Highlights
-
•
Metastatic vaginal cancer, rare cancer with limited treatment options.
-
•
Pembrolizumab and radiotherapy led to complete response in a patient with metastatic vaginal cancer.
-
•
Wall-eyed bilateral internuclear ophthalmoplegia, a new immune related adverse event.
Keywords: Pembrolizumab, Vaginal squamous cell carcinoma, Radiotherapy, Immune related adverse events, Wall-eyed internuclear ophthalmoplegia, Checkpoint inhibitors
Abstract
Primary vaginal cancer is a rare malignancy with a lack of international guidelines and supporting clinical trial evidence to guide decision making. Historical results have shown poor outcomes with chemotherapy for stage IVB vaginal squamous cell carcinoma (SCC). The evolving role of checkpoint inhibitors in rare gynaecological cancers prompted us to investigate the role of pembrolizumab in this setting. The efficacy of pembrolizumab in vaginal SCC has never been investigated in any clinical trial. There is established data to support the use of concurrent chemoradiotherapy in gynaecological cancers, however, the data for concurrent use of immunotherapy and radiotherapy is still lacking but is the subject of several clinical trials. We herein present the first reported case of chemotherapy refractory vaginal SCC with complete response to pembrolizumab and concurrent pelvic radiotherapy. We also present wall-eyed bilateral internuclear ophthalmoplegia (WEBINO) as a rare but new immune related adverse event.
1. Introduction
Primary vaginal cancer (PVCa) is a rare malignancy with 17,600 cases reported worldwide in 2018 (https://gco.iarc.fr/today/data/factsheets/cancers/22-Vagina-fact-sheet.pdf, 2021). Squamous cell carcinoma (SCC) accounts for the majority of vaginal cancers although rarer histological subtypes including melanoma, sarcoma and adenocarcinoma also occur. PVCa has the same risk factors as cervical cancer and is likely mediated by human papillomavirus (HPV) infection in about 66–80% of cases (Daling, 2002, Creasman et al., xxxx, Bertoli, 2019). Studies have shown that the prevalence for p16 positivity in vaginal SCC is around 72%, but higher at 89% in HPV positive cases (Bertoli, 2019). Fluorine-18-labeled fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) imaging is recommended for staging as its sensitivity for detecting PVCa and involved pelvic lymph nodes is greater than that of CT alone (Lamoreaux, 2005). For early-stage disease, surgery or definitive radiotherapy (RT) is recommended with reportedly similar but variable 5-year survival rates of 35–92% (Lee, 2013). Several small case series have reported poor outcomes for International Federation of Gynaecology and Obstetrics (FIGO) stage IV vaginal cancer following RT with a 5-year survival of 0% (Mock, 2003, Prempree and Amornmarn, 1985). For patients with stage IVB vaginal SCC, platinum-based chemotherapy is commonly used as first-line treatment despite limited evidence to support its efficacy (Thigpen, 1986). Given its rarity, no randomized controlled trials have been performed for vaginal cancer, instead, the treatment approach is largely extrapolated from cervical cancer management. However, vaginal SCC is a distinct entity with contrasting outcomes and varied treatment response when compared to cervical cancer necessitating an urgent need for novel therapies. Immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) are an exciting new class of cancer therapeutics that have shown promising preliminary activity in gynaecological cancers. Pembrolizumab, a humanised monoclonal anti-PD-1 antibody, has been approved by the United States Food and Drug Administration (FDA) for patients with PD-L1 expressing (Combined positive score (CPS) ≥ 1) recurrent or metastatic cervical cancer with disease progression on or after chemotherapy (Keytruda, 2020). Recently published phase II trials have reported encouraging efficacy of checkpoint inhibitors in a variety of rare gynaecological cancers including vaginal SCC, albeit in a handful of cases (Naumann, 2019, Klein et al., 2020).
PD-L1 positivity (>5% of the tumor cells) is observed in nearly 28–54%, 33–64% and 47% in cervical, vulvar and vaginal SCCs, respectively (Gadducci and Guerrieri, xxxx, Thangarajah, 2019, Winer et al., 2018). Efforts to improve outcomes with ICIs has led to a multimodality treatment approach combining them with RT to exploit their synergistic potential with a view to augmenting both local and systemic responses thereby resulting in improvement in overall disease control and survival. Several recent preclinical and clinical studies have elegantly demonstrated that the combination of RT and ICIs could be more potent than either treatment alone. RT may augment the immunogenicity of ICIs either through direct cytotoxic anti-tumour effect or by inducing multiple immunomodulatory changes in the tumour microenvironment eventually leading to immunogenic tumour cell death (Walle, et al., 2018).
We herein present the first reported case of chemotherapy refractory vaginal SCC with complete response (CR) to pembrolizumab and concurrent pelvic RT. We also report wall-eyed bilateral internuclear ophthalmoplegia (WEBINO) as a rare neurological immune related adverse event (irAE) which has never previously been reported.
2. Methods
This retrospective case study was performed at Tawam Hospital, United Arab Emirates. No evidence of disease (NED) was defined as PETCT showing no FDG avid lesions.
2.1. Ethics approval
This study was approved by Tawam Human Research Ethics Committee (T-HREC) at Tawam Hospital on 16th December 2020, reference no. MF2058-2020–762
2.2. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
2.3. Case report
A 45-year-old, multiparous, pre-menopausal female patient presented with a 2-week history of blood-stained vaginal discharge. She gave a previous history of vaginal wart excision 12 months prior for a benign squamous papilloma with low grade dysplasia. She was a life-long non-smoker and her Eastern Cooperative Oncology Group (ECOG) performance status was 1. Her past medical history and family history were unremarkable. On examination, she was found to have large vaginal warts occupying the anterior, right lateral and posterior vaginal walls. Cervical examination was normal and a Pap smear was negative for malignancy. Magnetic Resonance Imaging (MRI) of the pelvis revealed a large, circumferential vaginal tumour measuring 10 × 8 cm over the anterior and the posterior vaginal walls along with right iliac lymphadenopathy measuring 5.5 × 3.5 cm. Surgical excision of the vaginal warts was performed and histology confirmed this to be a moderately differentiated SCC of the vagina with focal keratinization. Immunohistochemistry (IHC) for P16 was negative and Ki-67 showed full-thickness positivity. IHC for Mismatch Repair proteins showed no loss of nuclear expression with low probability of microsatellite instability-high. PD-L1 IHC staining using Dako 22C3 assay showed combined positive score of 50. Tumour cells were negative for HPV 16 and 18. PET-CT scan revealed an intensely FDG avid vaginal tumour along with multiple liver metastases and right external iliac lymphadenopathy; FIGO stage IVB. Ultrasound-guided biopsy of the liver confirmed this to be metastatic SCC consistent with the vaginal origin. IHC was positive for CK (cytokeratin) 5/6 and P63 but negative for P16.
She was treated with carboplatin using an area under the curve of 5 and paclitaxel 175 mg/m2 intravenously (IV) every 21 days for 3 cycles. Unfortunately, post-chemotherapy PET-CT scan revealed disease progression with multiple new liver metastases, lung metastases and para-aortic lymphadenopathy (Fig. 1A-C). She developed multiple disease-related symptoms including abdominal pain and vaginal bleeding; and consequently, her ECOG performance status deteriorated to 2. Solid tumour panel sequencing of the liver biopsy tissue identified pathogenic variants in the PTEN ((c.388C > T p.(Arg130*)), GNAS (GNAS, c.2531G > A p.(Arg844His)), and NOTCH1 ((NOTCH1, c.3186G > A p.(Trp1062*)) genes. Although, these variants are considered driver mutations of cancer development, there are no specific PTEN, GNAS and/or NOTCH1-biomarker directed therapies for vaginal neoplasms currently.
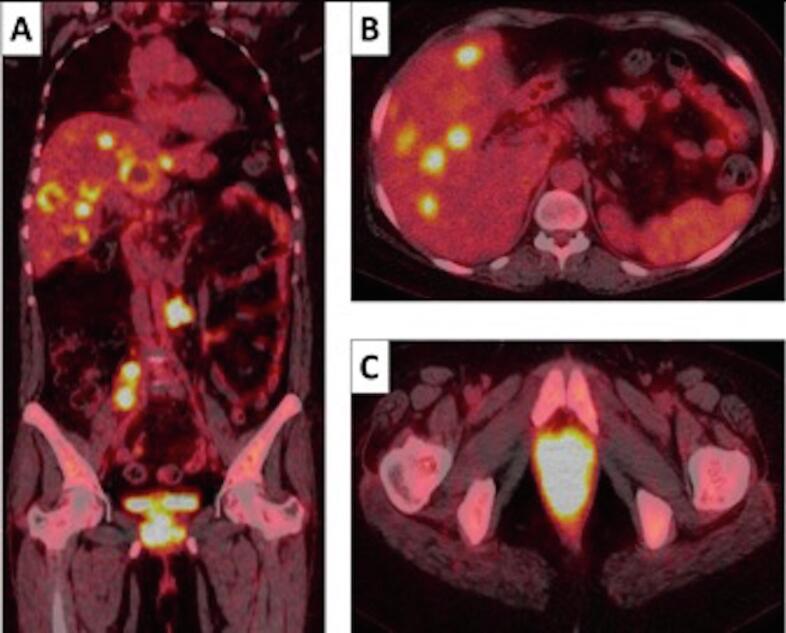
Fig. 1.
A-C: PET-CT scan images coronal (A), axial abdomen (B) and axial pelvis (C)- demonstrating locally advanced FDG avid Vaginal Carcinoma SUV max 18 along with FDG-avid right common iliac and left para-aortic lymphadenopathy and multiple liver metastases.
Extrapolating from cervical cancer management where pembrolizumab is a preferred second-line treatment option for PD-L1 positive patients, she was commenced on pembrolizumab 200 mg IV every 3 weeks. Six-weeks later, she noticed a significant improvement in her symptoms and her performance status improved to 0. PET-CT scan following 9-weeks of pembrolizumab showed CR in the liver and lung along with a partial response of the PVCa with an isolated focus of residual FDG activity (Fig. 2A-C). She then received radical RT- 45 Gy in 25 fractions to the PVCa and pelvic nodes + tumour bed boost of 5.4 Gy in 3 fractions followed by 2 sessions of high-dose rate brachytherapy boost (10 Gy each) concurrently with 3-weekly pembrolizumab, conceding the lack of prospective data for the use of this combination in such a clinical scenario. A PET-CT scan 6-weeks following completion of RT demonstrated a CR.
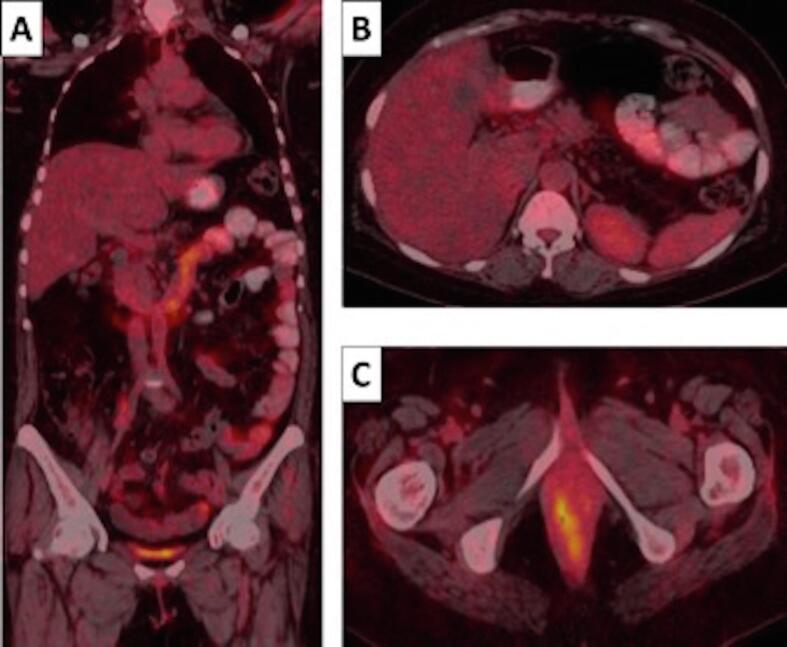
Fig. 2.
A-C: PET-CT scan images coronal (A), axial abdomen (B) and axial pelvis (C) following 9-weeks of pembrolizumab - demonstrating complete response of the liver metastases, right common iliac and left para-aortic lymphadenopathy but with low grade FDG uptake at the primary vaginal cancer.
Unfortunately, following 27-weeks of pembrolizumab, she presented with progressively worsening binocular diplopia. Ophthalmic examination revealed primary gaze exotropia and bilateral adduction impairment on conjugate horizontal eye movements (Fig. 3A-C). There was no evidence of nystagmus. Visual acuity and bilateral fundi examination was normal. There was no fatigability of eye movements and eyelids. The cover/uncover test showed alternating exotropia (the abducted eye re-fixates inwards when the contralateral eye is covered) (Fig. 4A and 4B). MRI of the orbit, brain and brainstem showed no evidence of metastases, ischaemic change, demyelination or orbital myositis. The diagnosis of wall-eyed bilateral internuclear ophthalmoplegia (WEBINO) as an irAE was made after excluding all other possible causes. Pembrolizumab was discontinued and she was treated with prednisolone 1 mg/kg orally daily. Steroid-related side effects included an episode of diabetic ketoacidosis needing intensive care admission and proximal myopathy. She also developed immune-related hypothyroidism after the second cycle of pembrolizumab necessitating thyroxine replacement.
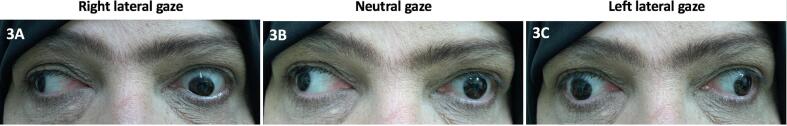
Fig. 3.
A-C: Wall-eyed bilateral internuclear ophthalmoplegia demonstrated by conjugate eye movement abnormalities in right lateral (A), neutral (B) and left lateral (C) gaze. Eye movements demonstrate primary gaze exotropia and bilateral adduction impairment on lateral gaze.
Fig. 4.
A and 4B: Cover/uncover test showing alternating exotropia. In primary gaze the left eye is abducted (A), however, once the right eye is covered (B) the left eye re-fixates inwards.
Within 7 months of receiving pembrolizumab, our patient achieved NED status and has remained with NED for 12 months following cessation of pembrolizumab with no FDG avid metastases on PET-CT along with a normal MRI Brain. On clinical assessment, her ECOG performance status is 0 and her conjugate eye movements have normalised albeit with a mild degree of residual binocular diplopia (Fig. 5A-C). She is currently on surveillance with 3-monthly PET-CT scans.
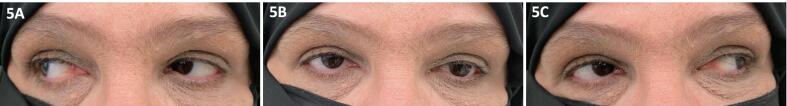
Fig. 5.
A-C: Improvement in conjugate eye movements in right lateral (A), neutral (B) and left lateral (C) gaze following treatment with high-dose corticosteroids and cessation of pembrolizumab.
3. Discussion
There is lack of high-quality data to inform decision-making with regards to systemic therapy for patients with FIGO stage IVB vaginal SCC. In the phase II Gynecologic Oncology Group trial of 16 patients with advanced or recurrent SCC of vagina, cisplatin at a dose of 50 mg/m2 IV every 3 weeks had insignificant activity with a response rate of 6% (9). There is currently no evidence to support second-line chemotherapy in patients with platinum-refractory SCC of vagina, therefore there is an urgent need to look for more effective therapies. ICIs have transformed the treatment paradigm of several cancers and have shown promising activity in several gynaecological malignancies. Pembrolizumab has already been approved by the FDA for cervical cancer based on the results of the pivotal Keynote-158 phase II trial, demonstrating an objective response rate of 14.3% in PD-L1–positive (combined positive score ≥ 1) recurrent/metastatic cervical cancer on or after chemotherapy (www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm6, 2021, Chung, 2019). There are no ongoing or completed trials for pembrolizumab in vaginal cancer. The efficacy and safety of nivolumab, a fully human immunoglobulin G4 PD-1 checkpoint inhibitor, was tested in the Checkmate 358 phase I/II trial (N = 24) for patients with recurrent or metastatic cervical, vaginal or vulvar carcinoma. Although the objective response rate was 26.3% for the cervical cancer cohort, the 2 patients with vaginal cancer did not respond to treatment with an 18-month overall survival (vaginal/vulvar cohort (n = 5)) of 20% (Naumann, 2019). Ipilimumab, an anti-CTLA-4 monoclonal antibody, has been tested in combination with nivolumab for this indication in the CA209-538 trial (12). In this trial, 41 patients with advanced rare gynaecological malignancies were treated with nivolumab (3 mg/kg) and ipilimumab (1 mg/kg) q3 weekly for four doses followed by maintenance nivolumab (3 mg/kg q2 weekly). Objective responses were noted in 28% of patients, including a CR in one of the three vaginal SCC patients. Although the above studies had limited number of vaginal SCC patients, the encouraging efficacy for PD-1 inhibitor monotherapy or its combination with a CTLA-4 inhibitor merits further evaluation.
Radical concurrent chemo-RT (CRT) is the standard of care for locally advanced cervical cancer but its role in vaginal SCC is not well defined. Miyamoto and colleagues, in their retrospective single-institution study (N = 71), demonstrated a potential improvement in overall survival and disease-free survival in favour of concurrent CRT as compared to RT alone for patients with PVCa (Miyamoto and Viswanathan, 2013). Several other smaller retrospective studies have reported on the efficacy and tolerability of CRT in PVCa with encouraging results (Dalrymple et al., 2004, Samant, 2007). There is now promising pre-clinical and clinical data for the synergistic combination of concurrent RT and ICIs in several cancers but its role in vaginal or cervical cancers has never been explored.
Our patient, despite having rapid disease progression on platinum-based chemotherapy, had an exceptional response to pembrolizumab, with CR of metastatic sites. The area of residual FDG avidity in the PVCa was targeted with concurrent radical RT leading to a CR. This treatment paradigm of combining ICIs and RT warrants further investigation in this setting, particularly for chemotherapy resistant tumours.
Despite the durable clinical benefits associated with checkpoint inhibitors, there are associated spectra of adverse effects, termed irAEs, reflecting a mechanism of action that is quite unique when compared to other systemic therapies. irAEs are typically transient and can affect any organ of the body, most commonly involving the skin and gastrointestinal tract; although other sites such as lungs, endocrine, musculoskeletal, renal, nervous, hematologic, cardiovascular, and ocular systems can also be affected. Neurologic irAEs are uncommon, with an overall incidence of 6% following treatment with anti-PD-1 antibodies (Puzanov, 2017, Dubey et al., 2019). Recognised neurological irAES include myasthenia gravis, Guillain-Barre syndrome, peripheral neuropathy, autonomic neuropathy, aseptic meningitis, encephalitis and transverse myelitis (Puzanov, 2017). WEBINO is a rare disorder of ocular motility that causes bilateral adduction deficits, bilateral abducting nystagmus, and exotropia in primary gaze (Wu et al., 2015). WEBINO is usually caused by the bilateral involvement of the medial longitudinal fasciculus by brainstem infarction or multiple sclerosis, however, other aetiologies include infection, vasculitis, trauma and metabolic syndromes. In our patient, all the above causes for WEBINO were excluded with serial MRI scans, negative autoimmune/ vasculitis screening and the absence of any metabolic abnormality or trauma. The patient’s eye movements normalised after discontinuation of pembrolizumab and following a prolonged course of high-dose steroids, in keeping with the typical resolution of neurological irAE. WEBINO has not been listed in the latest update of the Common Terminology Criteria for Adverse Events, version 5.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf, 2021). However, keeping in view that this is a severe/medically significant but not immediately life-threatening event and is disabling leading to limiting of self-care activities of daily living, this should be classified as a grade 3 toxicity. During ICI therapy there should be a high index of suspicion that any toxicity could be immune-related unless otherwise proven because prompt discontinuation of the immunological therapy and commencement of corticosteroids can be critical for symptom recovery (Brahmer et al., 2018). In a recent systematic review and meta-analysis of 30 studies involving 4324 patients receiving checkpoint inhibitors, the authors investigated the association between development of irAEs and cancer-related outcomes (Petrelli, 2020). This study demonstrated a reduction in risk of death (HR = 0.49, 95% CI 0.38–0.62; P < 0.001) and risk of progression (HR = 0.51, 95% CI:0.42–0.64; P < 0.001) for patients that developed irAEs. Several other studies have shown a positive correlation between irAEs and clinical outcomes. An exploratory study by Maher et al investigated 1741 advanced urothelial cancer patients receiving anti-PD-1/L1 antibodies, and demonstrated an improvement in overall survival for patients with irAEs compared to those without irAEs (HR, 0.45; 95% CI, 0.39 to 0.52) (Maher, 2019). Keynote-054, a phase III trial investigated the role of adjuvant pembrolizumab in 1019 patients with resected stage III melanoma and demonstrated that the occurrence of irAEs was associated with a longer recurrence free survival in the pembrolizumab arm (HR, 0.61; 95% CI, 0.39–0.95; P = 0.03) (Eggermont, 2020). The correlation between the occurrence and severity of irAEs to improvement in cancer-related outcomes has not been fully elucidated and needs further prospective investigation.
4. Conclusion
This is the first reported case of metastatic vaginal SCC with CR to pembrolizumab and concurrent RT. This case highlights the need for future studies of ICIs, particularly in PD-L1 positive patients, for this rare cancer. High degree of vigilance during ICI therapy is essential to promptly diagnose and manage rare irAEs such as the WEBINO reported here. Translation research is needed to identify appropriate biomarkers for ideal patient selection for ICIs in the future.
Funding
The authors did not receive any specific funding or sponsorship for this work.
Data availability
Data available on request from the authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- Bertoli H.K., et al. Human papillomavirus and p16 in squamous cell carcinoma and intraepithelial neoplasia of the vagina. Int. J. Cancer. 2019;145:78–86. doi: 10.1002/ijc.32078. [DOI] [PubMed] [Google Scholar]
- Brahmer, J.R., et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-68. https://doi.org/10.1200/jco.2017.77.6385. [DOI] [PMC free article] [PubMed]
- Chung H.C., et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2019 Jun 10;37(17):1470–1478. doi: 10.1200/jco.18.01265. [DOI] [PubMed] [Google Scholar]
- Creasman, W.T., Phillips, J.L., Menck, H.R. The National Cancer Data Base report on cancer of the vagina. Cancer 83(5):1033– 1040). doi: 10.1002/(SICI)1097-0142(19980901)83:5<1033::AID-CNCR30>3.0.CO;2-6. [PubMed]
- Daling J.R., et al. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol Oncol. 2002;84(2):263. doi: 10.1006/gyno.2001.6502. [DOI] [PubMed] [Google Scholar]
- Dalrymple, J.L., et al. Chemoradiation for primary invasive squamous carcinoma of the vagina. Int J Gynecol Cancer. 2004 Jan-Feb; 14(1):110-7. https://doi.org/10.1111/j.1048-891x.2004.014066.x. [DOI] [PubMed]
- Dubey D., David W.S., Amato A.A., Reynolds K.L., Clement N.F., Chute D.F., Cohen J.V., Lawrence D.P., Mooradian M.J., Sullivan R.J., Guidon A.C. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology. 2019;93(11) doi: 10.1212/wnl.0000000000008091. [DOI] [PubMed] [Google Scholar]
- Eggermont A.M.M., et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020;6(4):519. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadducci, A., Guerrieri, M.E. Immune Checkpoint Inhibitors in Gynecological Cancers: Update of Literature and Perspectives of Clinical Research. Anticancer Research, 37 (11) 5955-5965. https://doi.org/10.21873/anticanres.12042. [DOI] [PubMed]
- https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf (accessed 9th Sept 2021).
- https://gco.iarc.fr/today/data/factsheets/cancers/22-Vagina-fact-sheet.pdf (accessed 11th Sept 2021).
- Keytruda (pembrolizumab) injection for intravenous prescribing information, Merck and Co, Inc. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s084lbl.pdf. Accessed Nov 30th 2020.
- Klein O, et al. Combination immunotherapy with ipilumumab and nivolumab in patients with rare gynaecological malignancies. Journal of Clinical Oncology 2020 38:15_suppl, 6091-6091. https://www.doi.org/10.1200/jco.2020.38.15_suppl.6091.
- Lamoreaux W.T., et al. FDG-PET evaluation of vaginal carcinoma. Int J Radiat Oncol Biol Phys. 2005;62(3):733–737. doi: 10.1016/j.ijrobp.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Lee L.J., et al. ACR appropriateness criteria management of vaginal cancer. Oncology (Williston Park). 2013 Nov;27(11):1166–1173. doi: 10.1097/coc.0b013e318295af1d. [DOI] [PubMed] [Google Scholar]
- Maher V.E., et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J Clin Oncol. 2019;37(30):2730. doi: 10.1200/jco.19.00318. [DOI] [PubMed] [Google Scholar]
- Miyamoto D.T., Viswanathan A.N. Concurrent chemoradiation for vaginal cancer. PLoS One. 2013 Jun 7;8(6) doi: 10.1371/journal.pone.0065048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock U., et al. High-dose-rate (HDR) brachytherapy with or without external beam radiotherapy in the treatment of primary vaginal carcinoma: long-term results and side effects. Int J Radiat Oncol Biol Phys. 2003;56(4):950–957. doi: 10.1016/s0360-3016(03)00217-7. [DOI] [PubMed] [Google Scholar]
- Naumann R.W., et al. Safety and efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. Journal of Clinical Oncology. 2019;37(31):2825–2834. doi: 10.1200/jco.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli F., et al. Immune-related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J Immunother. 2020 Jan;43(1):1–7. doi: 10.1097/cji.0000000000000300. [DOI] [PubMed] [Google Scholar]
- Prempree T., Amornmarn R. Radiation treatment of primary carcinoma of the vagina. Patterns of failures after definitive therapy. Acta Radiol. Oncol. 1985;24(1):51–56. doi: 10.3109/02841868509134364. [DOI] [PubMed] [Google Scholar]
- Puzanov I., et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samant R., et al. Primary vaginal cancer treated with concurrent chemoradiation using Cis-platinum. Int J Radiat Oncol Biol Phys. 2007;69(3):746–750. doi: 10.1016/j.ijrobp.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Thangarajah F., et al. Clinical impact of PD-L1 and PD-1 expression in squamous cell cancer of the vulva. J Cancer Res Clin Oncol. 2019 Jun;145(6):1651–1660. doi: 10.1007/s00432-019-02915-1. [DOI] [PubMed] [Google Scholar]
- Thigpen J.T., et al. Phase II trial of cisplatin in advanced or recurrent cancer of the vagina: a Gynecologic Oncology Group Study. Gynecol Oncol. 1986;23(1):101–104. doi: 10.1016/0090-8258(86)90121-6. [DOI] [PubMed] [Google Scholar]
- Walle, T., et al. Radiation effects on antitumor immune responses: Current perspectives and challenges. Ther Adv Med Oncol 10: 1758834017742575, 2018. https://doi.org/10.1177/1758834017742575. [DOI] [PMC free article] [PubMed]
- Winer, I.S., Jones, N.L., Xiu, J., Ellerbrock, A., Brown, J., Herzog, T. Mutational burden, tumor PDL-1 expression, and microsatellite instability in gynecologic malignancies: Implications for immune Immune checkpoint expression, Poster 85 SGO 2018. https://www.sgo.org/wp-content/uploads/2018/03/Abstracts-2018-SGO-Annual-Meeting-on-Womens-Cancer-1.pdf.
- Wu Y.T., Cafiero-Chin M., Marques C. Wall-eyed bilateral internuclear ophthalmoplegia: review of pathogenesis, diagnosis, prognosis and management. Clin Exp Optom. 2015;98:25–30. doi: 10.1111/cxo.12200. [DOI] [PubMed] [Google Scholar]
- www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm610572.htm (Accessed 10th Sept 2021).