-responders) who had DWI performed prior to the start of chemoradiation and within 3 weeks of their treatment regime. We chose to include studies with DWI performed early in the therapy regime, as a practical biomarker should be able to predict response early enough to affect an appropriate change in treatment.
Data extraction was done independently by two authors, VH and SP. From each study, the following information was extracted: the name of the study, the first author, year of publication, study design, number of patients, mean age and range, histological type and tumor stage. Imaging data recorded included MRI field strength and the timing of the DWI sequences with respect to the treatment regime. Pre-treatment mean ADC values as well as mean ADC values obtained when imaged during the early phase of the therapy regime were recorded. The change in ADC (ΔADC) between these 2 timepoints was used and was calculated as early treatment ADC minus pre-treatment ADC divided by pre-treatment ADC and multiplied by 100.
Furthermore, information on the assessment of response before and after treatment was extracted. Responders (R) were defined as either complete or partial response, while non-responders (NR) were defined as stable disease and progressive disease as based on RECIST.
In the case of overlapping study populations published by the same author or institution, the larger study population was included. For duplicate publications, the most recent and complete report was used. The selected studies were assessed for the methodological quality using Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) criteria (Whiting, 2011).
2.5. Statistical methods
The analysis was carried out using RevMan 5.4 (Review Manager Version 5.4.1, The Cochrane Collaboration, 2020). Heterogeneity between studies was assessed using the Inconsistency index, I2, which represents the estimated proportion of unexplained inter-study variance. An I2 of ≤50% is suggestive of an absence of any substantial heterogeneity (Higgins, 2003). P values of <0.05 were considered statistically significant.
3. Results
3.1. Study selection
From a total of 326 citations, 114 records remained after removal of duplicates (Fig. 1). An additional 93 articles were removed on the basis of their titles and abstracts. Twenty-one articles were then subjected to full-text review to assess their suitability according to the inclusion criteria. Any study with only pre-treatment ADC values and no repeat early assessment of ADC during the therapy regime was excluded. The reasons for excluding the remaining studies are given in Fig. 1. Finally, 9 studies fully complied with the inclusion criteria and were included in the analysis.
Fig. 1.
Flow chart of literature search and study selection criteria adapted from PRISMA.
3.2. Quality of the included studies
The results of the QUADAS-2 assessment are shown in Fig. 2. Overall, following evaluation of the methodological quality of the included studies, the risk of bias was considered low.
Fig. 2.
QUADAS-2 quality assessment of the included studies.
Patient selection was considered well-defined within the respective methodology. However, some studies did not clearly or sufficiently define their inclusion criteria which may result in potential bias. The index test defined as DWI was clearly reported in all articles and should not be seen as a source of potential bias.
As DWI is not used routinely used for routine response monitoring, the reference standard of T1 and T2 sequences and their interpretation would not have introduced bias. Similarly, for flow and timing, there were no delays between imaging and treatment regimes, and all patients received a post therapy evaluation.
3.3. General study characteristics
Nine studies, which comprised a total of 270 patients with cervical cancer, used DWI to evaluate treatment response. There were 264 women with squamous cell cancers and 6 with adenocarcinomas. All studies included tumor staging ranging from FIGO stage IB to IV and a summary of other characteristics are included in Table 1.
Table 1.
Summary of characteristics of the included nine studies. (CRT: chemoradiation, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease).
| Author, year | Country | Study design | Number of Patients | Age mean (range) | Tumor type n | FIGO staging n | Treatment | Outcome n |
|---|---|---|---|---|---|---|---|---|
| Harry et al., 2008 (Harry et al., 2008) | UK | Prospective | 20 | 50 (34–80) | Squamous cell: 18 Adenocarcinoma: 2 | IB2: 1 IIA: 2 IIB: 3 IIIB: 13 IVB: 1 | Concurrent CRT | PR: 19 SD: 1 |
| Zhang et al., 2011 (Zhang et al., 2011) | China | Prospective | 14 | 44 (26–71) | Squamous cell: 14 | IIA:2 IIB:5 IIIB:5 IVA: 2 | Concurrent CRT | CR: 1 PR: 13 |
| Fu et al., 2012 (Fu et al., 2012) | China | Prospective | 30 | 42 (25–55) | Squamous cell: 30 | IB2:15 IIA:9 IIB: 6 | Neoadjuvant chemotherapy | CR: 1 PR: 22 SD: 5 PD: 2 |
| Kuang et al.,2014 (Kuang et al., 2014) | China | Retrospective | 75 | 49 (36–66) | Squamous cell: 75 | IIA:38 IIB: 15 III: 13 IV: 9 | Concurrent CRT | CR: 35 PR: 22 SD: 18 |
| Makino et al., 2014 (Makino et al., 2014) | Japan | Retrospective | 25 | 63.5 (36–91) | Squamous cell; 21 Adenocarcinoma: 4 | IB: 3 IIA: 1 IIB: 4 IIIA: 2 IIIB: 8 IVA: 3 IVB: 4 | Concurrent CRT: 16, RT: 9 | CR: 16 PD: 9 |
| Liu et al., 2015 (Liu, 2015) | China | Prospective | 33 | 53.6 (36–75) | Squamous cell: 33 | IIB: 10 IIIA: 1 IIIB: 22 | Concurrent CRT | CR: 7 PR: 26 |
| Das et al., 2015 (Das et al., 2015) | India | Prospective | 24 | 50 (36–61) | Squamous cell: 24 | IIB: 9 IIIB: 15 | Neoadjuvant CRT | CR: 9 PR: 6 SD: 9 |
| Zhu et al., 2016 (Zhu, 2016) | China | Prospective | 21 | 49.6 (24–76) | Squamous cell: 21 | II: 11 III: 6 IV: 4 | Concurrent CRT | CR: 18 PR: 3 |
| Bian et al., 2019 (Bian et al., 2019) | China | Prospective | 28 | 47.8 (31–69) | Squamous cell: 28 | IB: 1 IIB: 26 IIIB: 1 | Concurrent CRT | CR: 22 PD: 6 |
All studies were published between 2008 and 2019, were all written in English language and were conducted in four different countries: United Kingdom, China, India and Japan. The selected studies were all observational with 7 prospective (Harry et al., 2008, Zhang et al., 2011, Fu et al., 2012, Liu, 2015, Das et al., 2015, Zhu, 2016, Bian et al., 2019), and 2 retrospective designs (Kuang et al., 2014, Makino et al., 2014). There were no randomized studies.
All patients in six of the included studies were treated with concurrent chemoradiation (Harry et al., 2008, Kuang et al., 2014, Zhang et al., 2011, Liu, 2015, Zhu, 2016, Bian et al., 2019). In one study, 16 patients received concurrent chemoradiation while the remaining 9 received radiotherapy alone due to advanced age or poor performance status (Makino et al., 2014). In the other two studies, the patients received neoadjuvant chemotherapy followed by radical hysterectomy or radiotherapy in one report (Fu et al., 2012), and by concurrent chemoradiation in the other (Das et al., 2015).
Out of the entire study population of 270 patients, there were 181 responders (complete and partial response) and 89 non-responders (stable disease, progressive disease and residual disease).
MRI scans were performed on a field strength of 1.5 Tesla in four studies and 3.0 Tesla in five studies. All studies used b values between b = 0 and b = 1000 to calculate the ADC. Baseline or pre-treatment DWI was done on all patients and used to calculate ADC via regions of interest (ROI). For the timepoints of DWI (and ADC) assessment early in the treatment regime, this was done on day 14 in 6 studies (Harry et al., 2008, Kuang et al., 2014, Zhang et al., 2011, Fu et al., 2012, Liu, 2015, Zhu, 2016) and on day 21 in the other 3 studies (Makino et al., 2014, Das et al., 2015, Bian et al., 2019). A summary of the imaging characteristics, ADC values and response rates is given in Table 2.
Table 2.
A summary of study imaging characteristics, ADC values (units of ADC value: 10−3mm2/s), the percentage change in ADC and response rates.
| Author, year | MRI Tesla strength | b-value (s/mm2) | Timing of DWI assessments used | Study findings – ADC correlation with response | Pre-treatment ADC | Early treatment ADC | ΔADC/% | Response (n) |
|---|---|---|---|---|---|---|---|---|
| Harry et al. 2008 (Harry et al., 2008) | 1.5T | 0, 1000 | Pre-therapy Day 14 |
No correlation with response ADC correlated with response (p = 0.009) ΔADC correlated with response (p = 0.01) |
1.24 ± 0.18 1.15 ± 0.10 |
1.53 ± 0.28 1.43 ± 0.10 |
23 20 |
R = 19 NR = 1 |
| Zhang et al., 2011 (Zhang et al., 2011) | 1.5T | 0, 1000 | Pre-therapy Day 14 |
No correlation with response ADC – no correlation with response ΔADC correlated with response (p = 0.010) |
1.04 ± 0.11 1.11 ± 0.21 |
1.47 ± 0.10 1.35 ± 0.24 |
41 22 |
R = 1 NR = 13 |
| Fu et al., 2012 (Fu et al., 2012) | 3T | 0, 900 | Pre-therapy Day 14 |
No correlation with response ADC correlated with response (p = 0.022) |
0.89 ± 0.09 0.88 ± 0.08 |
0.98 ± 0.10 0.89 ± 0.09 |
10 1 |
R = 23 NR = 7 |
| Kuang et al., 2014 (Kuang et al., 2014) | 3T | 0, 1000 | Pre-therapy Day 14 |
No correlation with response ADC – no correlation with response ΔADC correlated with response (p < 0.05) |
0.80 ± 0.1 0.78 ± 0.05 |
1.02 ± 0.13 1.04 ± 0.08 |
28 33 |
R = 57 NR = 18 |
| Makino et al., 2014 (Makino et al., 2014) | 1.5T | 0, 1000 | Pre-therapy Day 21 |
No correlation with response ADC – no correlation with response ΔADC correlated with response (p < 0.05) |
0.87 ± 0.13 0.92 ± 0.10 |
1.30 ± 0.25 1.17 ± 0.15 |
46 27 |
R = 16 NR = 9 |
| Liu et al., 2015 (Liu, 2015) | 1.5T | 0, 1000 | Pre-therapy Day 14 |
No correlation with response ΔADC correlated with response (p = 0.007) |
0.810 ± 0.015 0.863 ± 0.088 |
1.213 ± 0.981 1.154 ± 0.131 |
49 34 |
R = 7 NR = 26 |
| Das et al., 2015 (Das et al., 2015) | 3T | 0, 800 | Pre-therapy Day 21 |
No correlation with response ADC correlated with response (p = 0.01) ΔADC correlated with response (p = 0.03) |
0.71 ± 0.11 0.74 ± 0.12 |
1.31 ± 0.26 1.00 ± 0.19 |
85 35 |
R = 15 NR = 9 |
| Zhu et al., 2016 (Zhu, 2016) | 3T | 0, 1000 | Pre-therapy Day 14 |
No correlation with response ADC correlated with response (p = 0.001) |
1.00 ± 0.11 | 1.39 ± 0.26 | 39 | R = 21 |
| Bian et al., 2019 (Bian et al., 2019) | 3T | 0, 850 | Pre-therapy Day 21 |
No correlation with response ADC – no correlation with response |
0.9775 ± 0.9542 0.9945 ± 0.2168 |
1.3915 ± 0.1804 1.2632 ± 0.1502 |
43 27 |
R = 22 NR = 6 |
3.4. Prediction of response before treatment
All nine studies evaluated pre-treatment ADC for the prediction of response to treatment. Of these, only one study found a correlation between pre-treatment ADC and outcome (Bian et al., 2019). Bian et al showed that pre-therapy ADC was significantly higher in their good response group than in those with a poor response (p < 0.05). The remaining eight studies did not find any correlation between pre-treatment ADC and response.
In our meta-analysis, we did not find a correlation between pre-treatment ADC values and response, p = 0.48, as shown in Fig. 3.
Fig. 3.
Forest plot showing the Std Mean Difference in pre-treatment ADC values (×10−3 mm2/s) between responders (R) and non-responders (NR). Heterogeneity: Tau2 = 0.01; Chi2 = 4.54, I2 = 10%. Test for overall effect: Z = 0.70 (p = 0.48). Weights are from random effects model.
3.5. Prediction of response during treatment
Response prediction during treatment was reported in all nine studies and is summarized in Table 2. Two of these studies found that the absolute values of ADC measured early in the treatment regime, as well as the change in ADC from pre- to early therapy values both significantly correlated with response (Harry et al., 2008, Das et al., 2015). For Harry et al., the ADC values after 14 days of chemoradiation showed a correlation with eventual MR-measured response (p = 0.009, ρ = 0.448) as did the change in ADC at that time point (p = 0.01, ρ = 0.56) (Harry et al., 2008). Similarly, Das et al showed both significant correlations with ADC values early in the regime as well as in the change in ADC, (p = 0.01 and p = 0.03 respectively) (Das et al., 2015).
Four studies looked at the change in ADC from pre-therapy values to early-treatment values, and a significant correlation with response was observed, although this was not demonstrated with the absolute values (Kuang et al., 2014, Zhang et al., 2011, Makino et al., 2014, Liu, 2015).
Alternatively, the remaining three studies reported a significant correlation with the ADC values at the early treatment timepoint with response but did not use change in ADC as an indicator (Fu et al., 2012, Zhu, 2016, Bian et al., 2019). In these studies, we calculated the change in ADC between the 2 time points in the way previously described and used this percentage change in our analysis.
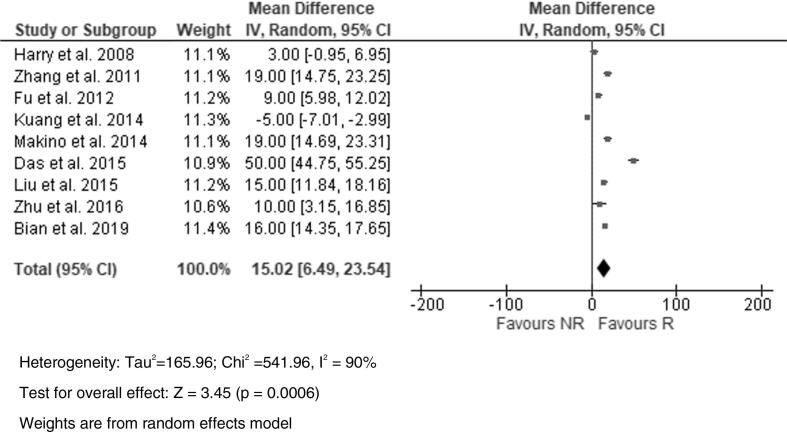
In our meta-analysis, there was a significant correlation with early treatment ADC values and response (Fig. 4) as well as a significant correlation with the percentage change in ADC (ΔADC) and response (Fig. 5). In addition, a higher mean percentage increase in ADC (49.7%) seen within the first 3 weeks of therapy was seen in responders compared to a mean percentage increase of 19.7% in non-responders (p = 0.016).
Fig. 4.
Forest plot showing the Std Mean Difference in early-treatment ADC values (x10−3mm2/s) between responders (R) and non-responders (NR).
Fig. 5.
Forest plot showing the Std Mean Difference in change in ADC values (ΔADC) percentage between responders (R) and non-responders (NR).
Only 3 of the included studies provided information on sensitivity and specificity of ADC in predicting response. Kuang et al found that a 21% increase in ADC after 2 weeks of chemoradiation had 85.7% sensitivity and 100% specificity in differentiating complete responders from partial responders and stable disease (Kuang et al., 2014). Das et al found their cut-off in ΔADC to be 0.48 for 70% sensitivity and 71% specificity for predicting response (Das et al., 2015). Similarly, Liu et al concluded that day 14 after therapy was the optimal time for ADC prediction of response and their cut-off of 35.4% for ΔADC at this time point was 100% sensitive and 73.1% specific for prediction of complete response (Liu, 2015). We were unable to assess an overall sensitivity and specificity of ADC or construct a ROC curve as only 3 studies provided this information.
4. Discussion
DWI has the ability to provide functional information on the microstructure of tissues by assessing the differences in water mobility within tumors and how this is impacted by treatment.
This meta-analysis systematically evaluated the role of DWI as a predictive biomarker of treatment response in patients with cervical cancer and found a statistically significant correlation between ADC values detected within three weeks of therapy as well as the change in ADC (ΔADC) at this time point with eventual response.
Pre-treatment ADC values however did not show the ability to predict response. Clearly, identification of a prognostic indicator before treatment would be valuable as this would guide initial treatment planning and therefore individualize therapy from the beginning.
In other tumors such as head and neck cancers, there have been promising results with pre-therapy ADCs (Camargo et al., 2017, Ellingson et al., 2014, Ravanelli et al., 2020, Palmisano et al., 2020), but this has not been borne out in the majority of studies with cervical carcinoma (MEYER et al., 2021). Similarly, this review has not detected any significant relationship between pre-therapy ADC and response.
This may be due to differences in pre-treatment cell density and other histopathological features that are detected by DWI and ADC, which may not necessarily influence treatment success (Surov et al., 2020). For instance, it is known that ADC values differ in squamous cell cancers and adenocarcinomas (Lin et al., 2015).
We however found significant correlation between ADC values documented early in the treatment regime as well as the percentage change in ADC at this time. In addition, the magnitude of the change was greater in responders than in non-responders.
Several other studies looking at different tumors have reported similar results (Amodeo et al., 2018, Vollenbrock et al., 2020, Chen et al., 2019) and it is likely that the relative change in ADC or percentage change in ADC is the more promising measure as this has the advantage that relative measurements suffer less from practice variations when compared to absolute values. DWI detects changes in the microstructure of biological tissues and ADC is sensitive to the dynamic motion of water particularly with treatment, hence the potential to be more useful as a marker of response during treatment, as opposed to before treatment.
The nine studies reviewed showed variable results both in the absolute value of ADCs and the magnitude of the changes in ADC, which may be explained by the techniques used, since diffusion values obtained from DWI and subsequent ADC mapping depend on the pulse sequence and post-processing methods used.
Unfortunately, we were unable to assess overall sensitivity and specificity of ADC since only 3 studies reviewed provided this data, and therefore we cannot provide a cut-off value to use to differentiate between responders and non-responders.
Our systematic review demonstrates that response prediction and assessment with quantification of ADC values on DWI in patients with cervical cancer can be challenging.
There were several limitations of this review. Firstly, as the analysis is based upon results of the literature search, there may be some degree of publication bias due to the trend of positive or significant reported results. Only articles in English Language were reviewed and two out of the nine studies were retrospectively designed, are also limiting factors.
A known challenge in interpreting quantitative measurements in these studies is the practice variations when dealing with image acquisitions, such a different field strengths (1.5T and 3T were used), different b-values used to calculate the ADC and different measurement techniques and ROI placement. For instance, some studies measured tumor ADC using single axial section while others used three-dimensional volume. Similarly, the experience of the radiologist is a crucial factor in evaluating these measurements and was not documented in all the studies.
The varying results of the included studies can also be explained by intrinsic tumor characteristics as well as different treatment regimes, as some patients received concurrent chemoradiation while others received neoadjuvant chemotherapy prior to radiotherapy.
Using the RECIST criteria for response may be viewed by some as a limitation as it may not be directly linked to clinical outcome, and it can be argued that changing a treatment regime not only depends on RECIST, but other factors such as FIGO stage, patient condition and metastatic disease. However, we feel that as the reference standard used to express response, and accepted in daily oncological and radiological practice, it remains an objective parameter.
The heterogeneity observed among the studies must also be considered. Different scanner technique, b-values, magnetic field strength and diverse methods of measuring ADC may contribute to this although it was not sufficient to prevent a meta-analysis from being performed. Differences in defining responders and non-responders will add to this as well, as the studies included were not completely uniform in the definition of complete responders.
Nonetheless, even though not all of the included studies reached significance individually, the change in ADC within three weeks of starting treatment showed a trend towards a larger increase in responders when compared to non-responders. This may be explained by the early effect of chemoradiation on the tumor microenvironment inducing necrosis and apoptosis, loss of cell membrane integrity, increase in extracellular space, lower cellularity, typically resulting in an increase in ADC.
However, we are unable to determine specific ADC cut-off values that can be applied in clinical practice on individualized patients. Larger prospective studies with accepted and standardized DWI sequences and consistent methods of ADC measurements are required to achieve comparable and functional results.
Also crucial would be studies to further evaluate DWI in predicting survival as some initial reports have suggested a role for this (Somoye et al., 2012), and in particular, reducing late relapse (Kalampokas et al., 2020).
5. Conclusion
The results of this systematic review and meta-analysis show that a change in ADC values, particularly a relative increase in ADC early in the treatment regime has the potential to predict response to treatment patients with cervical cancer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Harry V.N., Semple S.I., Gilbert F.J., Parkin D.E. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol. Oncol. 2008;111(2):213–220. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Dzik-Jurasz A., Domenig C., George M., Wolber J., Padhani A., Brown G., Doran S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360(9329):307–308. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- Šaňák D., Nosál′ V., Horák D., Bártková A., Zeleňák K., Herzig R., Bučil J., Školoudík D., Buřval S., Cisariková V., Vlachová I., Köcher M., Zapletalová J., Kurča E., Kaňovský P. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48(9):632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- Oppenheim C., et al. Do transient ischemic attacks with diffusion-weighted imaging abnormalities correspond to brain infarctions? AJNR Am. J. Neuroradiol. 2006;27(8):1782–1787. [PMC free article] [PubMed] [Google Scholar]
- Hayes C., Padhani A.R., Leach M.O. Assessing changes in tumour vascular function using dynamic contrast-enhanced magnetic resonance imaging. NMR Biomed. 2002;15(2):154–163. doi: 10.1002/nbm.756. [DOI] [PubMed] [Google Scholar]
- Padhani A.R. Functional MRI for anticancer therapy assessment. Eur. J. Cancer. 2002;38(16):2116–2127. doi: 10.1016/s0959-8049(02)00388-x. [DOI] [PubMed] [Google Scholar]
- Pickles M.D., Gibbs P., Lowry M., Turnbull L.W. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn. Reson. Imaging. 2006;24(7):843–847. doi: 10.1016/j.mri.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Chenevert T.L., Meyer C.R., Moffat B.A., Rehemtulla A., Mukherji S.K., Gebarski S.S., Quint D.J., Robertson P.L., Lawrence T.S., Junck L., Taylor J.M.G., Johnson T.D., Dong Q., Muraszko K.M., Brunberg J.A., Ross B.D. Diffusion MRI: a new strategy for assessment of cancer therapeutic efficacy. Mol. Imaging. 2002;1(4):336–343. doi: 10.1162/15353500200221482. [DOI] [PubMed] [Google Scholar]
- Chenevert T.L., et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J. Natl. Cancer Inst. 2000;92(24):2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- Hamstra D.A., Rehemtulla A., Ross B.D. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J. Clin. Oncol. 2007;25(26):4104–4109. doi: 10.1200/JCO.2007.11.9610. [DOI] [PubMed] [Google Scholar]
- Stejskal E.O., Tanner J.E. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965;42(1):288–292. [Google Scholar]
- Le Bihan D., Breton E., Lallemand D., Aubin M.L., Vignaud J., Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- Ross B.D., et al. Magnetic resonance imaging and spectroscopy: application to experimental neuro-oncology. Q. Magn. Reson. Biol. Med. 1994;1(2):89–106. [PMC free article] [PubMed] [Google Scholar]
- Ross B.D., et al. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol. Cancer Ther. 2003;2(6):581–587. [PubMed] [Google Scholar]
- Zhao M., Pipe J.G., Bonnett J., Evelhoch J.L. Early detection of treatment response by diffusion-weighted 1H-NMR spectroscopy in a murine tumour in vivo. Br. J. Cancer. 1996;73(1):61–64. doi: 10.1038/bjc.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.C., Moffat B.A., Schott A.F., Layman R., Ellingworth S., Juliar R., Khan A.P., Helvie M., Meyer C.R., Chenevert T.L., Rehemtulla A., Ross B.D. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin. Cancer Res. 2007;13(2):443–450. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang Y., Liang B., Yang Z. The utility of diffusion-weighted MR imaging in cervical cancer. Eur. J. Radiol. 2010;74(3):e101–e106. doi: 10.1016/j.ejrad.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Dashottar S., Pany T.P., Lohia N. Role of apparent diffusion coefficient as a biomarker in the evaluation of cervical cancer. Indian J. Radiol. Imaging. 2019;29(01):25–32. doi: 10.4103/ijri.IJRI_441_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameeduddin A., Sahdev A. Diffusion-weighted imaging and dynamic contrast-enhanced MRI in assessing response and recurrent disease in gynaecological malignancies. Cancer Imaging. 2015;15(1):3. doi: 10.1186/s40644-015-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang F., Yan Z., Wang J., Rao Z. The value of diffusion-weighted MRI to evaluate the response to radiochemotherapy for cervical cancer. Magn. Reson. Imaging. 2014;32(4):342–349. doi: 10.1016/j.mri.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Lee E.Y.P., Yu X., Chu M.M.Y., Ngan H.Y.S., Siu S.W.K., Soong I.S., Chan Q., Khong P.-L. Perfusion and diffusion characteristics of cervical cancer based on intraxovel incoherent motion MR imaging-a pilot study. Eur. Radiol. 2014;24(7):1506–1513. doi: 10.1007/s00330-014-3160-7. [DOI] [PubMed] [Google Scholar]
- McVeigh P.Z., Syed A.M., Milosevic M., Fyles A., Haider M.A. Diffusion-weighted MRI in cervical cancer. Eur. Radiol. 2008;18(5):1058–1064. doi: 10.1007/s00330-007-0843-3. [DOI] [PubMed] [Google Scholar]
- Yang W., Qiang J.W., Tian H.P., Chen B., Wang A.J., Zhao J.G. Multi-parametric MRI in cervical cancer: early prediction of response to concurrent chemoradiotherapy in combination with clinical prognostic factors. Eur. Radiol. 2018;28(1):437–445. doi: 10.1007/s00330-017-4989-3. [DOI] [PubMed] [Google Scholar]
- Zheng X., Guo W., Dong J., Qian L. Prediction of early response to concurrent chemoradiotherapy in cervical cancer: Value of multi-parameter MRI combined with clinical prognostic factors. Magn. Reson. Imaging. 2020;72:159–166. doi: 10.1016/j.mri.2020.06.014. [DOI] [PubMed] [Google Scholar]
- Meyer H.-J., Wienke A., Surov A. Pre-treatment apparent diffusion coefficient does not predict therapy response to radiochemotherapy in cervical cancer: a systematic review and meta-analysis. Anticancer Res. 2021;41(3):1163–1170. doi: 10.21873/anticanres.14873. [DOI] [PubMed] [Google Scholar]
- Amodeo S., Rosman A.S., Desiato V., Hindman N.M., Newman E., Berman R., Pachter H.L., Melis M. MRI-based apparent diffusion coefficient for predicting pathologic response of rectal cancer after neoadjuvant therapy: systematic review and meta-analysis. AJR Am. J. Roentgenol. 2018;211(5):W205–W216. doi: 10.2214/AJR.17.19135. [DOI] [PubMed] [Google Scholar]
- Vollenbrock S.E., Voncken F.E.M., Bartels L.W., Beets-Tan R.G.H., Bartels-Rutten A. Diffusion-weighted MRI with ADC mapping for response prediction and assessment of oesophageal cancer: a systematic review. Radiother. Oncol. 2020;142:17–26. doi: 10.1016/j.radonc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535. [PMC free article] [PubMed] [Google Scholar]
- Naganawa S., Sato C., Kumada H., Ishigaki T., Miura S., Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur. Radiol. 2005;15(1):71–78. doi: 10.1007/s00330-004-2529-4. [DOI] [PubMed] [Google Scholar]
- Duffaud F., Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull. Cancer. 2000;87(12):881–886. [PubMed] [Google Scholar]
- Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., Rubinstein L., Shankar L., Dodd L., Kaplan R., Lacombe D., Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Whiting P.F., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen J.-Y., Xie C.-M., Mo Y.-X., Liu X.-W., Liu Y.i., Wu P.-H. Diffusion-weighted magnetic resonance imaging for prediction of response of advanced cervical cancer to chemoradiation. J. Comput. Assist. Tomogr. 2011;35(1):102–107. doi: 10.1097/RCT.0b013e3181f6528b. [DOI] [PubMed] [Google Scholar]
- Fu C., Bian D., Liu F., Feng X., Du W., Wang X. The value of diffusion-weighted magnetic resonance imaging in assessing the response of locally advanced cervical cancer to neoadjuvant chemotherapy. Int J Gynecol Cancer. 2012;22(6):1037–1043. doi: 10.1097/IGC.0b013e31825736d7. [DOI] [PubMed] [Google Scholar]
- Makino H., Kato H., Furui T., Morishige K.-I., Kanematsu M. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for uterine cervical cancer. J. Obstet. Gynaecol. Res. 2014;40(4):1098–1104. doi: 10.1111/jog.12276. [DOI] [PubMed] [Google Scholar]
- Liu Y., et al. Time-window of early detection of response to concurrent chemoradiation in cervical cancer by using diffusion-weighted MR imaging: a pilot study. Radiat. Oncol. (Lond., Engl.) 2015;10:185. doi: 10.1186/s13014-015-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Chandramohan A., Rami Reddy J.K., Mukhopadhyay S., Kumar R.M., Isiah R., John S., Oommen R., Jeyaseelan V. Role of conventional and diffusion weighted MRI in predicting treatment response after low dose radiation and chemotherapy in locally advanced carcinoma cervix. Radiother. Oncol. 2015;117(2):288–293. doi: 10.1016/j.radonc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Zhu L., et al. Evaluating early response of cervical cancer under concurrent chemo-radiotherapy by intravoxel incoherent motion MR imaging. BMC Cancer. 2016;16:79. doi: 10.1186/s12885-016-2116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian H., Liu F., Chen S., Li G., Song Y., Sun M., Dong H. Intravoxel incoherent motion diffusion-weighted imaging evaluated the response to concurrent chemoradiotherapy in patients with cervical cancer. Medicine. 2019;98(46):e17943. doi: 10.1097/MD.0000000000017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo A., Schneider T., Liu L., Pakpoor J., Kleinberg L., Yousem D.M. Pretreatment ADC values predict response to radiosurgery in vestibular schwannomas. AJNR Am. J. Neuroradiol. 2017;38(6):1200–1205. doi: 10.3174/ajnr.A5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson B.M., Sahebjam S., Kim H.J., Pope W.B., Harris R.J., Woodworth D.C., Lai A., Nghiemphu P.L., Mason W.P., Cloughesy T.F. Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. AJNR Am. J. Neuroradiol. 2014;35(4):673–679. doi: 10.3174/ajnr.A3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanelli M., Grammatica A., Maddalo M., Ramanzin M., Agazzi G.M., Tononcelli E., Battocchio S., Bossi P., Vezzoli M., Maroldi R., Farina D. Pretreatment DWI with histogram analysis of the ADC in predicting the outcome of advanced oropharyngeal cancer with known human papillomavirus status treated with chemoradiation. AJNR Am. J. Neuroradiol. 2020;41(8):1473–1479. doi: 10.3174/ajnr.A6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano A., Di Chiara A., Esposito A., Rancoita P.M.V., Fiorino C., Passoni P., Albarello L., Rosati R., Del Maschio A., De Cobelli F. MRI prediction of pathological response in locally advanced rectal cancer: when apparent diffusion coefficient radiomics meets conventional volumetry. Clin. Radiol. 2020;75(10):798.e1–798.e11. doi: 10.1016/j.crad.2020.06.023. [DOI] [PubMed] [Google Scholar]
- Surov A., Wienke A., Meyer H.J. Pretreatment apparent diffusion coefficient does not predict therapy response to neoadjuvant chemotherapy in breast cancer. Breast. 2020;53:59–67. doi: 10.1016/j.breast.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Li H., Chen Z., Ni P., Zhong Q., Huang H., Sandrasegaran K. Correlation of histogram analysis of apparent diffusion coefficient with uterine cervical pathologic finding. AJR Am. J. Roentgenol. 2015;204(5):1125–1131. doi: 10.2214/AJR.14.13350. [DOI] [PubMed] [Google Scholar]
- Chen Y., Xie T., Ye Z., Wang F., Long D., Jiang M., Fang J., Lin Q., Li K., Wang Z., Fu Z. ADC correlation with Sirtuin1 to assess early chemoradiotherapy response of locally advanced esophageal carcinoma patients. Radiat. Oncol. 2019;14(1) doi: 10.1186/s13014-019-1393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somoye G., Harry V., Semple S., Plataniotis G., Scott N., Gilbert F.J., Parkin D. Early diffusion weighted magnetic resonance imaging can predict survival in women with locally advanced cancer of the cervix treated with combined chemo-radiation. Eur. Radiol. 2012;22(11):2319–2327. doi: 10.1007/s00330-012-2496-0. [DOI] [PubMed] [Google Scholar]
- Kalampokas E., Macdonald G., Young H., Bednarek A., Kennedy A.-M., Cairns M., Parkin D.E. Definitive chemoradiotherapy for cervical cancer: a 11-year population-based study. Eur. J. Cancer Care (Engl.) 2020;29(3) doi: 10.1111/ecc.v29.310.1111/ecc.13223. [DOI] [PubMed] [Google Scholar]