Highlights
-
•
PARP inhibitors (PARPi) reduce the risk of ovarian cancer progression and recurrence after response to platinum chemotherapy.
-
•
Rarely, PARPi are associated with increased risk of hematologic malignancies (MDS/AML) in some patients with ovarian cancer.
-
•
The causal relationship between PARPi and leukemogenesis is unclear but may be associated with cumulative chemotherapy.
-
•
We present 3 patients with germline BRCA-associated ovarian cancer and mutations potentially associated with risk of MDS/AML.
-
•
The clinical benefit of PARPi should be viewed with caution in patients with elevated baseline risk of developing MDS/AML.
Keywords: Ovarian cancer, BRCA mutation, PARP inhibitors, Myelodysplastic syndrome, Acute myeloid leukemia
Abstract
Poly(ADP-ribose) polymerase inhibitors (PARPi) are FDA approved as frontline maintenance for BRCA-associated advanced stage high-grade ovarian cancer (HGOC), having demonstrated an unprecedented improvement in relapse-free survival. Myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) are rare toxicities of PARPi. We describe three patients with germline BRCA-associated (gBRCA+) HGOC and alterations in AML driver genes. Although none evidenced overt hematologic malignancy, PARPi maintenance was cautiously considered given the potential risk of MDS/AML. A better understanding of the role of clonal hematopoiesis in the subsequent development of PARPi-associated MDS/AML will improve management of this patient population.
1. Introduction
Newly diagnosed high-grade ovarian cancer (HGOC) is treated with cytoreductive surgery and platinum-based chemotherapy (Moore et al., 2018). Approximately 70% of patients relapse within 3 years, ultimately dying of their disease. Poly(ADP-ribose) polymerase inhibitors (PARPi) are approved by the US Food and Drug Administration for HGOC. Maintenance PARPi have been shown to significantly reduce the risk of progression or recurrence following response to platinum-based chemotherapy (Moore et al., 2018). In the frontline setting for patients with germline alterations in BRCA1/2 (gBRCA+) HGOC, olaparib was associated with 70% lower risk of disease progression/death compared with placebo (hazard ratio [HR] 0.3, p < 0.001), and niraparib showed a similar benefit (HR 0.43, p < 0.001) (González-Martín et al., 2019, Moore et al., 2018). About 15% of HGOC is associated with gBRCA and homologous recombination repair deficiency (HRD). In HRD cells, PARP proteins are a critical component of DNA repair, acting on single-stranded breaks (Tutt et al., 2021). Suppression of PARP results in unrepaired single-stranded breaks, ultimately leading to replication fork collapse and double-strand breaks. Homologous recombination repair and PARP-dependent repair pathways are not independently essential in HGOC, but the loss of both leads to cell death (i.e., synthetic lethality). Synthetic lethality facilitates selective tumor cell death while minimizing toxicity to normal cells.
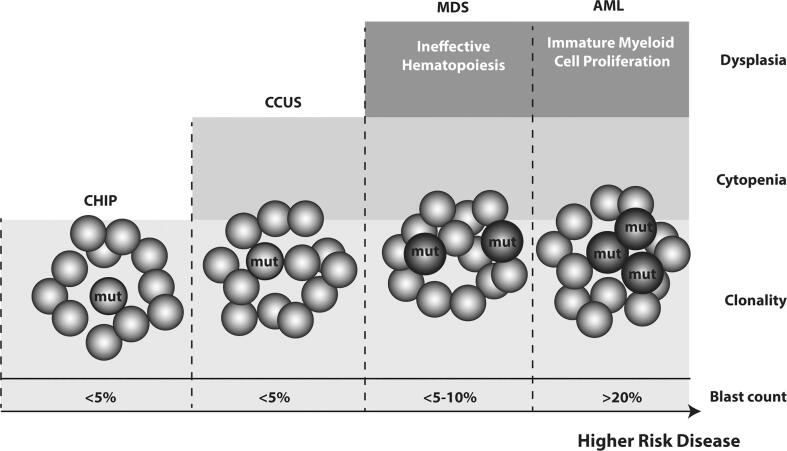
PARPi are associated with increased risk of secondary MDS/AML (Morice et al., 2021). The mechanism of PARPi-driven leukemogenesis is unclear and may be driven by previous chemotherapy exposure. PARPi may potentiate clonal hematopoiesis , selecting for clones with acquired mutations within the DNA damage response pathway, increasing the risk of treatment-related myeloid neoplasms (Bolton et al., 2020). Clonal hematopoiesis is the expansion of a clonal population of blood cells with one or more somatic mutations and is common in normal aging (Miller and Steensma, 2020). In patients exposed to cytotoxic therapy for non-myeloid malignancies, the rate of clonal hematopoiesis was 5–10% higher than age-matched controls with enrichment for DNA damage response pathway alterations. A clonal hematopoiesis clone may progress to clonal cytopenias of undetermined significance, MDS, and eventually AML (Fig. 1) (Bowman et al., 2018). Clonal hematopoiesis is associated with increased all-cause mortality and double the risk of atherosclerotic cardiovascular disease (Miller and Steensma, 2020).
Fig. 1.
The spectrum of clonal hematopoiesis from pre-neoplastic states to myeloid neoplasms. Pre-neoplastic states: clonal hematopoiesis of indeterminate potential (CHIP) is defined by the presence of somatic mutation(s) with variant allele frequency ≥ 2% without cytopenia; clonal cytopenias of undetermined significance (CCUS) has both a somatic variant and cytopenias. Myeloid neoplasms: myelodysplastic syndrome (MDS) is characterized by dysplasia (abnormal cellular morphology) on a bone marrow exam and peripheral cytopenia; acute myeloid leukemia (AML) further involves the excessive proliferation of blasts (immature myeloid cells) with somatic mutations blocking normal hematopoiesis.
Although rare, PARPi-associated MDS/AML is lethal. Increased baseline risk of MDS/AML poses challenges for frontline PARPi maintenance. Here we report three cases of patients with HGOC and alterations in MDS/AML driver genes (Table 1). gBRCA mutations were detected in all patients following cancer diagnosis. The patients had no personal or family history of hematologic malignancies.
Table 1.
Case characteristics. Germline BRCA1/2 (gBRCA1/2) mutations were detected in all patients after cancer diagnosis. Hematologic mutational profile was done on peripheral blood +/- bone marrow.
| BRCA status | Hematologic mutational profile | Hematologic diagnosis | PARPi maintenance | |
|---|---|---|---|---|
| Patient 1 | gBRCA1 | FLT3 A680V (VAF 49%) | Uncertain | Yes |
| Patient 2 | gBRCA1 | DNMT3A R882C (VAF 2.5%) | CHIP | No |
| Patient 3 | gBRCA2 |
TET2-G1370V (VAF 34%) TET2-L1899fs*8 (VAF 14.2%) |
CCUS | No |
VAF, variant allele frequency; CHIP, clonal hematopoiesis of indeterminate potential; CCUS, clonal cytopenias of undetermined significance
2. Case presentation
2.1. Patient 1
A 52-year-old woman who presented with a pelvic mass underwent optimal primary debulking surgery for stage IIIC HGOC. Family history was significant for breast cancer in her mother. Cycle 1 of postoperative chemotherapy with carboplatin/paclitaxel was complicated by eosinophilic gastroenteritis, which resolved with steroids. She was found to have gBRCA1 mutation and was considered for maintenance PARPi post-chemotherapy.
Hypereosinophilia (white blood cell (WBC) count 15.4 K/uL, eosinophils 62%) prompted further hematologic workup. Mutational analysis for hypereosinophilia-associated genetic aberrations (e.g., FIP1L1-PDGFRA fusion, PDGFRB, and JAK2 mutations) was unrevealing. At the time of HGOC diagnosis, she also had thrombocytosis (platelets [Plt] 992 K/uL) and leukocytosis (WBC 27 K/uL, absolute neutrophil count (ANC) 22.3 K/uL). To assess the risk for myeloproliferative neoplasm, targeted next-generation sequencing (NGS) on peripheral blood identified a FLT3 A680V variant (variant allele frequency 41.3%). A bone marrow evaluation showed FLT3 A680V (variant allele frequency 49%) with no other alterations, suggesting a germline heterozygous mutation. Flow cytometry and hematopathology were normal. Given negative MDS/AML workup and potential progression-free survival (PFS) benefit, the patient elected to begin PARPi maintenance. During the short interval follow-up of 3-months, she had not experienced toxicity or required dose reduction and remains on maintenance olaparib.
2.2. Patient 2
A 47-year-old woman with psoriatic arthritis on dexamethasone, and family history of HGOC in her mother and breast cancer in a maternal aunt, presented with a colonic perforation after colonoscopy. She underwent an exploratory laparotomy and was diagnosed with stage IVB HGOC. Subsequently, she had multiple surgical resections complicated by nonhealing abdominal wounds and bacteremia. She completed 8 cycles of carboplatin/paclitaxel with peg-filgrastim support. Cycles 3 and 5–7 were delayed for significant myelosuppression (nadir Plt 5 K/uL, hemoglobin 6.4 g/dL, WBC 3.7 K/uL). Therapeutic heparin for a history of superior mesenteric vein thrombus was stopped after cycle 3 due to thrombocytopenia. Folate supplementation was initiated, given significant gastrointestinal complications. Upon hematologic workup, NGS on peripheral blood was positive for gBRCA1 and clonal hematopoiesis with somatic DNMT3A R882C mutation (variant allele frequency 2.5%). The patient’s blood counts recovered, albeit slowly, after each chemotherapy cycle. The hematological derangements were attributed to a combination of chemotherapy, inflammation from recent infection, poor nutritional status, and decreased bone marrow reserve. Bone marrow evaluation was deferred. She was subsequently diagnosed with progressive HGOC and was no longer a candidate for PARPi maintenance.
2.3. Patient 3
A 57-year-old woman who presented with postmenopausal bleeding underwent surgical debulking for stage IIIC HGOC. She was found to have gBRCA2 mutation. Family history was significant for breast cancer in her mother, pancreatic cancer in her maternal grandmother, and prostate cancer in her father and paternal grandfather. She received postoperative carboplatin/paclitaxel and bevacizumab. Despite normal baseline blood counts, her treatment was complicated by persistent, prolonged neutropenia, requiring chemotherapy dose reduction and delays. Bone marrow evaluation showed normal cellularity with trilineage maturing hematopoiesis. Although few erythroid elements with dyspoietic changes were seen, MDS diagnostic criteria were not met. Molecular analysis was notable for TET2-G1370V (variant allele frequency 34%) and TET2-L1899fs*8 (variant allele frequency 14.2%). Cytogenetics showed a 20q deletion in 3/20 cells. Flow cytometry was negative for B- or T-cell clonal expansion and increased CD34 + blasts. These findings were consistent with cytopenias of undetermined significance. Given this diagnosis, the patient elected to continue close observation in lieu of PARPi maintenance. The cytopenias of undetermined significance will be monitored with blood counts every 3 months, and peripheral blood sequencing in 6 months.
3. Discussion
PARPi have transformed the management of advanced HGOC, having demonstrated significant improvement in PFS (González-Martín et al., 2019, Moore et al., 2018). Although overall survival data with frontline PARPi maintenance is not yet mature, there is potential for enhanced survival and even cure. However, a recent meta-analysis of 28 randomized clinical trials of PARPi in various cancers, mostly HGOC, demonstrated significantly increased risk of MDS/AML compared with placebo (OR 2.63, p = 0.026) (Morice et al., 2021). It remains unclear whether increased MDS/AML is a result of exposure to prior chemotherapy and/or PARPi. Data in gBRCA-mutated breast cancer with PARPi maintenance was not associated with the same magnitude of risk for MDS/AML (Tutt et al., 2021). Since BRCA mutations predispose patients to multiple cancer types that generally have an increased sensitivity to platinum-based agents, patients with gBRCA-associated HGOC are more likely to have undergone treatment with platinum and alkylating agents. Platinum chemotherapy specifically appears to cause clonal expansion that can lead to leukemia (Coorens et al., 2021). The platinum effect is suggested by the difference in the observed rates of MDS/AML on PARPi in newly diagnosed vs. relapsed HGOC (<1.5% vs. 8%) and the 4% rate of MDS/AML in the placebo group with relapsed disease (Poveda et al., 2021). Bolton et al failed to show a difference in the risk increase of DNA damage response clonal hematopoiesis, which can progress to leukemia, in patients on PARPi compared with those receiving other systemic therapies or radiation, after accounting for cumulative exposure to chemotherapy (Bolton et al., 2020). Yet, when studied prospectively, the DNA damage response clonal hematopoiesis rate was higher after PARPi exposure compared with the rate after other cytotoxic therapies. The median latency period from exposure to PARPi to treatment-related myeloid neoplasms following platinum or alkylating agents was 17.8 months, with a median exposure duration of 9.8 months, compared with the mean latency period of 5.3/5.1 years and 4.5/3.8 years for MDS and AML, respectively (Morice et al., 2021). Taken together, this would seem to indicate that cumulative exposure to chemotherapy in the setting of recurrent BRCA-associated cancers at least contributes to predisposition to treatment-related myeloid neoplasms in patients on PARPi for HGOC. This warrants further investigation.
Treatment-related myeloid neoplasms was a significant concern for these three patients with possible baseline risk of hematologic malignancy (Table 2). Patient 1 had unexplained hypereosinophilia, thrombocytosis, leukocytosis, and a FLT3 A680V (variant allele frequency 41.3%) alteration in peripheral blood. Bone marrow analysis showed FLT3 A680V mutation (variant allele frequency 49%) with otherwise normal morphology. FLT3 mutations occur in about 30% of AML cases, with the FLT3 internal tandem duplication (FLT3-ITD) responsible for 25% of all AML cases (Daver et al., 2019). Scant data exist on the significance of FLT3 A680V, an alteration in tyrosine kinase domain 1 (TKD1), rather than in TKD2 where most pathogenic FLT3 non-ITD mutations are found. In vitro, somatic FLT3 A680V resulted in auto-phosphorylation of FLT3, demonstrating dysregulated kinase activation and possible oncogenic capacity (Tarlock et al., 2015). Alterations with high variant allele frequency (40–60%) are often germline but may also be due to clonal hematopoiesis progression, most likely presenting as overt hematologic malignancy. Predisposition to AML in the context of germline mutations remains poorly understood, and cannot be excluded (SONG, 2017). Patient 2 had persistent and prolonged cytopenias; peripheral blood NGS was positive for clonal hematopoiesis with somatic DNMT3A R882C mutation (variant allele frequency 2.5%). DNMT3A is one of the most frequently mutated genes in hematological malignancies (Bowman et al., 2018). DMNT3A mutations are highly age-related, commonly present in clonal hematopoiesis with an average variant allele frequency of 14.3% and are usually benign. Importantly, DNMT3A R882 is a hotspot mutation with a higher prevalence in MDS (23%) and AML (58%) compared with the normal population. The R882 mutant was shown to act as a dominant-negative inhibitor of wild-type DNMT3A in vitro.
Table 2.
Relevant genes that are frequently mutated in AML (Di Nardo and Cortes, 2016).
| Gene | Functional class | Functional change | Mutation frequency | Common pathogenic mutations |
|---|---|---|---|---|
| FLT3 | Signaling and kinase pathway | Gain of function | ∼ 30% of AML | FLT3-ITD, activating TKD point mutations |
| DNMT3A | Epigenetic modification | Loss of function | ∼20% of de novo AML | R882 missense mutation |
| TET2 | Epigenetic modification | Loss of function | ∼30% of MDS; ∼10% of AML; ∼50% in CMML |
Throughout the gene |
FLT3, FMS-like tyrosine kinase 3; FLT3-ITD, FLT3-internal tandem duplication; TKD, tyrosine kinase domain; DNMT3A, DNA methyltransferase 3A; TET2, Tet methylcytosine dioxygenase 2; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CMML, chronic myelomonocytic leukemia
Patient 3 had clonal hematopoiesis with a clinically significant missense mutation TET2 G1370V (variant allele frequency 34%) and a frameshift mutation TET2 L1899fs*8 (variant allele frequency 14.2%) identified on bone marrow evaluation for persistent neutropenia. Reported in a range of hematopoietic cancers, TET2 mutations are also commonly seen in normal aging and are associated with a modest neutropenic effect (−6.73%, p = 0.017) (Bowman et al., 2018). For variant allele frequency > 10%, neutropenic effect (−9.15%, p = 0.012) and thrombocytopenia (−5.73%, p = 0.033) are increased. This could explain neutropenia in our patient; however, the significance of a 20q deletion in the absence of bone marrow myelodysplasia is unclear. Ultimately, the patient was diagnosed with cytopenias of undetermined significance, but not MDS, as she had no bone marrow myelodysplasia.
DMNT3A and TET2 are among the most commonly mutated genes in clonal hematopoiesis (Bowman et al., 2018). The absolute risk of clonal hematopoiesis progressing to a hematologic neoplasm is between 0.5 and 1% per year. Aging, chemotherapy, and genotoxic stress can serve as selective pressures on somatic variants. Although PARPi increases the risk of clonal hematopoiesis (Bolton et al., 2020), PARP inhibition also has the potential to treat certain clonal hematopoiesis (Maifrede et al., 2020). Within the DNA damage response pathway, TET2-mutant cells downregulated BRCA-mediated homologous recombination and mostly relied on the PARP-mediated DNA repair, while DMNT3A-mutant cells favored BRCA-mediated homologous recombination repair with downregulation of the PARP-mediated repair pathway. Consequently, when exposed to PARPi in vitro and animal models, TET2-mutants showed PARPi-sensitivity, but DMNT3A-mutants were resistant. That said, treatment-related AML with a TET2-mutant clone expansion following PARPi has also been previously reported (Martin et al., 2020).
We hypothesize that extensive exposure to platinum and alkylating agents contributes to the increased risk of treatment-related myeloid neoplasms in the setting of PARPi maintenance in patients with BRCA-associated HGOC, who are more likely to have received multiple lines of chemotherapy. The causal relationship between PARPi and leukemogenesis is unclear. An unbiased, systematic evaluation of cumulative chemotherapy exposure and the true incidence of MDS/AML in the setting of PARPi in patients with BRCA-associated cancer could facilitate a better understanding of risk factors that predispose to treatment-related myeloid neoplasms. This could help prevent such neoplasms by avoiding PARPi in the highest-risk patients, while providing frontline PARPi to patients who are most likely to benefit from it. Insufficient data exist to make recommendations, and further work is needed to ensure that patients who are candidates for PARPi are not prematurely denied a potentially curative treatment.
Informed consent
Informed consent was obtained from the patients for the publication of this report.
Funding
This study was supported in part by the NIH/NCI Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748
Author contributions
Conceptualization, Data Curation, Writing-original draft: A.N., D.H.A., K.C., R.E.O
Writing-review and editing, Approved the final version: All authors
CRediT authorship contribution statement
Anastasia Navitski: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Duaa H. Al-Rawi: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Ying Liu: Writing – review & editing. Maria M. Rubinstein: Writing – review & editing. Claire F. Friedman: Writing – review & editing. Raajit K. Rampal: Writing – review & editing. Diana L. Mandelker: Writing – review & editing. Karen Cadoo: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Roisin E. O’Cearbhaill: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bolton K.L., Moukarzel L.A., Ptashkin R., Gao T., Patel M., Caltabellotta N., Braunstein L.Z., Aghajanian C., Hyman D.M., Berger M.F., Diaz L.A., Li B.T., Abida W., Schram A.M., Weigelt B., Friedman C.F., Zehir A., Papaemmanuil E., Cadoo K.A., Levine R.L. The impact of poly ADP ribose polymerase (PARP) inhibitors on clonal hematopoiesis. J. Clin. Oncol. 2020 doi: 10.1200/jco.2020.38.15_suppl.1513. [DOI] [Google Scholar]
- Bowman R.L., Busque L., Levine R.L. Clonal Hematopoiesis and Evolution to Hematopoietic Malignancies. Cell Stem Cell. 2018 doi: 10.1016/j.stem.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorens T.H.H., Collord G., Lu W., Mitchell E., Ijaz J., Roberts T., Oliver T.R.W., Burke G.A.A., Gattens M., Dickens E., Nangalia J., Tischkowitz M., Anderson J., Shlien A., Godfrey A.L., Murray M.J., Behjati S. Clonal hematopoiesis and therapy-related myeloid neoplasms following neuroblastoma treatment. Blood. 2021 doi: 10.1182/blood.2020010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daver N., Schlenk R.F., Russell N.H., Levis M.J. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019 doi: 10.1038/s41375-018-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo C.D., Cortes J.E. Mutations in AML: Prognostic and therapeutic implications. Hematology. 2016 doi: 10.1182/asheducation-2016.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Martín A., Pothuri B., Vergote I., DePont Christensen R., Graybill W., Mirza M.R., McCormick C., Lorusso D., Hoskins P., Freyer G., Baumann K., Jardon K., Redondo A., Moore R.G., Vulsteke C., O’Cearbhaill R.E., Lund B., Backes F., Barretina-Ginesta P., Haggerty A.F., Rubio-Pérez M.J., Shahin M.S., Mangili G., Bradley W.H., Bruchim I., Sun K., Malinowska I.A., Li Y., Gupta D., Monk B.J. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019 doi: 10.1056/nejmoa1910962. [DOI] [PubMed] [Google Scholar]
- Maifrede S., Le B.V., Nieborowska-Skorska M., Golovine K., Sullivan-Reed K., Dunuwille W.M.B., Nacson J., Hulse M., Caruso L.B., Gazze Z., Lian Z., Padella A., Chitrala K., Bartholdy B., Matlawska-Wasowska K., Di Marcantonio D., Simonetti G., Greiner G., Sykes S.M., Valent P., Paietta E.M., Fernandez H.F., Tallman M.S., Litzow M., Minden M.D., Huang J., Martinelli G., Vassiliou G.S., Tempera I., Piwocka K., Johnson N., Challen G., Skorski T. TET2 and DNMT3A Mutations Exert Divergent Effects on DNA Repair and Sensitivity of Leukemia Cells to PARP Inhibitors. Blood. 2020 doi: 10.1182/blood-2020-137658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.-E., Khalife-Hachem S., Grinda T., Kfoury M., Garciaz S., Pasquier F., Vargaftig J., Uzunov M., Belhabri A., Bertoli S., Auger N., Saada V., Guillouf C., Antony-Debre I., Rouleau E., Salviat F., Caron O., Pautier P., Etienne G., Vey N., Rosselli F., De Botton S., Leary A., Marzac C., Micol J.-B. Therapy Related Myeloid Neoplasm Post PARP Inhibitors: Potential Clonal Selection. Blood. 2020 doi: 10.1182/blood-2020-139971. [DOI] [PubMed] [Google Scholar]
- Miller, P.G., Steensma, D.P., 2020. Implications of Clonal Hematopoiesis for Precision Oncology. JCO Precis. Oncol. Doi: 10.1200/po.20.00144. [DOI] [PubMed]
- Moore K., Colombo N., Scambia G., Kim B.-G., Oaknin A., Friedlander M., Lisyanskaya A., Floquet A., Leary A., Sonke G.S., Gourley C., Banerjee S., Oza A., González-Martín A., Aghajanian C., Bradley W., Mathews C., Liu J., Lowe E.S., Bloomfield R., DiSilvestro P. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018 doi: 10.1056/nejmoa1810858. [DOI] [PubMed] [Google Scholar]
- Morice P.M., Leary A., Dolladille C., Chrétien B., Poulain L., González-Martín A., Moore K., O’Reilly E.M., Ray-Coquard I., Alexandre J. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021 doi: 10.1016/S2352-3026(20)30360-4. [DOI] [PubMed] [Google Scholar]
- Poveda A., Floquet A., Ledermann J.A., Asher R., Penson R.T., Oza A.M., Korach J., Huzarski T., Pignata S., Friedlander M., Baldoni A., Park-Simon T.W., Tamura K., Sonke G.S., Lisyanskaya A., Kim J.H., Filho E.A., Milenkova T., Lowe E.S., Rowe P., Vergote I., Pujade-Lauraine E., Byrski T., Pautier P., Harter P., Colombo N., Scambia G., Nicoletto M., Nussey F., Clamp A., Penson R., Poveda Velasco A., Rodrigues M., Lotz J.P., Selle F., Ray-Coquard I., Provencher D., Prat Aparicio A., Vidal Boixader L., Scott C., Yunokawa M., Medioni J., Pécuchet N., Dubot C., De La Motte Rouge T., Kaminsky M.C., Weber B., Lortholary A., Parkinson C., Ledermann J., Williams S., Banerjee S., Cosin J., Hoffman J., Plante M., Covens A., Sonke G., Joly F., Hirte H., Amit A., Matsumoto K., Tjulandin S., Hoon Kim J., Gladieff L., Sabbatini R., O’Malley D., Timmins P., Kredentser D., Laínez Milagro N., Barretina Ginesta M.P., Tibau Martorell A., De Liaño G., Lista A., Ojeda González B., Mileshkin L., Mandai M., Boere I., Ottevanger P., Nam J.H., Filho E., Hamizi S., Cognetti F., Warshal D., Dickson-Michelson E., Kamelle S., McKenzie N., Rodriguez G., Armstrong D., Chalas E., Celano P., Behbakht K., Davidson S., Welch S., Helpman L., Fishman A., Bruchim I., Sikorska M., Słowińska A., Rogowski W., Bidziński M., Śpiewankiewicz B., Casado Herraez A., Mendiola Fernández C., Gropp-Meier M., Saito T., Takehara K., Enomoto T., Watari H., Choi C.H., Kim B.G., Weon Kim J., Hegg R. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021 doi: 10.1016/S1470-2045(21)00073-5. [DOI] [PubMed] [Google Scholar]
- SONG, S., 2017. Abstract 3565: Recurrently detected germline mutation of FLT3 (D358V) associated with prevalence of hematopoietic malignancy and prognosis of AML patients. Cancer Research. Doi: 10.1158/1538-7445.am2017-3565.
- Tarlock K., Hansen M.E., Hylkema T., Ries R., Farrar J.E., Auvil J.G., Gerhard D.S., Smith M.A., Davidsen T.M., Gesuwan P., Hermida L.C., Marra M.A., Mungall A.J., Mungall K., Ma Y., Zong S., Long W., Boggon T., Alonzo T.A., Kolb E.A., Gamis A.S., Meshinchi S. Discovery and Functional Validation of Novel Pediatric Specific FLT3 Activating Mutations in Acute Myeloid Leukemia: Results from the COG/NCI Target Initiative. Blood. 2015;126:87. doi: 10.1182/blood.V126.23.87.87. [DOI] [Google Scholar]
- Tutt A.N.J., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., Gelber R.D., de Azambuja E., Fielding A., Balmaña J., Domchek S.M., Gelmon K.A., Hollingsworth S.J., Korde L.A., Linderholm B., Bandos H., Senkus E., Suga J.M., Shao Z., Pippas A.W., Nowecki Z., Huzarski T., Ganz P.A., Lucas P.C., Baker N., Loibl S., McConnell R., Piccart M., Schmutzler R., Steger G.G., Costantino J.P., Arahmani A., Wolmark N., McFadden E., Karantza V., Lakhani S.R., Yothers G., Campbell C., Geyer C.E. Adjuvant Olaparib for Patients with BRCA1 - or BRCA2 -Mutated Breast Cancer. N. Engl. J. Med. 2021 doi: 10.1056/nejmoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]