Abstract
Previous research suggests more biomechanically demanding tasks (e.g., stair descent, hopping) magnify biomechanical asymmetries compared with walking after anterior cruciate ligament (ACL) reconstruction. However, it is unclear if modifying task-specific constraints, like walking speed also elicits greater biomechanical asymmetries in this population. We examined the effects of manipulating walking speed on ground reaction force (GRF) asymmetries in individuals with ACL reconstruction and uninjured controls. Thirty individuals with ACL reconstruction (Age= 20.6 ± 5.4 yr., body mass index [BMI]= 23.9 ± 3.3 kg/m2) and fifteen controls (Age= 23.1 ± 4.5 yr., BMI= 23.6 ± 2.7 kg/m2) were tested on an instrumented treadmill at three speeds (100%, 120%, and 80% self-selected speed). Bilateral vertical and posterior-anterior GRFs were recorded at each speed. GRF asymmetries were calculated by subtracting the uninjured from the injured limb at each percent of stance. Statistical parametric mapping was used to evaluate the effects of speed on GRF asymmetries across stance. We found vertical and posterior GRF asymmetries were exacerbated at faster speeds and reduced at slower speeds in ACL individuals but not controls (p < 0.05). No differences in anterior GRF asymmetries were observed between speeds in either group (p > 0.05). Our results suggest increasing walking speed magnifies GRF asymmetries in individuals with ACL reconstruction.
Statement of Clinical Significance:
Evaluating both preferred and fast walking speeds may aid in characterizing biomechanical asymmetries in individuals with ACL reconstruction which may be valuable in earlier rehabilitative timepoints when more difficult tasks like hopping and running are not feasible.
Keywords: Ground Reaction Force, Asymmetry, Task Complexity
Introduction
Appropriate joint loading is critical for maintaining knee joint health and mitigating osteoarthritis risk after anterior cruciate ligament (ACL) reconstruction 1-4. Underloading of the ACL-reconstructed limb (i.e., smaller vertical ground reaction forces [GRFs] relative to the uninjured limb) is associated with poorer patient-reported outcomes and lower knee cartilage proteoglycan density, and therefore, maybe a detrimental walking strategy employed by some individuals with ACL reconstruction3; 5. Given that asymmetrical GRFs during walking are known to negatively affect knee joint health and patient outcomes, it is critical to understand under what conditions GRF asymmetries are present in individuals with ACL reconstruction.
Gait biomechanics, such as GRF limb asymmetries, are most commonly evaluated at self-selected walking speeds to capture one’s habitual walking pattern and loading environment. Many studies have shown that those with ACL reconstruction walk with near “normal” vertical GRF symmetry (i.e., < 5% interlimb difference) as early as 6 months post-reconstruction 1; 2; 5-8. Although these data may indicate a resolution of biomechanical asymmetry, findings should be interpreted with caution. For example, early resolution of GRF asymmetry could be due to reductions in uninvolved limb GRF characteristics which would falsely suggest an improvement in gait characteristics1. Further, evidence suggests that asymmetry in the vertical GRF may be task-dependent 9 wherein more biomechanically demanding tasks (i.e., running, jump landing, etc.) reveal greater biomechanical differences between ACL-reconstructed and healthy knees 10-12. Therefore, it is plausible modifying the difficulty of a motor task may be advantageous when attempting to characterize biomechanical asymmetries in those after ACL reconstruction as it may provide a more rigorous clinical evaluation of a patient’s functional capabilities.
One way to increase task difficulty during walking is to increase walking speed, as it challenges the neuro-musculoskeletal system by requiring increased attentional and muscular demands 13-18. Many studies have suggested manipulating walking speeds may be useful to help better characterize gait impairments like asymmetries in pathological populations (e.g., stroke, amputees) 19-23. For example, previous work have shown that pathological populations often exhibit increased gait asymmetries at fast speeds compared to preferred 19-23. In able-bodied individuals, however, it has generally been shown that GRF symmetries are maintained across non-preferred walking speeds 23-25; though some evidence does exist showing that certain gait metrics like support and propulsive impulses are affected by non-preferred walking speeds 13; 26. Nonetheless it is not clear if manipulating gait speed from a self-selected pace alters GRF symmetry in individuals with ACL-reconstruction similar to that seen in other pathological gaits. It is plausible individuals with ACL reconstruction may indeed exhibit a differential response from healthy adults when manipulating walking speeds given the long-term neuromuscular deficits induced after ACL-reconstruction27-30. Previous work may lend some support for this notion as it has been shown that manipulating walking speed alters knee kinematic variability in those with ACL deficiency but not in healthy controls14; 31. As such, understanding the effect of walking speed on GRF asymmetries during gait may provide a unique insight into the effects of altered task difficulty on biomechanical function in those with ACL reconstruction.
The vertical GRF during gait is often used as an estimate of the total load applied up through the lower limbs in individuals with ACL reconstruction 1; 2; 5-8, and recent work has shown this gait parameter is a viable target for gait interventions using real-time biofeedback paradigms2; 32. However, evaluation of other components of the GRF during walking, such as the posterior-anterior GRF, have received far less attention in the literature despite evidence suggesting this parameter may provide additional insight into the extent of biomechanical asymmetries present after surgery. For example, Lim et al 33; 34 demonstrated the magnitude of asymmetry in the posterior (breaking) GRF was substantially higher than that seen in the vertical GRF component (i.e., 11% vs. 4%). Further, posterior GRF limb symmetry, but not vertical GRF symmetry has been shown to predict knee extensor moment symmetry in those with ACL reconstruction 33; 34. Given these findings, there is a need to further examine the posterior-anterior GRF during gait and how walking speeds may impact the magnitude of asymmetries observed in individuals with ACL reconstruction.
Therefore, the purpose of this study was to examine the effect of gait speed on asymmetry in vertical and posterior-anterior GRFs in individuals with ACL reconstruction and compare the results with uninjured controls. Although discrete pre-determined time points of the GRF waveform are commonly evaluated, we employed statistical parametric mapping (SPM) in order to more comprehensively evaluate the entirety of the GRF waveform. Consideration of GRFs across the entirety of the stance phase is important as it may provide a more robust evaluation of gait characteristics that may not be captured when using single discrete time points. We hypothesized that 1) asymmetry in GRFs would increase with an increase in walking speed in the ACL-reconstructed group, and 2) uninjured controls would be able to maintain symmetrical GRF characteristics regardless of walking speed.
Methods
The data presented herein are from a randomized clinical trial (Clinical trial #: NCT03282565) identifying the effect of functional resistance training on gait biomechanics in a cohort of ACL-reconstructed individuals (National Institutes of Health Grant # R21 HD092614-02). For this manuscript, data were collected from the ACL-reconstructed individuals during their first study visit prior to the administration of any intervention. All participants and legal guardian(s) of participants under the age of 18 provided informed written consent for this study. The University Medical School Institutional Review Board approved all procedures of the current study.
Participants:
Thirty individuals with unilateral ACL reconstruction (Time from surgery: 9.5 ± 2.8 weeks, Age = 20.6 ± 5.4 years, BMI = 23.9 ± 3.3 kg/m2, Table 1) and fifteen matched healthy control participants who were free from lower extremity injury in the past six months (Age = 23.1 ± 4.5 years, BMI = 23.6 ± 2.7 kg/m2, Table 1) volunteered for this study. Control participants were matched with one of the ACL subjects based on age (± 3 yrs.) and sex. All volunteers, regardless of injury status, had to be between 14-40 years of age to qualify for participation. If any interested person reported 1) pregnancy or actively trying to become pregnant, 2) a history of untreated diabetes or hypertension, and 3) a diagnosis of any neurological condition or chronic pain pathology, they were excluded from participation in this study. Additional exclusion criteria for the ACL reconstruction group included: 1) multiple ACL injuries or surgeries, 2) any lower-extremity injury or surgery other than the current ACL reconstruction, and 3) reconstruction with an allograft. Participants in the uninjured control group were excluded if they had any history of a previous knee injury.
Table 1.
Participant Demographics
| ACL-Reconstructed Group N=30, Mean [95% CI] |
Healthy Control Group N=15, Mean [95% CI] |
|
|---|---|---|
| Age (yrs.) | 20.63 [18.62, 22.63] | 23.1 [20.6, 25.6] |
| Height (cm.) | 172.04 [158.53, 175.43] | 173.29 [166.76, 179.82] |
| Weight (kg.) | 70.95 [66.40, 75.51] | 71.52 [63.76, 79.27] |
| BMI (kg/m2) | 23.91 [22.70, 25.13] | 23.64 [22.14, 25.14] |
| Self-selected Speed (m/s) | 1.30 [1.24, 1.36] a | 1.47 [1.36, 1.58] |
| 120% Self-selected Speed (m/s) | 1.56 [1.49, 1.63] a | 1.76 [1.63, 1.89] |
| 80% Self-selected Speed (m/s) | 1.04 [0.99, 1.09] a | 1.17 [1.09, 1.26] |
| Graft Type (n) | 25 patellar 4 hamstring tendon 1 quadriceps tendon |
N/A |
BMI: Body mass index.
Indicates significant difference between groups (α level = 0.05)
Experimental Procedures:
The experiment began by having participants perform a 10-meter walk test overground to determine their self-selected walking speed for future treadmill walking conditions similar to several recent studies 2; 35; 36. All participants wore their own athletic shoes during testing. The participants walked over a 12-meter walkway at their preferred walking speed while an experimenter used a hand-held stopwatch to time the intermediate 10-meter walk37. Participants were given one practice trial, and then the average of 3 successive trials was used to determine their self-selected walking speed. Once speed was determined, all participants completed one 65-second walking trial on a fully instrumented Bertec split-belt treadmill (ITC-11-20L/R, Bertec, Corp., Columbus, OH, USA) at each of the three treadmill speeds (80%, 100%, and 120% of self-selected speed). The vertical and posterior-anterior GRFs were recorded using a custom-written LabVIEW program (LabVIEW 2011, National Instruments Corp., Austin, TX, USA). The GRF data were lowpass filtered at 500 Hz using a National Instruments 8th order analog anti-aliasing Butterworth filter (SCXI 1143, National Instruments Corp., Austin, TX, USA) which were then sampled at 1000 Hz using 16-Bit National Instruments data acquisition hardware (USB-6255).
Participants were given standardized verbal instructions to facilitate normal walking by asking them to 1) maintain their position on the center of the treadmill, 2) walk as normal as possible and attempt not to scuff their feet or strike the opposite treadmill belt with their foot (i.e., cross-over step), and 3) keep their eyes forward and not look down at their feet for extended periods (other than checking body position). These standardized instructions were repeated to participants as needed during treadmill conditions. Once the treadmill was initiated and reached the set speed, participants were allotted 10 seconds to familiarize themselves with the treadmill speed before trial recording was initiated. Following each 65-second trial, the treadmill speed was changed to the subsequent walking speed (i.e., 120% and 80% of self-selected speed), and once the treadmill reached the set speed, participants were again given 10 seconds of familiarization before initiating the next 65-second trial recording.
Data Reduction:
Analog force data were recorded and further analyzed using an offline LabVIEW program (LabVIEW 2011, National Instruments Corp., Austin, TX, USA). Force data were low-pass filtered using a fourth-order zero-phase lag Butterworth filter (100 Hz cut-off frequency). The stance phase for each gait cycle was identified as the period between where the vertical GRF exceeded 20N and subsequently fell below 20N. On average, over 50 strides per limb were identified at each walking speed and subsequently used to generate time-normalized (101-point) ensemble averages of the vertical and posterior-anterior GRF curves. Ensemble GRF curves were normalized to percent body weight (% BW) by dividing each element of the GRF waveform by the participant’s body weight in Newtons and multiplying by 100. We chose to calculate interlimb differences (i.e., asymmetry) for each GRF component in our cohort instead of using other metrics of symmetry (i.e. limb symmetry indices) in order to more directly evaluate the magnitude of GRF changes (i.e. % BW) that may be occurring across walking speeds. For ACL-reconstructed participants, the uninjured limb GRF was subtracted from the injured limb GRF across each percent of the stance phase. For control participants, we considered the limb with the smaller peak vertical GRF from the self-selected speed as the “injured limb” and the limb with the greater peak vertical GRF as the “uninjured limb”. Once the “injured” and “uninjured” limbs were identified from the self-selected speed trial, we calculated asymmetry similar to ACL-reconstructed participants where we subtracted “uninjured limb” GRF from the “injured” limb GRF across each percent of stance phase shown below in equation 1. For reference, positive value represents higher GRFs in the “injured” relative to the “uninjured” limb whereas negative values indicate lower GRFs in the “injured” relative to the contralateral limb. The identified “injured” and “uninjured” limb in controls were kept consistent for the 120% and 80% speed conditions. We opted to identify “injured” and “uninjured” limbs for control participants in this fashion to give us a conservative estimate of gait GRF asymmetries when comparing the effects of walking speed conditions between ACL reconstructed participants and controls.
Statistical Analyses:
Demographic comparisons between individuals with ACL reconstruction and uninjured controls were performed using two-sample t-tests in IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, NY, USA). To evaluate the effects of walking speed on asymmetry in the vertical and posterior-anterior GRFs, we performed two separate 2 x 3 (group: [ACL reconstructed, Controls] by speed: [80%, 100%, and 120% self-selected speed]) repeated-measures analysis of variance (ANOVA2onerm; SPM[F]) using one-dimensional statistical parametric mapping (SPM1D) techniques. Analyses were completed via Matlab (R2020b, The Mathworks Inc. Natick, MA, USA) using open-source code (SPM1D, v.M0.1, www.spm1d.org) detailed elsewhere 38; 39. Briefly, SPM1D analysis first calculates a vector field of test statistics (F) at each instantaneous point across the GRF waveform. To evaluate the significance of the vector field (F), SPM techniques compute a critical F threshold (F*) using Random Field Theory (critical threshold 5%; α = 0.05) wherein portions of the computed vector field (F) that exceeded the computed critical F threshold (F*) were considered significant 39. We tested the null hypothesis that speed does not induce an effect on the observed asymmetry in vertical and posterior-anterior GRFs in individuals with ACL reconstruction and uninjured controls.
Following the inspection of SPM results, significant interactions were followed with post hoc paired t-tests (TTESTpaired; SPM[t]) to evaluate the effects of walking speed on asymmetry in GRFs within each respective group. The paired t-tests analyses tested three planned comparisons: 80% - 100% self-selected speed, 80% – 120% self-selected speed, and 100%-120% self-selected speed. Similar to procedures described above, SPM[t] analyses calculate a scalar field (t) at each instantaneous point across GRF waveforms, and significance was evaluated at a critical t threshold (t*, α = 0.05). If any portions of the GRF waveform exceed this critical threshold (t*), then a significant difference between speeds was considered present. We tested the null hypothesis that the magnitude of asymmetry in GRF is similar across walking speeds in both ACL-reconstructed participants and uninjured controls.
Results:
Participant Demographics
Participant demographics and walking speeds (Mean and 95% CI) are provided in Table 1. No significant differences were found between groups for age, body-mass, height, or BMI (p > 0.05). Individuals with ACL reconstruction walked with slower self-selected speeds compared with uninjured controls (1.30 m/s [95% CI: 1.24, 1.36] vs. 1.47 [95% CI: 1.36, 1.58], p <0.01, Cohen’s d= 0.98).
Vertical Ground Reaction Force
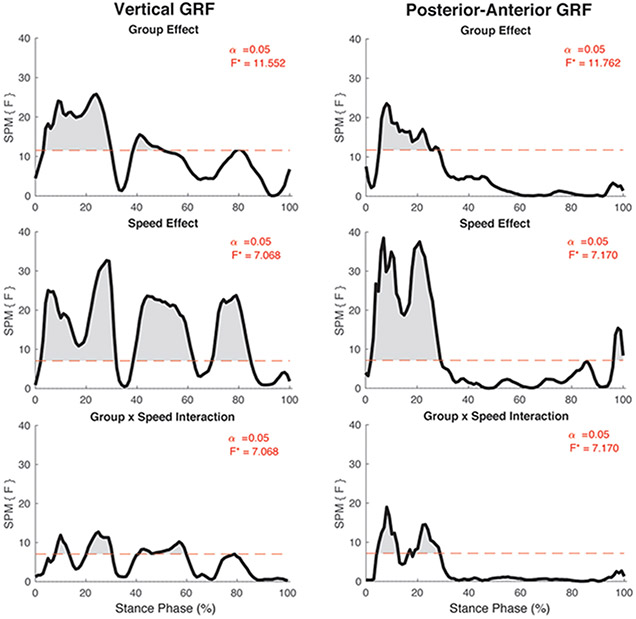
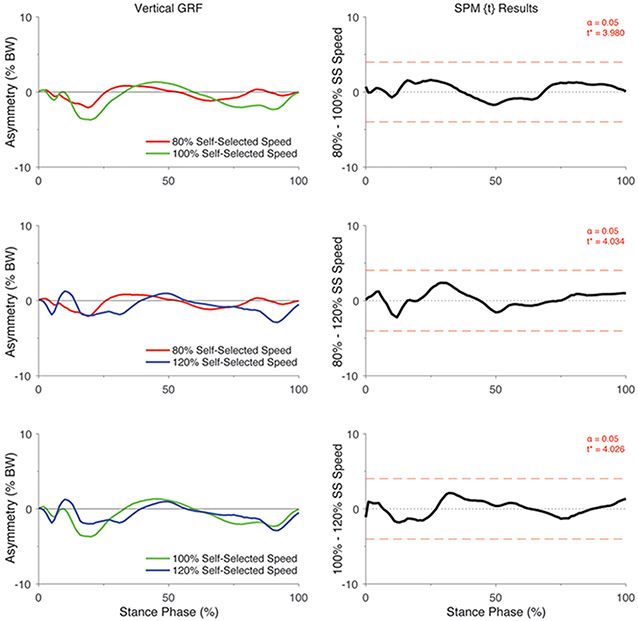
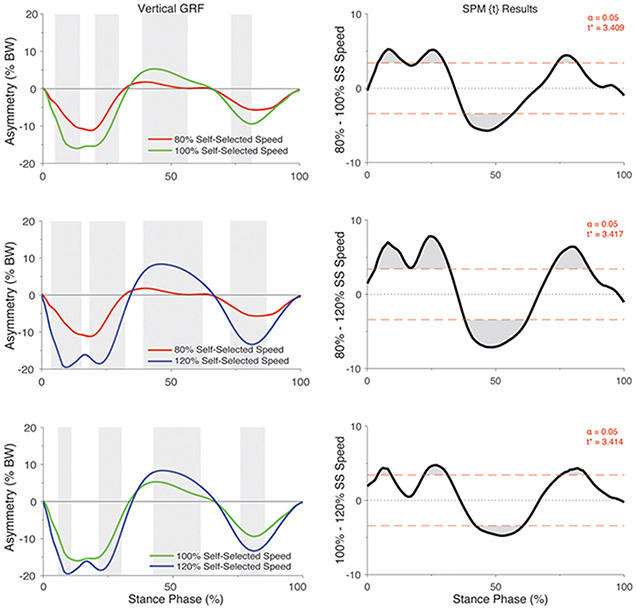
There was a significant group by speed interaction on the asymmetry in vertical GRF (F*[2,86] = 7.07, p <0.05; Figure 1). Post hoc analyses of the interaction indicated that the asymmetry in vertical GRF did not differ between speeds in the control participants (all p > 0.05; Figure 2) but differed significantly in individuals with ACL reconstruction (all p < 0.05; Figure 3). Specifically, asymmetry increased with increases in walking speed, with 80% self-selected speed showing the lowest asymmetry and 120% self-selected speed showing the greatest asymmetry (i.e., 100% > 80% of self-selected walking speed and 120% > 80% and 100% of self-selected walking speeds) (Figure 3). Ensemble averages of the vertical GRF waveforms for both limbs that were used to compute GRF asymmetries can be found in Supplemental Figure 1.
Figure 1:
SPM [F] main effects and interaction results for the vertical and posterior-anterior ground reaction force components. For each graph, the red dashed line corresponds to the critical F thresholds, denoted as F*. Any portion of the waveform that exceeded this threshold (shaded in grey) indicates a significance difference at p < 0.05.
Figure 2.
SPM[t] Post hoc paired t-tests showing the effect of walking speed on asymmetry in vertical GRF (asymmetry: % BW) for uninjured control participants. For column 1, positive values represent higher vertical GRFs in the “injured” relative to the contralateral limb whereas negative values indicate lower vertical GRFs in the “injured” relative to the contralateral limb. For each graph in column 2, the red dashed line corresponds to the critical t thresholds, denoted as t*. Any portion of the waveform exceeding this threshold (shaded in grey) indicates a significance difference between speeds at p < 0.05. Note that the limb that had lower peak vertical GRF during the self-selected speed walking condition was considered as the “injured limb” for the control participants. SS = self-selected speed.
Figure 3.
SPM[t] Post hoc paired t-tests showing the effect of walking speed on asymmetry in vertical GRF (asymmetry: % BW) for individuals with ACL reconstruction. For column 1, positive values represent higher vertical GRFs in the injured relative to the contralateral limb whereas negative values indicate lower vertical GRFs in the injured relative to the contralateral limb. Shaded regions in column 1 correspond to significant regions identified in the SPM [t] results in column 2. For each SPM [t] graph, the red dashed line corresponds to the critical t thresholds, denoted as t*. Any portion of the waveform exceeding this threshold (shaded in grey) indicates a significance difference between speeds p < 0.05. SS = Self-selected speed.
Posterior-Anterior Ground Reaction Force
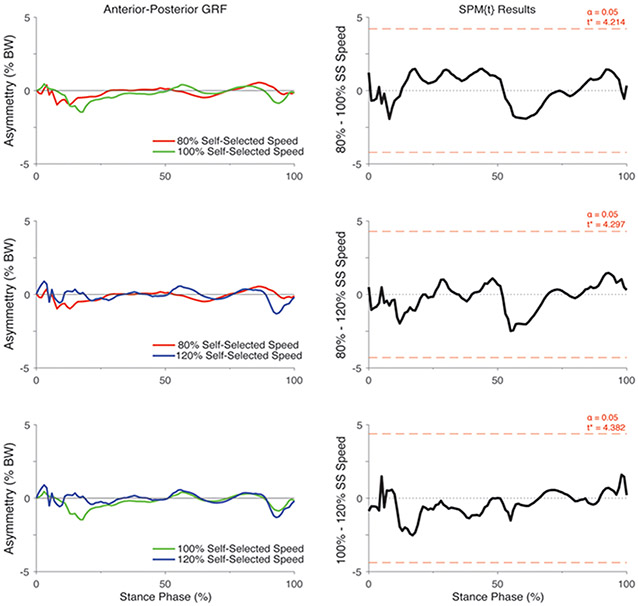
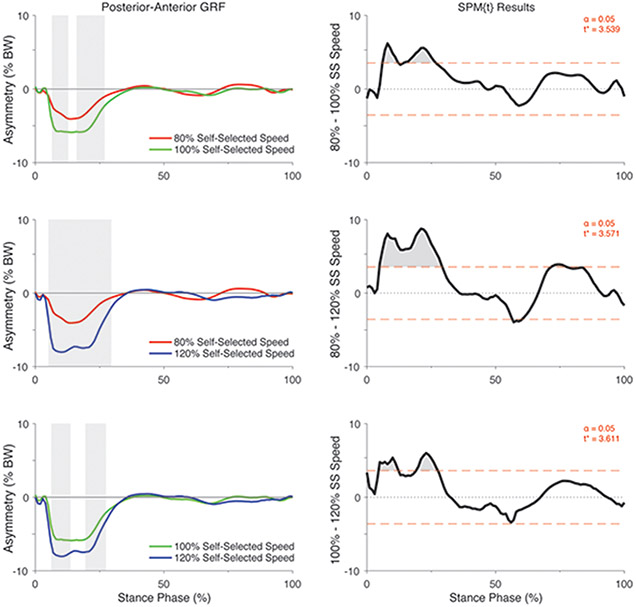
There was a significant group by speed interaction on the asymmetry in posterior-anterior GRF (F*[2,86] = 7.17, p <0.05; Figure 1). Post hoc analyses of the interaction indicated that the asymmetry in posterior-anterior GRF did not differ between speeds in the control participants (All p > 0.05; Figure 4) but differed significantly in individuals with ACL reconstruction (All p < 0.05; Figure 5). Specifically, asymmetry increased in the posterior GRF with increases in walking speed, with 80% self-selected speed showing the lowest asymmetry and 120% self-selected speed showing the greatest asymmetry (i.e., 100% > 80% of self-selected walking speed and 120% > 80% and 100% of self-selected walking speeds) (Figure 5). Ensemble averages of the posterior-anterior GRF waveforms for both limbs that were used to compute GRF asymmetries in each group can be found in Supplemental Figure 2.
Figure 4.
SPM [t] Post hoc paired t-tests showing the effect of walking speed on asymmetry in posterior-anterior GRF (asymmetry: % BW) for uninjured control participants. For column 1, positive values represent higher posterior-anterior GRFs in the “injured” relative to the contralateral limb whereas negative values indicate lower vertical GRF’s in the “injured” relative to the contralateral limb. For each SPM [t] graph, the red dashed line corresponds to the critical t thresholds, denoted as t*. Any portion of the waveform exceeding this threshold (shaded in grey) indicates a significance difference between speeds at p < 0.05. Note that the limb that had lower peak vertical GRF during the self-selected walking speed condition was considered as the “injured limb” for the control participants. SS = Self-selected speed.
Figure 5.
SPM [t] Post hoc paired t-tests showing the effect of walking speed on asymmetry in posterior-anterior GRF (asymmetry: % BW) for individuals with ACL reconstruction. For column 1, positive values represent higher vertical GRFs in the injured relative to the contralateral limb whereas negative values indicate lower vertical GRFs in the “injured” relative to the contralateral limb. Shaded regions in column 1 correspond to significant regions identified in the SPM [t] results in column 2. For each SPM [t] graph, the red dashed line corresponds to the critical t thresholds, denoted as t*. Any portion of the waveform exceeding this threshold (shaded in grey) indicates a significance difference between speeds p < 0.05. SS = Self-selected speed.
Discussion:
The purpose of this study was to examine the effect of gait speed on asymmetry in vertical and posterior-anterior GRFs in individuals with ACL reconstruction and compare the results with uninjured controls. We found that the asymmetry in both vertical and posterior-anterior GRFs increased at faster walking speeds but decreased at slower speeds in individuals with ACL reconstruction. Conversely, speed did not affect GRF asymmetries in uninjured controls. These findings are clinically meaningful as assessing gait biomechanics at multiple walking speeds may be necessary to magnify existing gait asymmetries in individuals with ACL reconstruction. Ultimately, this may improve our ability to identify the extent of underlying gait impairments simply by manipulating task-specific constraints like walking speed.
As hypothesized, asymmetry in the vertical GRF was affected by walking speed in individuals with ACL reconstruction, but not in healthy controls. We found that asymmetries in the vertical GRF were smallest at 80% of self-selected speed (approximately 10% BW, Figure 3) but greatest at 120% of self-selected speed (approximately 18-20% BW, Figure 3). Our results appear to be consistent with findings from previous investigations in several neurologic/pathological populations (i.e., post-stroke, amputees) which also show an augmentation of gait asymmetries when task-constraints like speed are increased 19-23. In healthy individuals, however, our results and others suggest the effects of increased speeds on gait symmetries appear to be less pronounced or non-existent 23-25. Although direct comparison of our results to other ACL cohorts is limited, several previous studies provide support that more biomechanically demanding tasks (i.e., stair walking, jump landing, and running) show greater biomechanical impairments in ACL knees compared to those seen in self-selected level walking 9; 11; 12. Nonetheless, it is important to recognize that tasks like hopping or landing, for example, may be contraindicated early after ACL reconstruction, and the biomechanical and neuromuscular control demands required during these tasks differ from those of walking9; 40. Thus, given the results of our study, we believe manipulating walking speed could be a viable option to impose increased (or decreased) tasks-demands when evaluating gait biomechanics. This may be particularly useful in earlier stages of rehabilitation when patients are yet to advance to running or hopping. It is important to point out, however, that our study limited speed manipulations to 20% above and below self-selected, in part, so that all patients were capable of completing each walking condition safely without the use of handrails for stabilization and/or support. Thus, it is not clear if using even greater speed changes (i.e. 30-40% above or below self-selected) may more effectively reveal compensatory gait strategies in those with ACL reconstruction. Future studies should examine the use of a wider range of speed manipulations when aiming to evaluate the effects of speeds on walking mechanics in this patient population.
We observed that individuals with ACL reconstruction walk with marked asymmetries in the vertical GRF across the majority of stance phase. Our results also demonstrate the magnitude of these asymmetries may not be consistent across multiple walking speeds. When examining the ensemble averages of the vertical GRF for each limb and both groups (Figure S-1), it is apparent that ACL subjects primarily modulate the vertical GRF of their injured limb by decreasing its magnitude in early and late stance phase while also undergoing substantially less unloading during midstance (i.e. greater GRFs in the injured relative to the contralateral). At the highest speed, the vertical GRF appears to disproportionately increase in the uninjured relative to the injured limb, while at the slowest speed there is the least modulation in GRF and the greatest symmetry between limbs. These alterations in the vertical GRF corroborate findings from several previous studies at self-selected speeds 1; 8 and have been generally described as a less “dynamic” or blunted GRF waveform (i.e., less midstance unloading). Overall, we reason these gait alterations are reflective of ACL participants attempting to avoid or limit loading of the reconstructed limb by stepping more lightly at faster speeds, possibly because of fear of re-injury or apprehension due to increased task-demands. This aberrant loading phenomenon has also been accompanied by and associated with greater quadriceps-to-hamstring co-contraction and less knee flexion excursion which is thought to reflect a stiffened knee strategy41. Based on our findings, it is plausible that these compensatory strategies may become magnified by increasing walking speed but additional research that includes knee-specific gait metrics (i.e., moments, angles etc.) are needed to substantiate our findings.
Further, as stated above, our data provide supporting evidence that vertical GRF alterations in ACL reconstruction individuals are not only apparent in early stance/load acceptance phases but also extend into mid- and late-stance 1; 8. While the clinical significance of the timing/duration of stance phase GRF alterations are not entirely clear, it is reasonable to surmise that consistent loading asymmetries could be more detrimental to patient outcomes or knee joint health than ones that occur more discretely (e.g. only at initial contact or at peak loading). Future research is needed, however, to elucidate the biomechanical factors associated with mid- and late- stance vertical GRF alterations and how these aberrant gait mechanics are associated with patient outcomes and/or knee joint health. Furthermore, although not measured here, interlimb differences in spatiotemporal parameters may partly contribute to these GRF asymmetries as recent findings have shown patients approximately 8 months post-ACL reconstruction walk with asymmetrical step lengths42. Future studies should consider evaluating the contributions of spatiotemporal parameters to loading characteristics in those with ACL reconstruction.
Although many studies have highlighted the importance of vertical GRF asymmetries after ACL reconstruction1; 2; 5-8, less data is available detailing asymmetries in other components like the posterior-anterior GRF 33; 34. Here, we found that asymmetries in the posterior GRF (i.e., breaking forces) became exacerbated when speeds were increased and reduced at slower speeds in individuals with ACL reconstruction, but not in uninjured controls. However, we did not observe differences across speeds in the anterior GRF (i.e., propulsive GRF) in either group. The increase in posterior GRF asymmetry observed in response to elevated walking speeds could be explained by findings that suggest posterior GRFs are primarily counteracted by the knee extensors during loading response, while other anti-gravity muscle groups like the hip extensors may also contribute43. Thus, it is reasonable that the stiffened-knee gait strategy often adopted by patients after ACL-reconstruction may underlie findings of asymmetrical posterior GRFs as this would aid in limiting knee extensor demands particularly when walking speeds are increased44. While it may appear that the observed changes in the posterior GRF between walking speeds were small (i.e. approximately 2-5% BW change), we argue that these changes may represent important and meaningful alterations in GRF asymmetries given that GRF peak magnitudes generally range between 15-25% BW (Figure S-2). Thus, a 2-5% BW change in the posterior GRF asymmetries between speeds would correspond to a relatively large reduction (or improvement) in GRF symmetry.
Similar to several recent investigations, our results also suggest that asymmetries in the posterior GRF may be more pronounced than the vertical component in individuals with ACL reconstruction 33; 34. Although only three studies have evaluated posterior GRFs (including the current investigation) to date, it appears that posterior forces may be modulated to a greater extent than vertical support forces at least in earlier phases of rehabilitation. These findings are important given the strong associations between posterior GRFs and knee-specific metrics like the knee extensor moment in ACL reconstructed individuals 33; 34. It is possible that posterior GRFs could serve as a potential target for gait rehabilitation strategies in individuals with ACL reconstruction 33. However, future research is needed to elucidate the factors contributing to posterior GRF alterations and to identify avenues to target posterior GRF asymmetries; knowledge critical to improve our ability to restore gait mechanics in this population.
There are some potential limitations to this study. Our study was cross-sectional in design and included individuals with acute ACL reconstruction. As such, it is unclear if our results are generalizable to individuals in more chronic time-periods after ACL reconstruction or if the effects of speed diminish as individuals progress through rehabilitation. Nonetheless, evaluating gait characteristics early in the rehabilitation process may important given that gait alterations also occur in the uninjured limb which may confound calculations of symmetry indices at later time points. Furthermore, identification of asymmetries early after ACL reconstruction and treating them appropriately may allow for more symmetrical movements in later periods after ACL reconstruction and may also be useful as a criterion to consider before patients are progressed to more difficult movement tasks (jogging, hopping, etc.). We also did not randomize speed conditions in our study (i.e., the order was always self-selected, 120%, 80%) which may have affected our study outcomes. However, we do not suspect this is likely given that speed demonstrated similar effects on gait asymmetries here as it has in other investigations with pathological populations 19-23. Each speed condition also consisted of 65 second trials and it is possible that longer walking and familiarization periods would have led to different results. One benefit of the treadmill is that it allows for capture of multiple successive gait cycles and as such, we captured on average over 50 gait cycles for each condition and subject which we believe offers a sufficient representation of an individual’s walking gait. Future investigations should consider randomizing speeds and using longer familiarization periods when assessing gait across walking speeds. We also recognize that the presence of quadriceps weakness and/or increased muscle co-activation may have influenced the magnitude of GRF asymmetry and speed effects observed in our cohort 45. While the aims of this study were not to evaluate the effects of impaired quadriceps function on GRF asymmetries across walking speeds, future research should determine if ACL-reconstructed individuals with stronger quadriceps can maintain GRF symmetries despite manipulating walking speeds, similar to that of control participants. Our data also considered GRF characteristics as global measures of gait function after ACL reconstruction. Thus, we are unable to ascertain if walking speed has a similar effect on other biomechanical characteristics like joint-specific or spatiotemporal outcomes in individuals with ACL-reconstruction. It would be beneficial to evaluate if manipulating walking speed similarly leads to greater (or lesser) asymmetries in joint-specific outcomes like the knee extensor moment, or if individuals with ACL reconstruction adopt compensatory strategies at the ankle or hip in order to meet the demands imposed by varying walking speeds. Lastly, we note our study design included unequal sample sizes and while controls were matched by age (±3) and sex, matching between groups was not 1 to 1 which could affect the power of our statistical analyses.
Conclusion:
Increased walking speed magnifies biomechanical asymmetries in the vertical and posterior-anterior GRF in individuals with ACL reconstruction. At slower speeds, individuals with ACL reconstruction can walk with more symmetrical GRFs (i.e., decreased interlimb differences) compared with self-selected and fast speeds. It may be beneficial to evaluate speeds at and above self-selected walking speeds to evaluate the extent of gait biomechanical alterations in those with ACL reconstruction.
Supplementary Material
Supplementary Figure 1. Bilateral mean ground reaction force and posterior-anterior waveforms across stance phase for all walking speeds for uninjured controls and individuals with anterior cruciate ligament reconstruction. Negative values represent the posterior component of the ground reaction force vector while positive values indicate an anteriorly directed force vector. GRF: ground reaction force, ACLR: anterior cruciate ligament reconstruction.
Acknowledgements.
This work was supported by the National Institute of Child Health and Human Development of the National Institutes of Health (Grant # R21 HD092614-02) and the University of Michigan Undergraduate Research Opportunity Program (UROP).
Footnotes
Conflicts of Interest Statement: The authors report no conflicts of interest for the current investigation.
References:
- 1.Davis-Wilson HC, Pfeiffer SJ, Johnston CD, et al. 2019. Bilateral Gait Six and Twelve Months Post-ACL Reconstruction Compared to Controls. Med Sci Sports Exerc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luc-Harkey BA, Franz JR, Blackburn JT, et al. 2018. Real-time biofeedback can increase and decrease vertical ground reaction force, knee flexion excursion, and knee extension moment during walking in individuals with anterior cruciate ligament reconstruction. J Biomech 76:94–102. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer SJ, Spang J, Nissman D, et al. 2019. Gait Mechanics and T1rho MRI of Tibiofemoral Cartilage 6 Months after ACL Reconstruction. Med Sci Sports Exerc 51:630–639. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Mundermann A, Smith RL, et al. 2004. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng 32:447–457. [DOI] [PubMed] [Google Scholar]
- 5.Pietrosimone B, Blackburn JT, Padua DA, et al. 2018. Walking gait asymmetries 6 months following anterior cruciate ligament reconstruction predict 12-month patient-reported outcomes. J Orthop Res 36:2932–2940. [DOI] [PubMed] [Google Scholar]
- 6.Luc-Harkey BA, Franz JR, Hackney AC, et al. 2018. Lesser lower extremity mechanical loading associates with a greater increase in serum cartilage oligomeric matrix protein following walking in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 60:13–19. [DOI] [PubMed] [Google Scholar]
- 7.Pietrosimone B, Blackburn JT, Harkey MS, et al. 2016. Greater Mechanical Loading During Walking Is Associated With Less Collagen Turnover in Individuals With Anterior Cruciate Ligament Reconstruction. Am J Sports Med 44:425–432. [DOI] [PubMed] [Google Scholar]
- 8.Pietrosimone B, Seeley MK, Johnston C, et al. 2019. Walking Ground Reaction Force Post-ACL Reconstruction: Analysis of Time and Symptoms. Med Sci Sports Exerc 51:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeiffer SJ, Blackburn JT, Luc-Harkey B, et al. 2018. Peak knee biomechanics and limb symmetry following unilateral anterior cruciate ligament reconstruction: Associations of walking gait and jump-landing outcomes. Clin Biomech (Bristol, Avon) 53:79–85. [DOI] [PubMed] [Google Scholar]
- 10.Tashman S, Collon D, Anderson K, et al. 2004. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med 32:975–983. [DOI] [PubMed] [Google Scholar]
- 11.Tashman S, Kolowich P, Collon D, et al. 2007. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res 454:66–73. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder MJ, Krishnan C, Dhaher YY. 2015. The influence of task complexity on knee joint kinetics following ACL reconstruction. Clin Biomech (Bristol, Avon) 30:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice J, Seeley MK. 2010. An investigation of lower-extremity functional asymmetry for non-preferred able-bodied walking speeds. Int J Exerc Sci 3:182–188. [PMC free article] [PubMed] [Google Scholar]
- 14.Georgoulis AD, Moraiti C, Ristanis S, et al. 2006. A novel approach to measure variability in the anterior cruciate ligament deficient knee during walking: the use of the approximate entropy in orthopaedics. J Clin Monit Comput 20:11–18. [DOI] [PubMed] [Google Scholar]
- 15.Chiu SL, Chou LS. 2012. Effect of walking speed on inter-joint coordination differs between young and elderly adults. J Biomech 45:275–280. [DOI] [PubMed] [Google Scholar]
- 16.Ghanavati T, Salavati M, Karimi N, et al. 2014. Intra-limb coordination while walking is affected by cognitive load and walking speed. J Biomech 47:2300–2305. [DOI] [PubMed] [Google Scholar]
- 17.Walsh GS, Taylor Z. 2019. Complexity, symmetry and variability of forward and backward walking at different speeds and transfer effects on forward walking: Implications for neural control. J Biomech 97:109377. [DOI] [PubMed] [Google Scholar]
- 18.Liu MQ, Anderson FC, Schwartz MH, et al. 2008. Muscle contributions to support and progression over a range of walking speeds. J Biomech 41:3243–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan L, Wit A, Dudzinski K, et al. 2003. Adjustments in gait symmetry with walking speed in trans-femoral and trans-tibial amputees. Gait Posture 17:142–151. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Mukaino M, Ohtsuka K, et al. 2020. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int J Rehabil Res 43:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanderson DJ, Martin PE. 1997. Lower extremity kinematic and kinetic adaptations in unilateral below-knee amputees during walking. Gait Posture 6:126–136. [Google Scholar]
- 22.Beaman CB, Peterson CL, Neptune RR, et al. 2010. Differences in self-selected and fastest-comfortable walking in post-stroke hemiparetic persons. Gait Posture 31:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bejek Z, Paroczai R, Illyes A, et al. 2006. The influence of walking speed on gait parameters in healthy people and in patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc 14:612–622. [DOI] [PubMed] [Google Scholar]
- 24.Silverman AK, Fey NP, Portillo A, et al. 2008. Compensatory mechanisms in below-knee amputee gait in response to increasing steady-state walking speeds. Gait Posture 28:602–609. [DOI] [PubMed] [Google Scholar]
- 25.Goble DJ, Marino GW, Potvin JR. 2003. The influence of horizontal velocity on interlimb symmetry in normal walking. Hum Mov Sci 22:271–283. [DOI] [PubMed] [Google Scholar]
- 26.Seeley MK, Umberger BR, Shapiro R. 2008. A test of the functional asymmetry hypothesis in walking. Gait Posture 28:24–28. [DOI] [PubMed] [Google Scholar]
- 27.Garcia SA, Moffit TJ, Vakula MN, et al. 2020. Quadriceps Muscle Size, Quality, and Strength and Self-Reported Function in Individuals With Anterior Cruciate Ligament Reconstruction. J Athl Train 55:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuenze CM, Hertel J, Weltman A, et al. 2015. Persistent neuromuscular and corticomotor quadriceps asymmetry after anterior cruciate ligament reconstruction. J Athl Train 50:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisee C, Lepley AS, Birchmeier T, et al. 2019. Quadriceps Strength and Volitional Activation After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Sports Health 11:163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otzel DM, Chow JW, Tillman MD. 2015. Long-term deficits in quadriceps strength and activation following anterior cruciate ligament reconstruction. Phys Ther Sport 16:22–28. [DOI] [PubMed] [Google Scholar]
- 31.Nazary-Moghadam S, Salavati M, Esteki A, et al. 2019. Gait speed is more challenging than cognitive load on the stride-to-stride variability in individuals with anterior cruciate ligament deficiency. Knee 26:88–96. [DOI] [PubMed] [Google Scholar]
- 32.Evans-Pickett A, Davis-Wilson HC, Luc-Harkey BA, et al. 2020. Biomechanical effects of manipulating peak vertical ground reaction force throughout gait in individuals 6-12 months after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 76:105014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin PE, Sigward SM. 2019. Subtle alterations in whole body mechanics during gait following anterior cruciate ligament reconstruction. Gait Posture 68:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin PE, Sigward SM. 2018. Contributors to knee loading deficits during gait in individuals following anterior cruciate ligament reconstruction. Gait Posture 66:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armitano-Lago C, Pietrosimone B, Davis-Wilson HC, et al. 2020. Biofeedback augmenting lower limb loading alters the underlying temporal structure of gait following anterior cruciate ligament reconstruction. Hum Mov Sci 73:102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutherford D, Baker M, Wong I, et al. 2017. The effect of age and knee osteoarthritis on muscle activation patterns and knee joint biomechanics during dual belt treadmill gait. J Electromyogr Kinesiol 34:58–64. [DOI] [PubMed] [Google Scholar]
- 37.Washabaugh EP, Kalyanaraman T, Adamczyk PG, et al. 2017. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 55:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pataky TC. 2012. One-dimensional statistical parametric mapping in Python. Comput Methods Biomech Biomed Engin 15:295–301. [DOI] [PubMed] [Google Scholar]
- 39.Pataky TC, Robinson MA, Vanrenterghem J. 2013. Vector field statistical analysis of kinematic and force trajectories. J Biomech 46:2394–2401. [DOI] [PubMed] [Google Scholar]
- 40.Winters JM, Crago PE. 2000. Biomechanics and neural control of posture and movement. New York: Springer; xxii, 683 p. p. [Google Scholar]
- 41.Blackburn T, Pietrosimone B, Goodwin JS, et al. 2019. Co-activation during gait following anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 67:153–159. [DOI] [PubMed] [Google Scholar]
- 42.Hunnicutt JL, McLeod MM, Slone HS, et al. 2020. Quadriceps Neuromuscular and Physical Function After Anterior Cruciate Ligament Reconstruction. J Athl Train 55:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu MQ, Anderson FC, Pandy MG, et al. 2006. Muscles that support the body also modulate forward progression during walking. J Biomech 39:2623–2630. [DOI] [PubMed] [Google Scholar]
- 44.Neptune RR, Sasaki K, Kautz SA. 2008. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 28:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewek M, Rudolph K, Axe M, et al. 2002. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 17:56–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Bilateral mean ground reaction force and posterior-anterior waveforms across stance phase for all walking speeds for uninjured controls and individuals with anterior cruciate ligament reconstruction. Negative values represent the posterior component of the ground reaction force vector while positive values indicate an anteriorly directed force vector. GRF: ground reaction force, ACLR: anterior cruciate ligament reconstruction.