Abstract
Diabetic neuropathy is one of the most prevalent chronic complications of diabetes, and up to half of diabetic patients will develop diabetic neuropathy during their disease course. Notably, emerging evidence suggests that glycemic variability is associated with the pathogenesis of diabetic complications and has emerged as a possible independent risk factor for diabetic neuropathy. In this review, we describe the commonly used metrics for evaluating glycemic variability in clinical practice and summarize the role and related mechanisms of glycemic variability in diabetic neuropathy, including cardiovascular autonomic neuropathy, diabetic peripheral neuropathy and cognitive impairment. In addition, we also address the potential pharmacological and non-pharmacological treatment methods for diabetic neuropathy, aiming to provide ideas for the treatment of diabetic neuropathy.
Subject terms: Diabetes, Pharmacology
Zhang et al. describe metrics for evaluating glycaemic variability (GV) in clinical practice and summarize the role and related mechanisms of GV in diabetic neuropathy, including cardiovascular autonomic neuropathy, diabetic peripheral neuropathy and cognitive impairment. They aim to stimulate ideas for the treatment of diabetic neuropathy.
Introduction
With the improvement of people’s living standard and the increase of competitive pressure, there is growing number of patients with diabetes and diabetes-related complications1. Diabetic neuropathy (DN) is among the most common long-term complications of diabetes, with significant morbidity and mortality. It includes both peripheral and autonomic neuropathy, and is estimated to affect more than 60% of diabetes patients2.
Although chronic hyperglycemia is traditionally considered as a major risk factor for diabetes-related complications, it has been suggested that frequent or large glucose fluctuations may independently lead to diabetes-related complications. In addition to hemoglobin A1c (HbA1c), glycemic variability (GV) could be another independent risk factor for diabetic complications3. Several large-scale clinical studies had identified that the greater degree of GV was significantly associated with the higher incidence of chronic complications of diabetes4. As for instance, an extensive HbA1c control cohort including 38 patients with type 2 diabetes mellitus (T2DM) identified that high GV was harmful to DN even in the context of normal HbA1c levels5. In recent years, GV has been paid extensive attention as an indicator to evaluate blood glucose control. Furthermore, GV, defined as the degree of blood glucose fluctuation and rarely caused by a single factor, was regarded as a potential independent risk factor for diabetic comlications3,6–8. Similarly, findings from studies in T2DM supported that there was a significant positive association between GV and the development or progression of diabetic retinopathy9,10, cardiovascular events, and mortality11–13. Notably, GV tended to be a better glycemic parameter for assessing the risk of future micro- and macro-vascular complications in patients with T2DM3,14,15.
Here, we elaborate the role and related mechanisms of GV in DN, including cardiovascular autonomic neuropathy (CAN), diabetic peripheral neuropathy (DPN), and cognitive impairment. In parallel, we also discuss the potential pharmacological and non-pharmacological treatment methods for GV, aiming to provide new strategies for the treatment of diabetes with DN.
Assessment of GV
Patients with diabetes face a life-long optimization problem of how to lower average blood sugar levels and postprandial hyperglycemia without causing hypoglycemia16. In the past decade, along with HbA1c, GV has been increasingly regarded as a primary marker of glycemic control17–19. With increasing interest in the importance of GV, a number of indicators have been proposed to characterize GV in clinical trials. The coefficient of variation (CV) and standard deviation (SD) are those adopted in the consensus of continuous glucose monitoring (CGM) indices of GV16,20. Some other currently used indices include mean amplitude of glycemic excursion (MAGE), continuous overall net glycemic action, mean of daily differences, high blood glucose index, low blood glucose index, glycemic risk assessment in diabetes equation16,21–27. Nonetheless, currently, there is little consensus on the standard method to assess GV. Notably, the Advanced Technologies & Treatments for Diabetes International Consensus recommends the use of CV to assess GV with a cutoff value of 36% in clinical practice28.
In addition to self-blood glucose monitoring, that mainly supports self-management and medication adjustment of diabetic patients, here we emphasize CGM29. HbA1c, which reflects overall glycemic control over the first 60–90 days, has been considered the gold standard for assessing the outcome of diabetes management since 199330. However, there is increasing recognition of the limitations of HbA1c as glucose control, due to the ignorance of fluctuation in blood glucose levels known as GV. The development of CGM systems has improved the analysis and interpretation of GV31. CGM provides detailed information on several aspects of glucose control, including GV32. Of note, CGM can reliably detect potential postprandial hyperglycemia with normal HbA1c level33. Furthermore, CGM can also systematically record daily glucose levels, making the data more representative without interfering with normal daily life34. Recently, CGM has been proven to be a useful indicator of GV in preclinical type 1 diabetes mellitus (T1DM)35,36. Taken together, CGM system has made it possible to accurately measure short-term GV and to investigate the role of glucose fluctuations in the development of diabetes-related complications3.
It was reported that these indices of GV were largely correlated with each other37,38. For example, a previous study enrolling 88 Japanese patients with diabetes mellitus revealed that the GV indices, including index of glycemic control, mean of daily differences, continuous overall net glycemic action, and MAGE, obtained by CGM were closely correlated with SD glucose39. Conversely, more recently, a retrospective review found that daily GV and visit-to-visit GV was differently correlated with clinical parameters, and there was almost no connection between them40. Hence, further analysis is necessary to clarify the relationships among indices of GV.
Roles of GV in DN
According to clinical reports, patients with diabetes will develop several types of DN damage, including CAN, DPN, and cognitive impairment31,41,42. Presently, the pathogenesis of DN is complicated, and possible mechanisms can be categorized as follows: oxidative stress, mitochondrial dysfunction, advanced level of glycation endproducts, polyol pathway, hexosamine, and protein kinase C pathways, etc43 (Fig. 1). Moreover, accumulating evidence has revealed that the dysfunction of Schwann cells plays a significant role in the pathogenesis of DPN, such as apoptosis, lipid metabolism abnormality, oxidative stress, inflammatory reactions, and endoplasmic reticulum stresss44,45. In parallel, there is growing evidence supporting that GV has drawn a great attention for its role in CAN, DPN, and cognitive impairment.
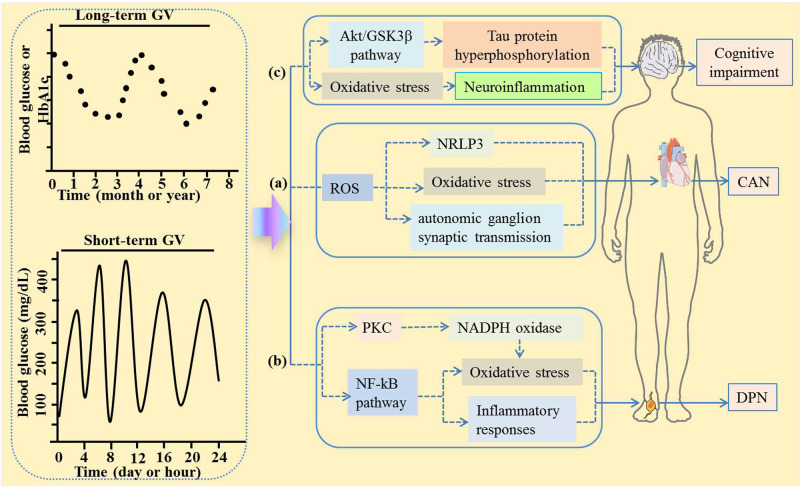
Fig. 1. Possible mechanism of GV causing DN.
The pathogenesis of DN is complex, and the possible mechanisms can be divided into oxidative stress, inflammatory reactions, etc. The possible mechanisms of GV causing CAN, DPN, and cognitive impairment are as follows: a GV increased ROS, which activated the NRLP3 inflammasome and inhibited autonomic ganglion synaptic transmission, thereby leading to CAN; b GV induces oxidative stress and inflammatory response by activating the NF-kB pathway or PKC, thereby causing DPN; c GV causes cognitive impairment by inhibiting Akt/GSK3β pathway to hyperphosphorylate Tau protein. GV glycemic variability, ROS reactive oxygen species, CAN cardiovascular autonomic neuropathy, DPN diabetic peripheral neuropathy.
GV and CAN
It is well known that there is bidirectional regulation between autonomic nervous activity and glucose metabolism46,47. Autonomic imbalance was prevalent and might develop to diabetic autonomic neuropathy in patients with diabetes48. Of note, recent evidence suggested that GV was involved in CAN with T1DM. A pilot study enrolling 44 T1DM patients from the University of Michigan Health System identified that the indices of GV reflective of hypoglycemic stress, low blood glucose index, and area under the curve for hypoglycemia, were significantly negative correlated with the low and high-frequency power of heart rate variability (CAN indicator), suggesting that GV was likely to contribute to CAN49. Similarly, Nyiraty et al. revealed that GV marker calculated by SD and mean absolute glucose were associated with the severity of CAN in patients with T1DM50. Nevertheless, in a cross-sectional observational study including 133 young adults with T1DM from 18 to 24 years, Christensen et al. reported that after adjusting for risk factors and multiple tests, only higher MAGE was associated with slightly increasing measures of heart rate variability, indicating that GV might not be a risk factor for CAN in young adults with T1DM51.
Furthermore, reports have indicated that GV are considered important risk factors for CAN in subjects with T2DM. For instance, a previous study reported that the fluctuation of fasting sympathetic nerve activity around wake-up assessed by heart rate variability was positively correlated with short-term GV in T2DM patients46. Likewise, a Korea prospective study showed that short and long-term GV, such as CGM-SD, CGM-CV, SD of HbA1c and log CV of HbA1c etc, had significantly higher association with the presence of CAN in patients with T2DM than in those without T2DM, indicating that GV was independently associated with the presence of CAN in patients with T2DM52. In parallel, a retrospective cohort study including 681 subjects with T2DM reported that CAN was significantly associated with the risk of developing higher HbA1c variability measured by SD53. Moreover, baroreflex sensitivity, as a sensitive indicator of CAN in T2DM, was found to be inversely related to long-term GV represented by visit-to-visit HbA1c variability in patients with T2DM54. Analogously, Lai et al. showed that the HbA1c variability measured by SD was not only strongly related to the presence but also to the severity of CAN55. However, it was worth to note that in a non-insulin-treated T2DM cohort study consisting of 39 women and 48 men, the indicators of CAN including the standard deviation of normal-to-normal intervals, the root mean square of successive differences, total power, and expiration-to-inspiration ratio etc, were significantly correlated with the increase in MAGE in only women, implying that GV have gender-specific effects on CAN in patients with T2DM56 (Table 1). As a consequence, further prospective studies are needed to confirm the role of GV and CAN in both T1DM and T2DM.
Table 1.
Roles of GV in CAN.
| Metrics of GV | Individuals | Results | References |
|---|---|---|---|
| Low blood glucose index and Under the curve | 44 T1DM patients | Significantly negative correlated with heart rate variability | 49 |
| SD and MAGE | 20 T1DM patients | Correlated with CAN | 50 |
| MAGE | 133 young adults with T1DM | Slightly increase heart rate variability | 51 |
| CV in CGM and all parameters of HbA1c | 110 T2DM patients | Independently associated with CAN | 52 |
| SD of HbA1c | 681 T2DM patients | Significantly associated with CAN | 53 |
| Visit-to-visit HbA1c | 57 T2DM patients | Inversely related to baroreflex sensitivity | 54 |
| Intrapersonal mean, SD, CV for HbA1c | 238 T2DM patients | Strongly associated with CAN | 55 |
| MAGE | 48 men and 39 women with T2DM | Increased GV was associated with CAN in women | 56 |
CV coefficient of variation, SD standard deviation, T2DM type 2 diabetes mellitus, T1DM type 1 diabetes mellitus, HbA1c hemoglobin A1c, MAGE mean amplitude of glycemic excursion, CAN cardiovascular autonomic neuropathy.
Limited by the current knowledge, the pathogenesis of CAN is not fully understood. However, emerging evidence suggested that CAN might be caused by changes in GV due to inflammatory reactions and oxidative stress (Fig. 1a)57–59. On the one hand, autonomic dysfunction occurred in the early stages of diabetes, which might be accompanied by changes in various inflammatory cytokines, including interleukin-657. On the other hand, previous findings from in vitro studies showed that acute blood glucose fluctuations induced a greater trigger effect on oxidative stress through reactive oxygen species overproduction at the mitochondrial electron transport chain60. Moreover, increased reactive oxygen species activated the NRLP3 inflammasome and inhibited autonomic ganglion synaptic transmission by oxidizing the nAch receptor α3 subunit, thereby leading to diabetic CAN58.
GV and DPN
DPN is a common chronic complication of long-term diabetes, and its incidence increases with the overall incidence of diabetes. DPN is associated with neuropathic pain, foot ulcers, and subsequent gangrene and amputation, severely affecting the patient’s quality of life. In recent years, accumulating evidence has indicated GV as a risk factor of DPN with T1DM. In an 11-year follow-up of 100 patients with T1DM, the standard deviation of blood glucose was proved to be an independent predictor of the prevalence of DPN1. In addition, Kwai et al. found that MAGE was strongly related to excitability markers of altered motor and sensory axonal function, such as super excitability, strength duration time constant, minimum I/V slope etc, indicating that GV may be a key mediator of axonal degeneration as well as a contributing factor in development of DPN with T1DM61. Even more importantly, a cross-sectional study found that patients with DPN had higher variability in HbA1c, including HbA1c-SD and HbA1c-CV compared those without DPN62. Consistent with this result, a systematic literary review revealed that the increased variability of HbA1c could be used as a biomarker for DPN in foot63.
Concurrently, multiple cross-sectional studies have shown that the variability of HbA1c is strongly associated with DPN in patients with T2DM64–66. Another observational cohort study enrolled 90 T2DM and with/without DPN, and found that MAGE was significantly correlated with DPN with well-controlled HbA1c67. Analogously, Hu et al.68 enrolled 982 T2DM patients who were screened for DPN and monitored by a continuous glucose monitoring system, and demonstrated MAGE as a significant independent contributor to DPN in type 2 diabetic patients. Furthermore, a retrospective case-control study conducted in Taiwan showed that greater long-term GV was clearly associated with DPN in adults with T2DM69. Intriguingly, it was worth to note that GV was also closely linked to the risk of painful DPN in T2DM. A case-control, retrospective study including 275 T2DM with or without painful DPN as well as 351 T2DM without DPN showed that the fasting plasma glucose-CV was significantly correlated with painful DPN risk after multivariate adjustment64. Shortly after, similar results found that increased postprandial glycemic exposure, defined as high HbA1c and near-normal fasting plasma glucose levels, significantly increased the risk of painful DPN in T2DM patients70. More recently, Yang et al. revealed that the GV represented by time in range decreased significantly in the mild/moderate/severe pain groups compared with the pain-free group, suggesting that time in range could be used as a valuable clinical evaluation index for painful DPN71 (Table 2).
Table 2.
Roles of GV in DPN.
| Metrics of GV | Individuals | Results | References |
|---|---|---|---|
| SDBG | 100 T1DM patients | A predictor of the prevalence of DPN | 1 |
| MAGE | 17 T1DM patients | Strongly correlated with excitability markers of DPN | 61 |
| SD and CV of HbA1c | 50 T1DM patients | Long-term GV is associated with DPN | 62 |
| CV-HbA1c, M-HbA1c | 563 T2DM patients | Closely associated with DPN | 65 |
| SD and CV of HbA1c | 223 T2DM patients | Strongly associated with the severity of DPN | 66 |
| SD-blood glucose, MODD, and MAGE | 90 T2DM patients | MAGE is significantly correlating with DPN | 67 |
| MODD, MAGE, and SD | 982 T2DM patients | Significant independent contributor to DPN | 68 |
| Fasting plasma glucose -CV | 2773 T2DM patients | Significantly associated with a risk of painful DPN | 70 |
| Time in range | 364 individuals with DPN | Correlated with painful DPN | 71 |
DPN diabetic peripheral neuropathy, CV coefficient of variation, SD standard deviation, T2DM type 2 diabetes mellitus, T1DM type 1 diabetes mellitus, HbA1c hemoglobin A1c, MAGE mean amplitude of glycemic excursion, MODD mean of daily differences.
As yet, there is little information regarding the effect of GV on DPN. However, it should not be overlooked that there is one of the mechanisms of DPN induced by GV through activating protein kinase C dependent NADPH oxidase, which further lead to oxidative stress72. In parallel, GV-induced Schwann cells apoptosis might be involved in this process73. Notably, emerging preclinical research showed that GV could weaken the motor nerve conduction velocity of the sciatic nerve, and destroy the microstructure structures of the myelin sheath and axons of the sciatic nerve74. In addition, GV significantly reduced the expression of superoxide dismutase, increased the expression levels of malondialdehyde, TNF-a, interleukin-6, and NF-kB. Altogether, studies above indicate that GV induces oxidative stress and inflammatory response by activating the NF-kB pathway, thereby causing DPN (Fig. 1b)74.
GV and cognitive impairment
Apart from CAN and DPN, the impact of GV on cognitive impairment such as Alzheimer’s disease and vascular dementia has also been addressed75. Strikingly, cognitive impairment is twice more frequent in elderly with T2DM. Previous clinical studies had shown that the MAGE was significantly associated with mini-mental status examination, cognition composite score, and brain atrophy in older patients with T2DM76,77. Moreover, a cross-sectional study conducted by Kim et al. showed that higher SD or CV of HbA1c was significantly associated with low mini-mental status examination78. Furthermore, HbA1c ≥ 8% in elderly adults with diabetes was related to a worse cognitive ability79. Of note, neuroimaging studies had examined the neural relevance of T2DM cognitive impairment and found white matter hyperintensitie might be the basis of the observed cognitive changes. Indeed, a survey from the Israel Diabetes and Cognitive Decline Study shown that HbA1c variability was significantly associated with APOE4 carrier white matter hyperintensitie volume80. Shortly after, a similar result indicated that GV was associated with a higher number of white matter hyperintensitie volume in the multiethnic Washington Heights Inwood Columbia Aging Project81. Consistent with these results, Tamura et al. found that high Glycoalbumin/HbA1c, a marker of high GV, was an important determinant factor for large white matter hyperintensitie volumes in a cross-sectional study82. Alongside, AGP showed distinctively different in diabetes-related dementia but not in Alzheimer’s disease associated with diabetes, suggesting that diabetes-related dementia group was potentially more susceptible to the deleterious effects of GV on the brain83 (Table 3). It is important to highlight that patients with T1DM have also been reported to have cognitive impairment84. For instance, in a T1DM Exchange Clinic Network including 18 research centers on diabetes, 48% of the participants had clinically significant cognitive impairment. In addition, higher HbA1c and continuous glucose monitoring average nocturnal blood glucose were both associated with the increased incidence of clinically significant cognitive impairment85. Taken together, all these findings indicate that GV may be a contributor to cognitive impairment in diabetic patients.
Table 3.
Roles of GV in cognitive impairment.
| Metrics of GV | Individuals | Results | References |
|---|---|---|---|
| MAGE | 121 T2DM patients | Significantly correlated with cognitive impairment | 76 |
| Multi-Scale GV | 43 older adults with and 26 without T2DM | Might contribute to brain atrophy and cognitive impairment | 77 |
| SD and CV of visit-to-visit HbA1c | 68 T2DM patients | Visit-to-visit GV influenced cognitive impairment | 78 |
| SD of HbA1c | 124 T2DM patients | Significantly associated with white matter hyperintensitie | 80 |
| Glycoalbumin/HbA1c | 178 elderly patients with diabetes | Independently associated with white matter hyperintensitie | 82 |
| SD and MAGE of HbA1c | 40 patients with AD-related diabetes and 19 patients with diabetes-related dementia | GV is more involved in the pathophysiology of diabetes-related dementia than Alzheimer’s disease associated with diabetes | 83 |
CV coefficient of variation, SD standard deviation, T2DM type 2 diabetes mellitus, HbA1c hemoglobin A1c, MAGE mean amplitude of glycemic excursion.
There is growing evidence that GV significantly drives increased oxidative stress, leading to neuroinflammation and cognitive impairment86. With the continuous in-depth research on GV risk factors, neuropathology and neuroimaging provides important mechanism clues for cognitive impairment. Notably, abnormal hyperphosphorylation of Tau protein was thought to play a key role in cognitive impairment87. Further support for the idea that GV affecting the risk of cognitive impairment came from another study conducted by Yang et al.74. In that study, the authors observed that both learning and memory abilities were disrupted in the fluctuant hyperglycemia rat model, and the mechanism might be that GV inhibited the Akt/GSK3β pathway to hyperphosphorylate Tau protein in the hippocampus, thereby inducing cognitive impairment. Besides, Xia et al. observed that excessive GV was associated with cognitive impairment, as well as significantly reduced degree centrality in the left middle frontal gyrus (Fig. 1c)88.
Therapeutic strategies for GV
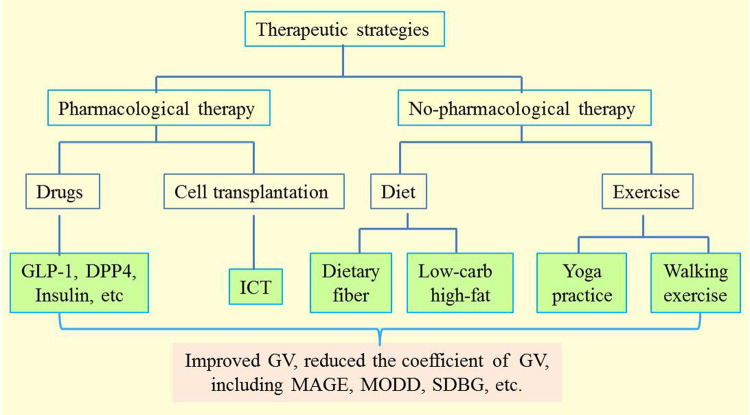
In light of these above findings, it is time to reconsider the therapeutic strategies for DN. Unfortunately, although several non-pharmacological treatments of DN have been discussed89, therapeutic strategies targeting the cause of DN are lacking. Therefore, the recommended pharmacological and non-pharmacological strategies for GV have gained more and more attention (Fig. 2).
Fig. 2. Therapeutic strategies to improve GV.
Pharmacological therapy including drugs and insulin as well as non-pharmacological therapy including diet, cell transplantation, and exercise are recommended to improve GV. MAGE mean amplitude of glycemic excursion, MODD mean of daily differences, GLP-1 glucagon-like peptide-1, DPP4 dipeptidyl peptidase 4, ICT islet cell transplantation, SDBG standard deviation of blood glucose.
Non-pharmacological therapy
Nutrition therapy is essential in the management of diabetes, where dietary carbohydrate intake is the main factor for postprandial hyperglycemia and GV. It has been shown that lipid and protein ingested before carbohydrate can significantly improve glucose tolerance by slowing gastric emptying and enhancing insulin secretion90,91. Interestingly, Chang et al. found that a very-low-carbohydrate, high-fat breakfast meal could significantly reduce MAGE and SD levels for 24 h, and improve GV in patients with T2DM92. Moreover, dietary fiber has shown to reduce postprandial GV in individuals with diabetes. In evaluating the acute effect of fiber-enriched buckwheat and corn pasta on postprandial GV, Vetrani et al.93 showed that there was a higher stability postprandial GV after fiber-enriched buckwheat pasta in subjects with T1DM. Besides, it is worth noting that use of a moderate amount of sucrose, as part of a balanced diet, does not affect the GV or insulin requirements in T1DM94. As such, manipulating the sequence of food intake or choosing more naturally dietary fiber food can improve GV in diabetic patients. In addition, exercise training, including aerobic exercise, resistance exercise and combined exercise sessions, can also decrease GV in T2DM95. In an observational study, Van Dijk et al. showed that prolonged walking exercise could greatly reduce the daily insulin administration in persons with TIDM, but does not necessarily impair 24-h GV96. Noteworthily, yoga-assisted treatment of T2DM has multiple benefits, including reduction in fasting plasma glucose, postprandial glucose, oxidative stress, and proinflammatory markers61. Similarly, in another study, Vijayakumar et al. assessed that there was a significant reduction in GV and a higher duration of time within the glycemic target after one week of yoga practice in T2DM97.
Pharmacological therapy
Although different diabetes treatments may reduce HbA1c to similar degrees, their effectiveness in reducing GV may significantly differ98. Since hyperglycemia is the main cause of the clinical manifestations and related complications of diabetes, lowering blood glucose levels through pharmacological therapy is the cornerstone of diabetes management.
Glucagon-like peptide-1 (GLP-1) receptor agonists
The oral hypoglycemic drug metformin is the first-line treatment for T2DM. When blood glucose was poorly controlled in patients with T2DM receiving metformin alone and poorly controlled blood glucose, GLP-1 receptor agonists and basal insulin are used as optional anti-diabetic drugs99,100. In contrast to Western countries, exenatide is a commonly and widely used short-term GLP-1 receptor agonist in China101. Exenatide significantly reduces not only standard deviation of the mean blood glucose value and largest amplitude of glycemic excursions, but also highest and mean blood glucose levels101.
Dipeptidyl peptidase 4 inhibitors
Vildagliptin is a dipeptidyl peptidase 4 inhibitor that can reduce not only average glycemia but also glucose fluctuation within 24 h by restoring the physiological pattern of insulin and glucagon secretion102. Meanwhile, vildagliptin more effectively improved glucose levels with a significantly greater reduction in GV and hypoglycemia than glimepiride in patients with T2DM ongoing metformin therapy103. Vianna et al.104 demonstrated that vildagliptin and gliclazide MR reduced GV(as measured by the MAGE, p = 0.007 and 0.034, respectively). Furthermore, an open-label, parallel-group, exploratory study indicated that once-weekly trelagliptin and once-daily alogliptin improved glycemic control and reduced GV without inducing hypoglycemia105.
Insulin
Unstable metabolic control and high risk of hypoglycemia due to GV is frequently observed in patients with diabetes on intensive insulin therapy106. Thus, the evidence on effectiveness and safety of insulin in patients with diabetes is a priority. Insulin degludec (IDeg), a novel ultra-long-acting basal insulin, has been extensively tested in a comprehensive study involving a wide range of diabetes patients107–109. One clinical trial demonstrated that IDeg achieved similar improvements in glycemic control to Iglar in insulin-deficient patients with T2DM, and the day-to-day variation of fasting blood glucose was smaller in patients receiving IDeg110. Interestingly, similar findings were reported in another observational longitudinal study64. Extensive evidence addresses that IDeg is not only able to reduce GV in patients with T2DM, but also effective in patients with T1DM. Iga et al.111 found that there were no differences in HbA1c, total insulin dosage, body weight changes, and basal to bolus ratio between the IDeg and IGlar arms. Notably, the day-to-day variability in fasting interstitial GV on the CGM curves was significantly smaller in the IDeg than IGlar treatment period111.
There has been a large amount of literature on the efficacy and safety of IDeg within basal-bolus regimens in non-hospitalized patients with diabetes, whereas the impact of treatment with IDeg on inpatients has rarely been investigated. An observational longitudinal retrospective study represented that IDeg had the potential to maintain stable levels of blood glucose and reduce GV in hospitalized patients with or without T2DM who require nutritional support112.
Cell transplantation
Cell transplantation is being investigated as a possible method of addressing the underlying cause of DN113,114. In a pilot clinical study, Mao et al.115 observed that autologous transplantation of bone marrow mononuclear cells significantly improved the signs and symptoms of DPN. Consistently, another group116 found that autologous transplantation of bone marrow mononuclear cells improved diabetic sensorimotor polyneuropathy in patients with T2DM, indicating that autologous transplantation of bone marrow mononuclear cells might be an effective and promising treatment for DPN. Of note, islet cell transplantation is another promising treatment for patients with T1DM and severe hypoglycemia that is resistant to other therapies117. Islet cell transplantation may reduce complications through both improved glycemic control and reduction in GV117,118. The results of one prospective, crossover study demonstrated that islet cell transplantation could slow the progression of diabetic retinopathy and nephropathy compared with intensive medical therapy119. In addition, Azmi et al. found that HbA1c, neuropathy symptoms and peroneal nerve conduction velocity were improved in T1DM patients after simultaneous pancreas and kidney transplantation120. However, additional clinical studies will be required to confirm the safety and efficacy of cell transplantation in the treatment of DN.
Combination therapy
Misra et al. reported a young woman with a KCNJ11-G334V mutation who showed significant improvements in glycaemic control when treated with high-dose sulfonylureas therapy combined with insulin121. Notably, this combination therapy also resulted in marked improvements in GV and hypoglycemic awareness. Similarly, a proof-of-concept study confirmed efficacy and safety of mealtime exenatide treatment for a high-risk insulin-requiring population and demonstrated a reduction of GV using this approach98. A double-blind randomized phase 2 study demonstrated that empagliflozin significantly reduced GV and increased time spent in the glucose targetrange without increasing time spent in hypoglycemia122. Nomoto et al. proposed that combination therapy of dapagliflozin and insulin injection did not show glucose fluctuation superiority over dipeptidyl peptidase 4 inhibitors on insulin therapy123. If reduced glycemic excursions are the treatment priority, a regimen using GLP-1 is preferable to basal insulin alone because of the shorter time above range and reduced GV124. Particularly, GV decreased only when GLP-1 was a part of the treatment regimen124. Nevertheless, in a prospective substudy of the Qatar Study, Ponirakis et al. found that treatment with exenatide plus pioglitazone or insulin reduced HbA1c and promoted small fiber regeneration, but had no impact on neuropathic pain over 1 year125.
Strikingly, a recent study reported that administration of hyocholic acid in diabetic mouse could improve fasting GLP-1 secretion and glucose homeostasis. Subsequently, in a clinical cohort, it was further confirmed that low concentration of hyocholic acid in serum was related to diabetes126. Thus, hyocholic acid is expected to be developed as another new drug for the treatment of diabetes GV, thereby improving DN. In addition, in terms of blood glucose control, the aim of treatment for T2DM is to reduce HbA1c to the target level and reduce GV in order to avoid both hypoglycemia and wide fluctuations of postprandial glucose127. Therefore, DN can be slowed down by early detection of autonomic imbalance, attention to diabetes control, and elimination of risk factors for neuropathy48.
Summary and further perspectives
Currently, assessment of GV in routine clinical practice remains a challenge. Indeed, there is no gold standard for evaluation of GV. Therefore, it becomes essential for clinical practice to adopt the best methods available for the evaluation of GV to provide the most relevant feedback to improve glycemic control128. Moreover, patients with diabetes face a life-long optimization problem of how to avoid hypoglycemia as well as lower average blood sugar levels and postprandial hyperglycemia16. This optimization can be achieved if GV is reduced. Consequently, in the past decade, along with HbA1c, GV has been increasingly regarded as a primary marker of glycaemic control17–19. However, for the same plasma insulin concentrations, hypoglycemic effects may differ, depending on insulin sensitivity, even after intravenous administration. In a relatively short period of time, insulin sensitivity varies considerably within and between individuals, leading to different metabolic effects in patients with diabetes106. Therefore, no-pharmacological therapy has become an effective way to improve blood GV. Altogether, DN is one of the most common long-term complications of diabetes, and good GV control may be essential in prevention of such complications.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China Grant 82104307 (to S.B.) and the Natural Science Foundation of Hunan Province Grant 2021JJ40865 (to S.B.).
Author contributions
Z.X.C. and Y.X. contributed towards the concept and manuscript writing; Z.C.S. and S.B. revised and supervised overall project.
Peer review information
Communications Biology thanks Rayaz Malik and Yoshifumo Saisho for their contribution to the peer review of this work. Primary Handling Editors: Gabriela Da Silva Xavier and Karli Montague-Cardoso. Peer reviewer reports are available.
Data availability
There were no data used in this study and thus no data are available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaochun Zhang, Xue Yang.
Contributor Information
Bao Sun, Email: scy_csu2016@csu.edu.cn.
Chunsheng Zhu, Email: zhuchunsheng6@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02896-3.
References
- 1.Bragd J, et al. Can glycaemic variability, as calculated from blood glucose self-monitoring, predict the development of complications in type 1 diabetes over a decade? Diabetes Metab. 2008;34:612–616. doi: 10.1016/j.diabet.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 3.Mi SH, et al. Comparison of glycemic variability and glycated hemoglobin as risk factors of coronary artery disease in patients with undiagnosed diabetes. Chin. Med J. 2012;125:38–43. [PubMed] [Google Scholar]
- 4.Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes. Metab. 2010;12:288–298. doi: 10.1111/j.1463-1326.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi F, Taniguchi M, Kosaka A, Uetake H, Tavakoli M. Improvement in neuropathy outcomes with normalizing HbA(1c) in patients with type 2 diabetes. Diabetes Care. 2019;42:110–118. doi: 10.2337/dc18-1560. [DOI] [PubMed] [Google Scholar]
- 6.Kohnert K-D, Heinke P, Vogt L, Salzsieder E. Utility of different glycemic control metrics for optimizing management of diabetes. World J. Diabetes. 2015;6:17–29. doi: 10.4239/wjd.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab. J. 2015;39:273–282. doi: 10.4093/dmj.2015.39.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Service FJ, et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 9.Gimeno-Orna, J. A., Castro-Alonso, F. J., Boned-Juliani, B. & Lou-Arnal, L. M. Fasting plasma glucose variability as a risk factor of retinopathy in Type 2 diabetic patients. J. Diabetes Complicat.17, 78–81. [DOI] [PubMed]
- 10.Picconi F, et al. Activation of retinal Müller cells in response to glucose variability. Endocrine. 2019;65:542–549. doi: 10.1007/s12020-019-02017-5. [DOI] [PubMed] [Google Scholar]
- 11.Cavalot F, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J. Clin. Endocrinol. Metab. 2006;91:813–819. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 12.Zoppini G, et al. Variability of body weight, pulse pressure and glycaemia strongly predict total mortality in elderly type 2 diabetic patients. The Verona Diabetes Study. Diabetes/Metab. Res. Rev. 2008;24:624–628. doi: 10.1002/dmrr.897. [DOI] [PubMed] [Google Scholar]
- 13.Bruginski D, Précoma D, Sabbag A, Olandowski M. Impact of glycemic variability and hypoglycemia on the mortality and length of hospital stay among elderly patients in Brazil. Curr. diabetes Rev. 2020;16:171–180. doi: 10.2174/1573399815999190619141622. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovascular Diabetol. 2018;17:33. doi: 10.1186/s12933-018-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pu Z, et al. Acute glycemic variability on admission predicts the prognosis in hospitalized patients with coronary artery disease: a meta-analysis. Endocrine. 2020;67:526–534. doi: 10.1007/s12020-019-02150-1. [DOI] [PubMed] [Google Scholar]
- 16.Kovatchev BP. Metrics for glycaemic control - from HbA(1c) to continuous glucose monitoring. Nat. Rev. Endocrinol. 2017;13:425–436. doi: 10.1038/nrendo.2017.3. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch IB. Glycemic variability and diabetes complications: does it matter? Of course it does! Diabetes Care. 2015;38:1610–1614. doi: 10.2337/dc14-2898. [DOI] [PubMed] [Google Scholar]
- 18.DeVries JH. Glucose variability: where it is important and how to measure it. Diabetes. 2013;62:1405–1408. doi: 10.2337/db12-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr. Rev. 2010;31:171–182. doi: 10.1210/er.2009-0021. [DOI] [PubMed] [Google Scholar]
- 20.Nusca A, et al. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 2018;34:e3047. doi: 10.1002/dmrr.3047. [DOI] [PubMed] [Google Scholar]
- 21.Joshi A, et al. Patterns of glycemic variability during a diabetes self-management educational program. Med. Sci. 2019;7:undefined. doi: 10.3390/medsci7030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakarova N, Dimova R, Grozeva G, Tankova T. Assessment of glucose variability in subjects with prediabetes. Diabetes Res. Clin. Pract. 2019;151:56–64. doi: 10.1016/j.diabres.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 23.Monnier L, Colette C, Owens DR. The application of simple metrics in the assessment of glycaemic variability. Diabetes Metab. 2018;44:313–319. doi: 10.1016/j.diabet.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Umpierrez GE, Kovatchev BP. Glycemic variability: how to measure and its clinical implication for type 2 diabetes. Am. J. Med. Sci. 2018;356:518–527. doi: 10.1016/j.amjms.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Z, Sun B, Huang S, Zhu C, Bian M. Glycemic variability: adverse clinical outcomes and how to improve it? Cardiovascular Diabetol. 2020;19:102. doi: 10.1186/s12933-020-01085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabris C, Patek SD, Breton MD. Are risk indices derived from CGM interchangeable with SMBG-based indices? J. Diabetes Sci. Technol. 2015;10:50–59. doi: 10.1177/1932296815599177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molnar GD, Taylor WF, Ho MM. Day-to-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8:342–348. doi: 10.1007/BF01218495. [DOI] [PubMed] [Google Scholar]
- 28.Danne T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovatchev BP. Metrics for glycaemic control—from HbA to continuous glucose monitoring. Nat. Rev. Endocrinol. 2017;13:425–436. doi: 10.1038/nrendo.2017.3. [DOI] [PubMed] [Google Scholar]
- 30.Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 31.Jin HY, Lee KA, Park TS. The impact of glycemic variability on diabetic peripheral neuropathy. Endocrine. 2016;53:643–648. doi: 10.1007/s12020-016-1005-7. [DOI] [PubMed] [Google Scholar]
- 32.Leelarathna L, et al. Evaluating glucose control with a novel composite continuous glucose monitoring index. J. Diabetes Sci. Technol. 2020;14:277–283. doi: 10.1177/1932296819838525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klonoff DC, et al. Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011;96:2968–2979. doi: 10.1210/jc.2010-2756. [DOI] [PubMed] [Google Scholar]
- 34.Law GR, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care. 2019;42:810–815. doi: 10.2337/dc18-2212. [DOI] [PubMed] [Google Scholar]
- 35.Helminen O, et al. Continuous glucose monitoring and HbA1c in the evaluation of glucose metabolism in children at high risk for type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2016;120:89–96. doi: 10.1016/j.diabres.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Van Dalem A, et al. Relationship between glycaemic variability and hyperglycaemic clamp-derived functional variables in (impending) type 1 diabetes. Diabetologia. 2015;58:2753–2764. doi: 10.1007/s00125-015-3761-y. [DOI] [PubMed] [Google Scholar]
- 37.Rodbard D. The challenges of measuring glycemic variability. J. Diabetes Sci. Technol. 2012;6:712–715. doi: 10.1177/193229681200600328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int. J. Mol. Sci. 2014;15:18381–18406. doi: 10.3390/ijms151018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saisho Y, et al. Relationships among different glycemic variability indices obtained by continuous glucose monitoring. Prim. Care Diabetes. 2015;9:290–296. doi: 10.1016/j.pcd.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchiya T, et al. Relationship between daily and visit-to-visit glycemic variability in patients with type 2 diabetes. Endocr. J. 2020;67:877–881. doi: 10.1507/endocrj.EJ20-0012. [DOI] [PubMed] [Google Scholar]
- 41.Freeman R. Diabetic autonomic neuropathy. Handb. Clin. Neurol. 2014;126:63–79. doi: 10.1016/B978-0-444-53480-4.00006-0. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Huang E, Gao S. Type 1 diabetes mellitus and cognitive impairments: a systematic review. J. Alzheimers Dis. 2017;57:29–36. doi: 10.3233/JAD-161250. [DOI] [PubMed] [Google Scholar]
- 43.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du W, et al. STAT3 phosphorylation mediates high glucose-impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy. FASEB J. 2019;33:8008–8021. doi: 10.1096/fj.201900127R. [DOI] [PubMed] [Google Scholar]
- 45.Liu YP, Shao SJ, Guo HD. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020;248:117459. doi: 10.1016/j.lfs.2020.117459. [DOI] [PubMed] [Google Scholar]
- 46.Matsushita Y, et al. The fluctuation in sympathetic nerve activity around wake-up time was positively associated with not only morning but also daily glycemic variability in subjects with type 2 diabetes. Diabetes Res. Clin. Pr. 2019;152:1–8. doi: 10.1016/j.diabres.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Thorens B. Neural regulation of pancreatic islet cell mass and function. Diabetes Obes. Metab. 2014;16 Suppl 1:87–95. doi: 10.1111/dom.12346. [DOI] [PubMed] [Google Scholar]
- 48.Fleischer J. Diabetic autonomic imbalance and glycemic variability. J. Diabetes Sci. Technol. 2012;6:1207–1215. doi: 10.1177/193229681200600526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaiswal M, et al. Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2014;37:2616–2621. doi: 10.2337/dc14-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyiraty S, et al. Cardiovascular autonomic neuropathy and glucose variability in patients with type 1 diabetes: is there an association? Front. Endocrinol. 2018;9:174. doi: 10.3389/fendo.2018.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christensen MMB, Hommel EE, Jørgensen ME, Fleischer J, Hansen CS. Glycemic variability and diabetic neuropathy in young adults with type 1 diabetes. Front. Endocrinol. 2020;11:644. doi: 10.3389/fendo.2020.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jun JE, et al. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:70. doi: 10.1186/s12933-015-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, et al. Cardiovascular autonomic neuropathy predicts higher HbA1c variability in subjects with type 2 diabetes mellitus. Diabetes Metab. J. 2018;42:496–512. doi: 10.4093/dmj.2018.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsutani D, et al. Visit-to-visit HbA1c variability is inversely related to baroreflex sensitivity independently of HbA1c value in type 2 diabetes. Cardiovasc Diabetol. 2018;17:100. doi: 10.1186/s12933-018-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai YR, et al. HbA1C variability is strongly associated with the severity of cardiovascular autonomic neuropathy in patients with type 2 diabetes after longer diabetes duration. Front. Neurosci. 2019;13:458. doi: 10.3389/fnins.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleischer J, et al. Glycemic variability is associated with reduced cardiac autonomic modulation in women with type 2 diabetes. Diabetes Care. 2015;38:682–688. doi: 10.2337/dc14-0654. [DOI] [PubMed] [Google Scholar]
- 57.Lieb DC, Parson HK, Mamikunian G, Vinik AI. Cardiac autonomic imbalance in newly diagnosed and established diabetes is associated with markers of adipose tissue inflammation. Exp. Diabetes Res. 2012;2012:878760. doi: 10.1155/2012/878760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ. Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab. J. 2019;43:3–30. doi: 10.4093/dmj.2018.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piconi L, et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab. Res. Rev. 2006;22:198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- 61.Kwai NC, Arnold R, Poynten AM, Krishnan AV. Association between glycemic variability and peripheral nerve dysfunction in type 1 diabetes. Muscle Nerve. 2016;54:967–969. doi: 10.1002/mus.25274. [DOI] [PubMed] [Google Scholar]
- 62.Pinto MV, et al. HbA1c variability and long-term glycemic control are linked to peripheral neuropathy in patients with type 1 diabetes. Diabetol. Metab. Syndr. 2020;12:85. doi: 10.1186/s13098-020-00594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casadei, G., Filippini, M. & Brognara, L. Glycated hemoglobin (HbA1c) as a biomarker for diabetic foot peripheral neuropathy. Diseases9, 10.3390/diseases9010016 (2021). [DOI] [PMC free article] [PubMed]
- 64.Pai YW, Lin CH, Lee IT, Chang MH. Variability of fasting plasma glucose and the risk of painful diabetic peripheral neuropathy in patients with type 2 diabetes. Diabetes Metab. 2018;44:129–134. doi: 10.1016/j.diabet.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 65.Lai YR, et al. HbA1C variability is strongly associated with the severity of peripheral neuropathy in patients with type 2 diabetes. Front. Neurosci. 2019;13:90. doi: 10.3389/fnins.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su JB, et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc. Diabetol. 2018;17:47. doi: 10.1186/s12933-018-0693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu F, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol. Metab. Syndr. 2014;6:139. doi: 10.1186/1758-5996-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu YM, et al. Association of glycaemic variability evaluated by continuous glucose monitoring with diabetic peripheral neuropathy in type 2 diabetic patients. Endocrine. 2018;60:292–300. doi: 10.1007/s12020-018-1546-z. [DOI] [PubMed] [Google Scholar]
- 69.Pai YW, Lin CH, Lin SY, Lee IT, Chang MH. Reconfirmation of newly discovered risk factors of diabetic peripheral neuropathy in patients with type 2 diabetes: a case-control study. PLoS ONE. 2019;14:e0220175. doi: 10.1371/journal.pone.0220175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pai YW, et al. Glycaemic control for painful diabetic peripheral neuropathy is more than fasting plasma glucose and glycated haemoglobin. Diabetes Metab. 2021;47:101158. doi: 10.1016/j.diabet.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, et al. Association of time in range, as assessed by continuous glucose monitoring, with painful diabetic polyneuropathy. J. Diabetes Investig. 2021;12:828–836. doi: 10.1111/jdi.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quagliaro L, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 73.Sun LQ, et al. The protective effect of alpha lipoic acid on Schwann cells exposed to constant or intermittent high glucose. Biochem. Pharm. 2012;84:961–973. doi: 10.1016/j.bcp.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, et al. The mechanisms of glycemic variability accelerate diabetic central neuropathy and diabetic peripheral neuropathy in diabetic rats. Biochem. Biophys. Res. Commun. 2019;510:35–41. doi: 10.1016/j.bbrc.2018.12.179. [DOI] [PubMed] [Google Scholar]
- 75.Świątoniowska-Lonc N, Polański J, Tański W, Jankowska-Polańska B. Impact of cognitive impairment on adherence to treatment and self-care in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2021;14:193–203. doi: 10.2147/DMSO.S284468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rizzo MR, et al. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33:2169–2174. doi: 10.2337/dc10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui X, Abduljalil A, Manor BD, Peng CK, Novak V. Multi-scale glycemic variability: a link to gray matter atrophy and cognitive decline in type 2 diabetes. PLoS ONE. 2014;9:e86284. doi: 10.1371/journal.pone.0086284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim C, et al. Association between visit-to-visit glucose variability and cognitive function in aged type 2 diabetic patients: a cross-sectional study. PLoS ONE. 2015;10:e0132118. doi: 10.1371/journal.pone.0132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mimenza-Alvarado AJ, et al. Effect of poor glycemic control in cognitive performance in the elderly with type 2 diabetes mellitus: The Mexican Health and Aging Study. BMC Geriatr. 2020;20:424. doi: 10.1186/s12877-020-01827-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Livny A, et al. Long-term variability in glycemic control is associated with white matter hyperintensities in APOE4 genotype carriers with type 2 diabetes. Diabetes Care. 2016;39:1056–1059. doi: 10.2337/dc15-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reitz C, et al. Relation of dysglycemia to structural brain changes in a multiethnic elderly cohort. J. Am. Geriatr. Soc. 2017;65:277–285. doi: 10.1111/jgs.14551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamura Y, et al. White matter hyperintensity in elderly patients with diabetes mellitus is associated with cognitive impairment, functional disability, and a high glycoalbumin/glycohemoglobin ratio. Front Aging Neurosci. 2017;9:220. doi: 10.3389/fnagi.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogawa Y, et al. Ambulatory glucose profile in diabetes-related dementia. Geriatr. Gerontol. Int. 2019;19:282–286. doi: 10.1111/ggi.13612. [DOI] [PubMed] [Google Scholar]
- 84.Bispham JA, Hughes AS, Driscoll KA, McAuliffe-Fogarty AH. Novel challenges in aging with type 1 diabetes. Curr. Diab Rep. 2020;20:15. doi: 10.1007/s11892-020-01298-9. [DOI] [PubMed] [Google Scholar]
- 85.Chaytor NS, et al. Clinically significant cognitive impairment in older adults with type 1 diabetes. J. Diabetes Complications. 2019;33:91–97. doi: 10.1016/j.jdiacomp.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 86.Watt, C., Sanchez-Rangel, E. & Hwang, J. J. Glycemic variability and CNS inflammation: reviewing the connection. Nutrients12, 10.3390/nu12123906 (2020). [DOI] [PMC free article] [PubMed]
- 87.Zhang Q, et al. CK2 phosphorylating I(2)(PP2A)/SET mediates tau pathology and cognitive impairment. Front. Mol. Neurosci. 2018;11:146. doi: 10.3389/fnmol.2018.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xia W, et al. Glucose fluctuations are linked to disrupted brain functional architecture and cognitive impairment. J. Alzheimers Dis. 2020;74:603–613. doi: 10.3233/JAD-191217. [DOI] [PubMed] [Google Scholar]
- 89.Cox AA, et al. Low-dose pulsatile interleukin-6 as a treatment option for diabetic peripheral neuropathy. Front. Endocrinol. 2017;8:89. doi: 10.3389/fendo.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma J, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32:1600–1602. doi: 10.2337/dc09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tricò D, et al. Mechanisms through which a small protein and lipid preload improves glucose tolerance. Diabetologia. 2015;58:2503–2512. doi: 10.1007/s00125-015-3710-9. [DOI] [PubMed] [Google Scholar]
- 92.Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am. J. Clin. Nutr. 2019;109:1302–1309. doi: 10.1093/ajcn/nqy261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vetrani C, et al. Fibre-enriched buckwheat pasta modifies blood glucose response compared to corn pasta in individuals with type 1 diabetes and celiac disease: acute randomized controlled trial. Diabetes Res. Clin. Pr. 2019;149:156–162. doi: 10.1016/j.diabres.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 94.Souto, D. L. et al. Does sucrose affect the glucose variability in patients with type 1 diabetes? a pilot crossover clinical study. Nutrition 179–184, 10.1016/j.nut.2018.05.009 (2018). [DOI] [PubMed]
- 95.Figueira FR, et al. Aerobic and combined exercise sessions reduce glucose variability in type 2 diabetes: crossover randomized trial. PLoS ONE. 2013;8:e57733. doi: 10.1371/journal.pone.0057733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Dijk JW, et al. Glycemic control during consecutive days with prolonged walking exercise in individuals with type 1 diabetes mellitus. Diabetes Res. Clin. Pr. 2016;117:74–81. doi: 10.1016/j.diabres.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 97.Vijayakumar V, Mavathur R, Sharma MNK, Kannan S. Reduced glycemic variability with yoga in patients with type 2 diabetes mellitus: results of a pilot study. J. Diabetes Sci. Technol. 2019;13:803–804. doi: 10.1177/1932296819852064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flat-Sugar Trial Investigators. Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care39, 973–981 (2016). [DOI] [PubMed]
- 99.Garber A, et al. CONSENSUS statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr. Pract. 2017;23:207–238. doi: 10.4158/EP161682.CS. [DOI] [PubMed] [Google Scholar]
- 100.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228–2234. doi: 10.1016/S0140-6736(14)61335-0. [DOI] [PubMed] [Google Scholar]
- 101.Yin TT, et al. Comparison of glycemic variability in Chinese T2DM patients treated with exenatide or insulin glargine: a randomized controlled trial. Diabetes Ther. 2018;9:1253–1267. doi: 10.1007/s13300-018-0412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koyanagawa N, et al. Comparative effects of vildagliptin and sitagliptin determined by continuous glucose monitoring in patients with type 2 diabetes mellitus. Endocr. J. 2016;63:747–753. doi: 10.1507/endocrj.EJ16-0266. [DOI] [PubMed] [Google Scholar]
- 103.Kim G, et al. The efficacy and safety of adding either vildagliptin or glimepiride to ongoing metformin therapy in patients with type 2 diabetes mellitus. Expert Opin. Pharmacother. 2017;18:1179–1186. doi: 10.1080/14656566.2017.1353080. [DOI] [PubMed] [Google Scholar]
- 104.Vianna AGD, et al. A randomized controlled trial to compare the effects of sulphonylurea gliclazide MR (modified release) and the DPP-4 inhibitor vildagliptin on glycemic variability and control measured by continuous glucose monitoring (CGM) in Brazilian women with type 2 diabetes. Diabetes Res. Clin. Pract. 2018;139:357–365. doi: 10.1016/j.diabres.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 105.Nishimura R, Osonoi T, Koike Y, Miyata K, Shimasaki Y. A randomized pilot study of the effect of trelagliptin and alogliptin on glycemic variability in patients with type 2 diabetes. Adv. Ther. 2019;36:3096–3109. doi: 10.1007/s12325-019-01097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodacki M, Carvalho RM, Zajdenverg L. The potential effect of ultra-long insulin degludec on glycemic variability. Diabetes Res. Clin. Pr. 2017;133:92–103. doi: 10.1016/j.diabres.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 107.Garber AJ, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498–1507. doi: 10.1016/S0140-6736(12)60205-0. [DOI] [PubMed] [Google Scholar]
- 108.Zinman B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long) Diabetes Care. 2012;35:2464–2471. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meneghini L, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36:858–864. doi: 10.2337/dc12-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aso Y, et al. Effect of insulin degludec versus insulin glargine on glycemic control and daily fasting blood glucose variability in insulin-naïve Japanese patients with type 2 diabetes: I’D GOT trial. Diabetes Res. Clin. Pract. 2017;130:237–243. doi: 10.1016/j.diabres.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 111.Iga, R. & Uchino, H. Glycemic variability in type 1 diabetes compared with degludec and glargine on the morning injection: an open-label randomized controlled trial. 8, 783–792, 10.1007/s13300-017-0269-0 (2017). [DOI] [PMC free article] [PubMed]
- 112.Fatati G, et al. Impact of insulin degludec in hospitalized patients with and without type 2 diabetes requiring parenteral/enteral nutrition: an observational study. Adv. Ther. 2018;35:809–816. doi: 10.1007/s12325-018-0709-x. [DOI] [PubMed] [Google Scholar]
- 113.Liu W, et al. Autologous bone marrow-derived stem cells for treating diabetic neuropathy in metabolic syndrome. Biomed. Res. Int. 2017;2017:8945310. doi: 10.1155/2017/8945310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naruse K, Nakamura J. Cell transplantation therapy for diabetic neuropathy. Nihon Rinsho. 2010;68 Suppl 9:627–631. [PubMed] [Google Scholar]
- 115.Mao H, et al. Efficacy of autologous bone marrow mononuclear cell transplantation therapy in patients with refractory diabetic peripheral neuropathy. Chin. Med. J. 2019;132:11–16. doi: 10.1097/CM9.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei W, et al. Autologous bone marrow mononuclear cell transplantation therapy improved symptoms in patients with refractory diabetic sensorimotor polyneuropathy via the mechanisms of paracrine and immunomodulation: a controlled study. Cell Transpl. 2020;29:963689720949258. doi: 10.1177/0963689720949258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holmes-Walker DJ, et al. Islet transplantation provides superior glycemic control with less hypoglycemia compared with continuous subcutaneous insulin infusion or multiple daily insulin injections. Transplantation. 2017;101:1268–1275. doi: 10.1097/TP.0000000000001381. [DOI] [PubMed] [Google Scholar]
- 118.Bertuzzi F, et al. Long-term effect of islet transplantation on glycemic variability. Cell Transplant. 2018;27:840–846. doi: 10.1177/0963689718763751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thompson DM, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91:373–378. doi: 10.1097/TP.0b013e31820437f3. [DOI] [PubMed] [Google Scholar]
- 120.Azmi S, et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia. 2019;62:1478–1487. doi: 10.1007/s00125-019-4897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Misra S, et al. Permanent neonatal diabetes: combining sulfonylureas with insulin may be an effective treatment. Diabet. Med. 2018 doi: 10.1111/dme.13758. [DOI] [PubMed] [Google Scholar]
- 122.Famulla S, et al. Glucose exposure and variability with empagliflozin as adjunct to insulin in patients with type 1 diabetes: continuous glucose monitoring data from a 4-week, randomized, placebo-controlled trial (EASE-1) Diabetes Technol. Ther. 2017;19:49–60. doi: 10.1089/dia.2016.0261. [DOI] [PubMed] [Google Scholar]
- 123.Nomoto H, et al. A randomized controlled trial comparing the effects of dapagliflozin and DPP-4 inhibitors on glucose variability and metabolic parameters in patients with type 2 diabetes mellitus on insulin. Diabetol. Metab. Syndr. 2017;9:54. doi: 10.1186/s13098-017-0255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carlson AL, et al. Evaluation of insulin glargine and exenatide alone and in combination: a randomized clinical trial with continuous glucose monitoring and ambulatory glucose profile analysis. Endocr. Pract. 2019;25:306–314. doi: 10.4158/EP-2018-0177. [DOI] [PubMed] [Google Scholar]
- 125.Ponirakis, G. et al. Effect of treatment with exenatide and pioglitazone or basal-bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res. Care8, 10.1136/bmjdrc-2020-001420 (2020). [DOI] [PMC free article] [PubMed]
- 126.Zheng X, et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 127.Zenari L, Marangoni A. What are the preferred strategies for control of glycaemic variability in patients with type 2 diabetes mellitus? Diabetes, Obes. Metab. 2013;null:17–25. doi: 10.1111/dom.12143. [DOI] [PubMed] [Google Scholar]
- 128.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There were no data used in this study and thus no data are available.