Abstract
Benign and malignant tumours may arise from eccrine and apocrine sweat glands. Hidradenocarcinoma is a rare malignant eccrine sweat gland tumour representing <0.01% of all skin cancers. There are 6 case reports in the literature of hidradenocarcinoma arising on the vulva, none of which are classified as poroid hidradenocarcinoma. Hidradenocarcinoma is thought to be an aggressive tumour with poor prognosis and high levels of local recurrence and systemic metastases. Conversely, hidradenoma papilliferum is a common benign apocrine sweat gland tumour found on the vulva. The prevalence and significance of atypical changes, however, is unknown. Distinguishing between these tumour types can be difficult. The authors present two cases, a poroid hidradenocarcinoma and an atypical hidradenoma papilliferum with necrosis and increased mitotic activity, to illustrate the diagnostic challenges associated with rare tumours of the vulva in the absence of an established histopathological classification system.
Keywords: Hidradenocarcinoma, Hidradenoma papilliferum, Sweat gland carcinoma, Vulva, Dermatopathology
1. Introduction
Cutaneous sweat gland tumours are uncommon and may be of eccrine or apocrine origin (Obaidat et al., 2007). Eccrine sweat glands are abundant and can be found all over the body, producing mostly sweat. Apocrine sweat glands are associated with hair follicles and found in the axillae, anogenital region, around the nipple and eye lid. They secrete a thicker fat droplet filled fluid which can mix with surface bacteria to produce body odour. Benign and malignant neoplasms can develop in both eccrine and apocrine sweat glands. Examples of benign eccrine neoplasms include poroma, hidradenoma, spiradenoma, cylindroma and syringoma, with porocarcinoma, hidradenocarcinoma, spiradenocarcinoma, malignant cylindroma and syringoid carcinoma representing their malignant counterparts (Obaidat et al., 2007). Examples of benign apocrine neoplasms include syringocystadenoma papilliferum and hidradenoma papilliferum, with syringocystadenocarcinoma and apocrine carcinoma representing their malignant counterparts (Obaidat et al., 2007).
Malignant sweat gland tumours of the vulva are very rare. Diagnosis, management and prognosis are poorly understood and most evidence has been derived from case reports and case series (Kazakov et al., 2011). Hidradenocarcinoma may also be referred to as malignant clear cell myoepithelioma, malignant acrospiroma, clear cell hidradenocarcinoma, malignant eccrine carcinoma, malignant clear cell hidradenoma, and primary mucoepidermoid cutaneous carcinoma. Hidradenocarcinomas account for <0.001% of all tumours and <0.01% of all skin cancers (Soni et al., 2015) and represent 1/13,000 tumours received by a major dermatopathology laboratory (Aziz et al., 2020). Six case reports have been identified in the literature of hidradenocarcinoma arising on the vulva (Messing et al., 1993, Massad et al., 1996, Smith and DeSimone, 2021, Kim et al., 2021, Wick et al., 1985, Giannelli et al., 2017). Hidradenocarcinoma often presents as a painless papule or nodule with slow clinical evolution (Wick et al., 1985) and are therefore rarely suspected clinically. Diagnosis of hidradenocarcinoma is challenging due to a wide spectrum of histopathological features that have been described and no established classification system in existence (Aziz et al., 2020) In addition, while accepted histological criteria are reported, not all lesions will possess all the criteria (Nazarian et al., 2009). Five-year survival for hidradenocarcinoma is reported between 30% (Avraham et al., 2013) and 73.9% (Avraham et al., 2013). Local recurrence after surgery has been reported at 50% (Moore et al., 2021). It is, therefore, an important diagnosis to make so that appropriate treatment and surveillance can be undertaken.
Hidradenoma papillipferum is a benign apocrine neoplasm involving anogenital mammary like glands and commonly occurs on the vulva, most commonly on the inner aspect of the labia (Sington et al., 2006). Malignant transformation is very rare. Benign tumours with atypical features have been described in the literature (Sington et al., 2006) but the clinical course and long-term outcomes are poorly understood.
We present two cases, one of a poroid hidradenocarcinoma of the vulva, and one of hidradenoma papilliferum with increased mitotic activity and necrosis, possibly representing carcinoma in situ, to illustrate pathological features which contribute to a diagnosis of a malignant sweat gland tumour and associated diagnostic challenges.
2. Case report
2.1. Case 1: Poroid hidradenocarcinoma
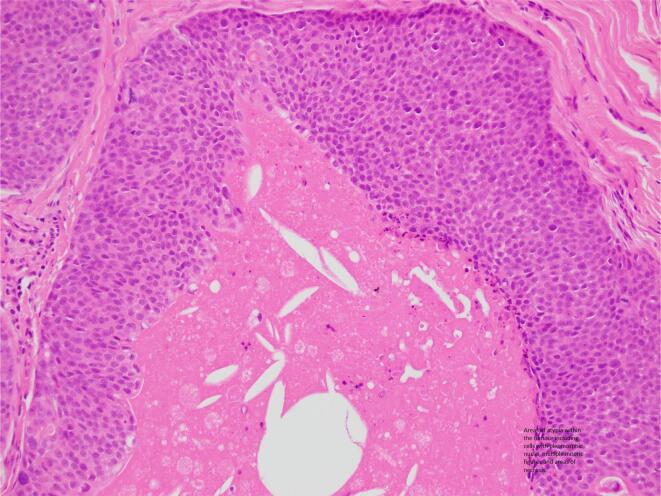
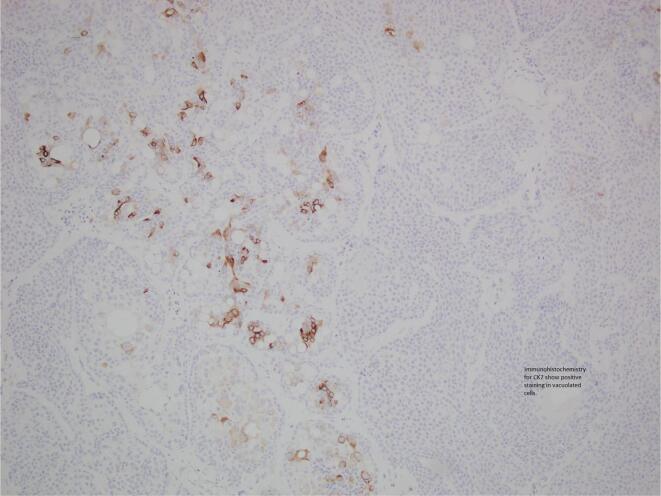
A 52-year-old female presented with an itchy, ulcerating, papular lesion on her right labia majora that had been present for 2 years. An excisional biopsy was performed. Histopathology demonstrated a hidradenocarcinoma arising within a poroid hidradenoma with the closest margin 0.1 mm. The lesion was characterised by ulceration, necrosis, and cells with pleomorphic nuclei and mitotic activity. One area of the tumour had cells with cytoplasmic vacuoles and this area stained positively for CK7, supporting the presence of ductal differentiation and the diagnosis of a primary cutaneous adnexal neoplasm (Qureshi et al., 2004), and BerEP4, which is commonly expressed in sweat gland tumours and in particular, in porocarcinomas (Afshar et al., 2013). The patient was referred to a Gynaecological Oncologist who performed a wide local excision of the scar. No residual malignancy or hidradenoma was identified. There was been no evidence of recurrence or metastatic disease after 9 months of observation (Fig. 1, Fig. 2).
Fig. 1.
Case 1 – Areas of atypia within the tumour including cells with pleomorphic nuclei, multiple mitotic figures and areas of necrosis.
Fig. 2.
Case 1 – Immunohistochemistry: CK7 shows positive staining in the lumina of vacuolated cells.
2.2. Case 2: Hidradenoma papilliferum with possible carcinoma in situ
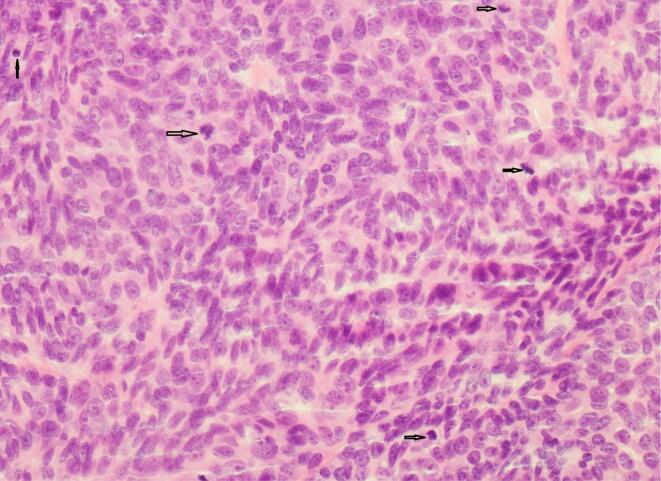
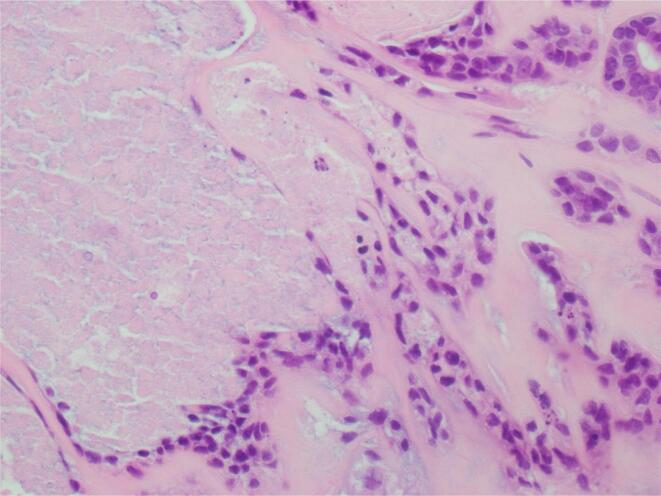
A 54-year-old female presented with a persistent lesion on her right posterior vulva. An excisional biopsy was performed. Histopathology was initially reported as hidradenocarcinoma with close margins. The patient was referred to a Gynaecological Oncologist and underwent a pelvic ultrasound which demonstrated no concerning features and a PET scan which demonstrated no FDG avid lymph nodes, locoregional or metastatic disease. A specialist histopathology review was conducted, and the diagnosis revised to hidradenoma pepilliferum with increased mitotic activity and necrosis (possibly carcinoma in situ) due to the absence of significant pleomorphism and stromal invasion. A wide local excision of the scar was performed. No residual malignancy or hidradenoma was identified (Fig. 3, Fig. 4).
Fig. 3.
Case 2 – Round to ovoid and minimally pleomorphic cells with increased mitotic activity.
Fig. 4.
Case 2 – High power view of central necrosis.
3. Discussion
3.1. Hidradenocarcinoma
Diagnosis of hidradenocarcinoma is challenging due to a wide spectrum of histopathological features that have been described and no established classification system in existence (Aziz et al., 2020). In addition, there is a wide range of differential diagnoses including atypical hidradenoma, eccrine porocarcinoma, clear cell neoplasms and squamous cell carcinoma (Jinnah et al., 2016). Features consistent with a diagnosis of hidradenocarcinoma include loss of circumscription, infiltration and deep extension, nuclear pleomorphism, increased mitotic activity, necrosis, perineural and vascular invasion. Not all tumours possess all the features listed, making the diagnosis challenging. In a study by Nazarian et al. (2009), all tumours with ≥3 of these features were classified as malignant. Due to the very limited data available, the prognostic significance of histopathological features is poorly understood. The authors suggest, however, that metastasising hidradenocarcinomas were more likely to be larger in size and demonstrate vascular invasion (Nazarian et al., 2009). The tumour presented in Case 1 demonstrates nuclear pleomorphism, increased mitotic activity and necrosis in keeping with a diagnosis of malignant hidradenocarcinoma. Features traditionally consistent with increased risk of metastasis such as perineural and vascular invasion, infiltration and deep extension are absent. It should be noted that an infiltrative growth pattern is not always seen with hidradenocarcinoma (Obaidat et al., 2007). Immunohistochemistry usually demonstrates positivity for cytokeratins (CK7), carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA) and S100 protein (Soni et al., 2015, Emanuel, xxxx).
3.2. Poroid hidradenoma
Poroid hidradenoma, such as that within which the hidradenocarcinoma evolved in Case 1, is a rare benign eccrine sweat gland neoplasm (Lim et al., 2021). Poroid hidradenomas account for approximately 5% of hidradenomas (Lim et al., 2021) and demonstrate differentiation towards the intradermal portion of the sweat gland (Lim et al., 2021, Macagno et al., 2021). No accounts of poroid hidradenomas arising on the vulva were found in the literature, with most cases arising on the trunk, upper limb, lower limb and head and neck (Lim et al., 2021). Treatment of poroid hidradenoma is excision and recurrence after excision has rarely been reported (Lim et al., 2021).
3.3. Atypical hidradenoma papilliferum
Hidradenoma papilliferum is a benign lesion of apocrine sweat glands most commonly found on the vulva and perianal region. It is understood that hidradenocarcinoma papilliferum or apocrine carcinoma does present with mitotic activity, necrosis and nuclear atypia (Obaidat et al., 2007). Little is known about those hidradenoma papilliferum which demonstrate atypical features. Nazarian et al. (2009) also describe the challenge posed by benign hidradenomas with focal atypical features such as that presented in Case 2. No follow up data has been reported on these patients but there is concern regarding possible recurrence risk and increased malignant potential (Nazarian et al., 2009). Sington et al. (2006) discuss cases of hidradenoma papilliferum that show pleomorphism. In a case series of 19, they found that nuclear abnormalities did not predict malignant behaviour. Features reported in the literature to be associated with hidradenocarcinoma papilliferum or apocrine carcinoma include infiltrative growth pattern, prominent cytologic atypia, solid sheets of cells and cribriform structures. There are too few cases, however, to provide definitive criteria (Sington et al., 2006). Vazmitel et al. (2008) describe a case of ductal carcinoma in situ (DCIS) arising in hidradenoma papilliferum. The features of the focal DCIS demonstrated crowded pleomorphic epithelial cells, hyperchromatic nuclei imparting a blastic appearance and atypical mitotic figures.
3.4. Management challenges
While the diagnosis of hidradenocarcinoma remains difficult to make, the management of hidradenocarcinoma also poses challenges. There is no specific staging system for cutaneous adnexal carcinomas therefore they are staged similarly to basal and squamous cell cancers (Avraham et al., 2013). There is no consensus regarding the management of hidradenocarcinoma. Lymph node sampling is recommended by some due to the anecdotally high rate of regional lymph node metastases (Ambe and Sondak, 2014), however a population database study showed nodal staging was rarely undertaken and did not demonstrate decreased survival associated with positive lymph nodes (Avraham et al., 2013). Wide local excision is regarded to be the mainstay of treatment (Avraham et al., 2013). There are case reports where radiation has been used successfully (Giannelli et al., 2017), however no difference in overall survival between those who had surgical excision and those who had surgery and adjuvant radiation was found in a population database study (Avraham et al., 2013). Chemotherapy has also been used to treat metastatic hidradenocarcinoma, with 5-FU and Capecitabine being examples of agents used successfully (Lerner et al., 2011). As outlined, malignant potential and risk of recurrence is not well understood in the case of hidradenoma papilliferum with atypical features. Excision is the mainstay of management.
4. Conclusion
Sweat gland neoplasms are found on the vulva and in cases where malignant transformation has occurred, or atypical features are present, insufficient evidence poses challenges in both diagnosis and management. Multidisciplinary histopathology review and discussion must be undertaken to optimise histopathological diagnosis and management in cases where these diagnoses are suspected. In addition, cases should, where possible, be contributed to the literature to increase our understanding of the disease.
Informed consent
Informed consent was obtained from the patients in both cases presented in this paper.
Author contribution
All authors contributed to the conception of this paper. MM wrote the initial draft of the manuscript and undertook the literature review. TM provided case information for case two and reviewed and made changes to the manuscript. BT provided case information for case one and reviewed and made changes to the manuscript. DJ provided case information for case two and reviewed and made changes to the manuscript. JL provided case information for case one and reviewed and made changes to the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Afshar M., Deroide F., Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J. Cutan. Pathol. 2013;40(2):259–264. doi: 10.1111/cup.12043. [DOI] [PubMed] [Google Scholar]
- Ambe C.M., Sondak V.K. Sentinel lymph node biopsy in melanoma and other cutaneous malignancies. Am. J. Haematol. Oncol. 2014;10(3):1–9. [Google Scholar]
- Avraham J.B., Villines D., Maker V.K., August C., Maker A.V. Survival after resection of cutaneous adnexal carcinomas with eccrine differentiation: risk factors and trends in outcomes. J. Surg. Oncol. 2013;108(1):57–62. doi: 10.1002/jso.23346. [DOI] [PubMed] [Google Scholar]
- Aziz K.T., Levin A.S., Cuda J.D., Ficke J.R. Poroid hidradenocarcinoma of the ankle: case report of a rare malignant cutaneous adnexal neoplasm. J. Foot Ankle Surg. 2020;59(2):423–426. doi: 10.1053/j.jfas.2019.08.019. [DOI] [PubMed] [Google Scholar]
- Emanuel, P. Hidradenocarcinoma pathology. DermNetNZ, Internet: [URL} https://dermnetnz.org/topics/hidradenocarcinoma-pathology/ (Last accessed: 4/3/2021 10:31AM).
- Giannelli F., Chiola I., Belgioia L., et al. Complete response in a patient with gynaecological hidradenocarcinoma treated with exclusive external beam radiotherapy and brachytherapy: a case report. J. Contemp. Brachyther. 2017;8(6):572–578. doi: 10.5114/jcb.2017.71554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah A.H., Emory C.L., Mai N.H., et al. Hidradenocarcoma presenting as soft tissue mass: a case report with cytomorphologic description, histologic correlation, and differential diagnosis. Diagn. Cytopathol. 2016;44(5):438–441. doi: 10.1002/dc.23449. [DOI] [PubMed] [Google Scholar]
- Kazakov D., Spagnolo D.V., Kacerovska D., Michal M. Lesions of Anogenital Mammary-like Glands: An Update. Adv. Anat. Pathol. 2011;18(1):1:28. doi: 10.1097/PAP.0b013e318202eba5. [DOI] [PubMed] [Google Scholar]
- Kim G.Y., Solanki M.H., Guo R. Vulvar apocrine hidradenocarcinoma arising in a hidranenoma papilleferum – a case report. J. Cutan. Pathol. 2021 doi: 10.1111/cup.14033. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Lerner A., Beckford A., Ugent S. Complete response of metastatic malignant hidradenocarcinoma to capecitabine treatment. Arch. Dermatol. 2011;147(8):998–999. doi: 10.1001/archdermatol.2011.210. [DOI] [PubMed] [Google Scholar]
- Lim J.S., Kwon E.S., Myung K.B., Cheong S.H. Poroid hidradenoma: a two-case report and literature review. Ann. Dermatol. 2021;33(3):289–292. doi: 10.5021/ad.2021.33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macagno N., Kervarrec T., Sohier P., Poirot B., Haffner A., Carlotti A., Balme B., Castillo C., Jullie M.-L., Osio A., Lehmann-Che J., Frouin E., Battistella M. NUT is a specific immunohistochemical marker for the diagnosis of YAP1-NUTM1-rearranged cutaneous poroid neoplasms. Am. J. Surg. Pathol. 2021 doi: 10.1097/PAS.0000000000001693. (Published online) [DOI] [PubMed] [Google Scholar]
- Massad L.S., Bitterman P., Clarke-Pearson D.L. Metastatic clear cell eccrine hidradenocarcinoma of the vulva: survival after primary surgical resection. Gynecol. Oncol. 1996;61(2):287–290. doi: 10.1006/gyno.1996.0141. [DOI] [PubMed] [Google Scholar]
- Messing M.J., Richardson M.S., Smith M.T., King L., Gallup D.G. Metastatic clear-cell hidradenocarcinoma of the vulva. Gynecol. Oncol. 1993;48(2):264–268. doi: 10.1006/gyno.1993.1045. [DOI] [PubMed] [Google Scholar]
- Moore J.A., Cui S., Berger N., Kim S., O'Guinn D., Labow D., Kamath A. Hidradenocarcinoma: a rare but challenging diagnosis. Clin. Imaging. 2021;75:138–142. doi: 10.1016/j.clinimag.2021.01.024. [DOI] [PubMed] [Google Scholar]
- Nazarian R.M., Kapur P., Rakheja D., et al. Atypical and malignant hidradenoarcinomas: a histological and immunohistochemical study. Mod. Pathol. 2009;22:600–610. doi: 10.1038/modpathol.2009.18. [DOI] [PubMed] [Google Scholar]
- Obaidat N.A., Alsaad K.O., Ghazarian D. Skin adnexal neoplasms – part 2: an approach to tumours of cutaneous sweat glands. J. Clin. Pathol. 2007;60(2):145–159. doi: 10.1136/jcp.2006.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi H.S., Ormsby A.H., Lee M.W., Zarbo R.J., Ma C.K. The diagnostic utility of p62, CK5/6, CK7, and CK20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J. Cutan. Pathol. 2004;31(2):145–152. doi: 10.1111/j.0303-6987.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- Sington J., Chandrapala R., Manek S., Hollowood K. Mitotic count is not predictive of clinical behaviour in hidradenoma papilliferum of the vulva: a clinicopathologic study of 19 cases. Am. J. Dermatopathol. 2006;28(4):322–326. doi: 10.1097/00000372-200608000-00005. [DOI] [PubMed] [Google Scholar]
- Smith C.G., DeSimone C.P. Invasive hidradenocarcinoma of the vulva: a case report and literature review. J. Gynaecol. Surg. 2021;37(1):82–85. [Google Scholar]
- Soni A., Bansal N., Kaushal V., et al. Current management approach to hidradenocarcinoma; a comprehensive review of the literature. Ecancermedicalscience. 2015;9:517. doi: 10.3332/ecancer.2015.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazmitel M., Spagnolo D.V., Nemcova J., Michal M., Kazakov D.V. Hidradenoma papilliferum with a ductal carcinoma in situ component: case report and review of the literature. Am. J. Dermatopathol. 2008;30(4):392–394. doi: 10.1097/DAD.0b013e31817c6a7d. [DOI] [PubMed] [Google Scholar]
- Wick M.R., Goellner J.R., Wolge J.T., Su W.P.D. Vulva sweat gland carcinomas. Arch. Pathol. Lab. Med. 1985;109:43–48. [PubMed] [Google Scholar]