Abstract
Objectives
An estimated 33–37% of incident cancers in Canada are attributable to modifiable risk factors. Interventions targeting these risk factors would minimize the substantial health and economic burdens Canadians face due to cancer. We estimate the future health and economic burden of cancer in Canada by incorporating data from the Canadian Population Attributable Risk of Cancer (ComPARe) study into OncoSim, a web-based microsimulation tool.
Methods
Using the integrated OncoSim population attributable risk and population impact measures, we evaluated risk factor-targeted intervention scenarios implemented in 2020, assuming the targeted risk factor prevalence reduction would be achieved by 2032 with a 12-year latency period.
Results
We estimate that smoking will be the largest contributor to cancer-related costs, with a cost of CAD $44.4 billion between 2032 and 2044. An estimated CAD $3.3 billion of the cost could be avoided with a 30% reduction in smoking prevalence by 2022. Following smoking, the next highest cancer management costs are associated with inadequate physical activity and excess body weight, accounting for CAD $10.7 billion ($2.7 billion avoidable) and CAD $9.8 billion ($3.2 billion avoidable), respectively. Avoidable costs for other risk factors range from CAD $90 million to CAD $2.5 billion.
Conclusion
Interventions targeting modifiable cancer risk factors could prevent a substantial number of incident cancer cases and billions of dollars in cancer management costs. With limited budgets and rising costs in cancer care in Canada, these simulation models and results are valuable for researchers and policymakers to inform decisions and prioritize and evaluate intervention programs.
Supplementary Information
The online version contains supplementary material available at 10.17269/s41997-021-00502-x.
Keywords: Cancer management, Cancer cost, Cancer prevention, Economic burden
Résumé
Objectifs
Il est estimé que de 33 % à 37 % des cancers incidents au Canada sont imputables à des facteurs de risque modifiables. Des interventions ciblant ces facteurs de risque réduiraient le fardeau sanitaire et économique considérable du cancer dans la population canadienne. Nous avons estimé le futur fardeau sanitaire et économique du cancer au Canada en intégrant les données de l’étude ComPARe (Canadian Population Attributable Risk of Cancer) dans l’outil de microsimulation en ligne OncoSim.
Méthode
À l’aide des indicateurs d’impact dans la population et du risque attribuable dans la population intégrés dans OncoSim, nous avons évalué des scénarios d’intervention mis en œuvre en 2020 axés sur les facteurs de risque, en partant de l’hypothèse que la réduction de la prévalence des facteurs de risque ciblés serait atteinte d’ici 2032 avec une période de latence de 12 ans.
Résultats
Nous estimons que le tabagisme sera le facteur qui contribuera le plus aux coûts du cancer, avec un coût de 44,4 milliards $ CA entre 2032 et 2044. Il est estimé qu’une part de 3,3 milliards $ CA de ce coût pourrait être évitée en réduisant de 30 % la prévalence du tabagisme d’ici 2022. Après le tabagisme, les coûts de prise en charge du cancer les plus élevés sont associés à l’inactivité physique et au surpoids, qui représentent respectivement 10,7 milliard $ CA (dont 2,7 milliards $ évitables) et 9,8 milliards $ CA (dont 3,2 milliards $ évitables). Les coûts évitables pour d’autres facteurs de risque vont de 90 millions $ CA à 2,5 milliards $ CA.
Conclusion
Des interventions ciblant les facteurs de risque de cancer modifiables pourraient prévenir un nombre considérable de cas de cancers incidents et épargner des milliards de dollars en coûts de prise en charge du cancer. Avec les budgets serrés et la hausse des coûts des soins du cancer au Canada, ces modèles de simulation et leurs résultats permettent aux chercheurs et aux responsables des politiques d’éclairer les décisions et de hiérarchiser et d’évaluer les programmes d’intervention.
Mots-clés: Prise en charge du cancer, coût du cancer, prévention du cancer, fardeau économique
Introduction
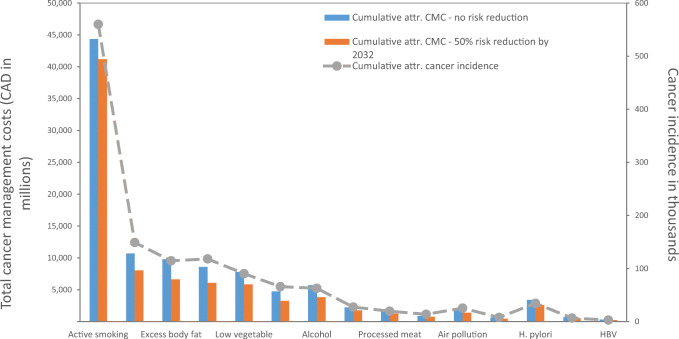
Cancer is the leading cause of mortality among Canadians (Public Health Agency of Canada 2019). Recent statistics show that approximately one in two Canadians is expected to develop cancer in their lifetimes, and that one in four Canadians will die from the disease (Brenner et al. 2020). Lung, colorectal, breast and prostate cancers accounted for approximately half of all cancer diagnoses and deaths in the Canadian population in 2019 (Brenner et al. 2020). The burden of cancer in Canada is expected to continue to rise because of population growth, changing population demographics, and cancer incidence increases for specific (or common) types of cancer (Brenner et al. 2020) (Fig. 1).
Fig. 1.
Cumulative cancer incidence and cancer management costs attributable to selected modifiable risk factors in Canada, 2032-2044. Abbreviations: attr., attributable; CMC, cancer management costs; H. pylori, Helicobacter pylori; HBV, hepatitis B virus; HCV, hepatitis C virus
Systematic reviews and meta-analyses by the International Agency for Research on Cancer (IARC) and the World Cancer Research Fund (WCRF) have provided sufficient evidence that modifiable lifestyle factors and exposures alter the risk of multiple common and less common cancers (International Agency for Research on Cancer (IARC) 2018; World Cancer Research Fund/American Institute for Cancer Research 2018). We previously estimated the current and future burden of cancer in Canada due to modifiable lifestyle and environmental risk factors in the Canadian Population Attributable Risk of Cancer (ComPARe) study (Brenner et al. 2018; Poirier et al. 2019). We also estimated cancer mortality due to these factors (Pader et al. 2021). Specifically, for the current burden, we estimated that 33% of cancer cases diagnosed in 2015 were attributable to risk factors such as smoking, alcohol, low consumption of fruit and vegetables, excess body weight and inadequate physical activity (Poirier et al. 2019). For the future burden, we estimated that up to 40,000 cases of cancer per year could be prevented by 2042 by reducing exposure to lifestyle and environmental risk factors and infections. The ComPARe study did not include estimates of the associated current and future costs of cancer or the potential cost savings associated with cancer prevention due to those modifiable risk factors.
The provision of resources for cancer management is a key concern for a growing and aging Canadian population. Cancer management costs are increasing faster than inflation (de Oliveira et al. 2018) and entail both direct (e.g., diagnosis and treatment) and indirect (e.g., patient’s lost days of productivity) costs, as well as quality of life impacts for patients. In Canada, cancer costs increased from $2.9 billion in 2005 to $7.5 billion in 2012 (de Oliveira et al. 2018). Organizations that fund cancer treatments and related cancer care have access to finite government or financial resources (de Oliveira et al. 2018). Estimates of the future economic cancer burden and the health and economic impacts of various changes to modifiable cancer risk factors provide decision makers with an understanding of cost-benefit and the potential outcomes associated with various planning scenarios. For example, estimating future cancer management costs may provide supporting evidence for investments in population-based interventions for modifiable risk factors in resource-constrained environments. Interventions for different modifiable risk factors can yield varying outcomes in terms of incidence, deaths, and cancer management cost savings. Estimating cancer incidence, deaths, and management cost on varying levels of interventions compared to baseline (no new intervention) provides evidence for policy- and decision makers regarding which interventions are most beneficial and cost-effective for the population.
Microsimulation models can be a powerful tool in cancer research due to their capability to provide answers that are not easily fulfilled through typical observational studies or randomized control trials. These models can retain memory of prior behaviours and states which allows for a clear representation and understanding of how various inputs and interventions over time impact the outcomes in a population (Çağlayan et al. 2018). Here, we report our work integrating the findings from the ComPARe study into OncoSim to assess the future cancer management costs that can be attributed to modifiable risk factors and costs that could be saved with effective interventions and prevention programs in Canada from 2032 to 2044.
Methods
OncoSim is a health and economic microsimulation model led and supported by the Canadian Partnership Against Cancer (CPAC), with model development by Statistics Canada and funded by Health Canada. Using Canadian-specific data, the OncoSim model is tailored for projecting and evaluating cancer programs, their costs and their impact among the Canadian population (Gauvreau et al. 2017). It is a free, web-based simulation tool that evaluates cancer control strategies. Users can change input parameters through a web interface to create scenarios to answer their policy questions. Combining data from the real world, expert opinion and the published literature, OncoSim projects health measures (e.g., cancer incidence, deaths, quality-adjusted life-years) and health costs, and attributes them to 27 risk factors in the Canadian population (https://www.partnershipagainstcancer.ca/tools/oncosim/). It models four cancer sites (breast, cervical, colorectal and lung) in detail and provides high-level projections for 28 other cancer sites (Table S1). The OncoSim suite of models has been used to inform various cancer policies and decisions across Canada (Gauvreau et al. 2017). Earlier versions of OncoSim used data from the ComPARe study to estimate cancer burden attributable to risk factors (Poirier et al. 2019). Costs from de Oliveira 2016 were integrated into the model (de Oliveira et al. 2016). Mean costs of care were categorized by phase of care, tumour site and sex. Phases of care include pre-diagnosis phase (3 months prior to diagnosis), initial phase (6 months after diagnosis), continuing care phase (following initial phase and prior to the last 12 months of life phase) and terminal phase (up to last 12 months of life). These costs are taken as direct inputs to the simulation and are applied to individuals diagnosed with cancer according to type and how they progress through the phases in the simulation. A summary of cancer management costs by phase of cancer progression, cancer site and sex is presented in Table S2.
Integration of the ComPARe study data into the OncoSim platform
To integrate the ComPARe study results into the OncoSim framework, the ComPARe study team provided the estimated population attributable risks (PAR), attributable cases (AC), potential impact fractions (PIF), and preventable cases (PC) for each risk factor, sex, age group (PAR and AC only), year (PIF and PC only) and geographic region, as well as the data to estimate these measures (i.e., risk factor prevalence (current and projected), relative risk (RR)). A list of acronyms used in this article is presented in Table S3. The OncoSim development team then integrated these data into the all-cancer model of the microsimulation tool by following the methods used in the ComPARe study (Brenner et al. 2019; Brenner et al. 2018).
In the integrated OncoSim model (version 3.3.3 and onwards), users can estimate PAR and PIF by applying input parameters associated with risk factor exposure prevalence and the RR, as well as assumed latency periods between exposure and outcomes, with default values taken from the ComPARe study. PARs and PIFs were then multiplied by simulation model outputs (incidence, deaths and cancer treatment costs) to generate attributable cases, deaths and costs. The cancer treatment costs in OncoSim were direct health care costs, including the costs of screening, clinical diagnosis, hospital and physicians, surgery, chemotherapy and radiotherapy, and palliative care.
The integrated OncoSim model also facilitates the estimation of counterfactual scenarios to assess potential impacts of various risk factor interventions on projected outcomes (incidence, deaths and cancer treatment costs). The default model contains the projected risk factor prevalence trends from the ComPARe study (e.g., the prevalence of active and passive smoking is projected to decrease by 2032; the prevalence of alcohol drinking and excess body weight is projected to increase by 2032; the prevalence of red and processed meat consumption, and of UV risk behaviours are assumed to remain unchanged by 2032) (O’Sullivan et al. 2019). Users are able to modify risk factor prevalence distributions through input parameters to set up various counterfactuals. The model can generate outputs for the preventable cases, mortality, and cancer management costs (the difference between default scenario and counterfactuals). In addition, per-capita intervention costs (which can vary by province) are multiplied by the corresponding population counts to generate the total cost of risk factor intervention programs. Together, the model allows calculation of cumulative clinical outcomes and cancer treatment cost, as well as net budgetary impacts of implementing prevention programs over time. The model provides the projection of clinical and economic outcomes up to year 2051. All the costs presented in our analyses were in 2019 Canadian dollars.
Two risk factors, residential radon and human papillomavirus (HPV), were modeled by the in-depth modules of OncoSim and not modeled in the integrated PAR/PIF module, hence not included in this report (Gauvreau et al. 2017; Smith et al. 2019).
Risk factor scenario parameter inputs
We simulated the attributable cancer management costs of 15 modifiable risk factors: active and passive smoking, alcohol consumption, excess body weight indicated by body mass index, inadequate physical activity, leisure-time sedentary behaviour, low fruit and vegetable consumption, red and processed meat consumption, ultraviolet (UV) risk behaviours (including sun tanning, sun bathing and indoor tanning), air pollution (particulate matter exposure indicated by PM2.5), Helicobacter pylori (H. pylori) infection, and hepatitis B and C virus (HBV and HCV) infection.
For each risk factor, we simulated a base/status quo scenario where all parameters were set at default, in which the risk factor prevalence would continue from the historical trend but unaltered by interventions. The intervention targets were adapted from the ComPARe study (Poirier et al. 2019) and summarized in Table S4. In this study, we did not specify details of the interventions themselves or the effectiveness of the interventions. Instead, we specified the intervention targets for the reduction in risk factor prevalence that would be achieved if the interventions were effective on a population level. All interventions started in the year 2020 and lasted up to and including 2032. A 12-year latency period between the onset of the intervention and the time when the intervention effects were reached was used for most risk factors, with the following exceptions: UV risk behaviours, where a 5-year latency was applied (O’Sullivan et al. 2019), and infectious agents (H. pylori, HBV and HCV), where a 15-year latency was used (Volesky et al. 2019b). We assumed that the interventions would be sustainable; i.e., after the intervention ends, the exposure prevalence would remain at the level to which it had decreased. Therefore, we only modeled the cancer incidence up to the year 2032 plus the latency period. For example, with a 12-year latency, we estimated the preventable cancer cases and management costs between the years 2032 and 2044, annually and cumulatively. Cancer risk factor pairs classified as having a probable or suggestive association by the IARC (International Agency for Research on Cancer (IARC) 2018) or the WCRF (World Cancer Research Fund/American Institute for Cancer Research 2018) were included in this analysis (Table S5).
Results
All summary results are presented in Table 1, and detailed preventable incidence and management cost results by sex and year are included in the Online Resources Tables S6a–o.
Table 1.
Cumulative attributable cancer cases and the associated cancer management costs due to modifiable risk factors in Canada and the preventable cancer cases and the associated preventable cancer management costs with various intervention scenarios
| Exposure | Intervention scenarios | Female | Male | Both | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC | PCC | CMC (CAD in millions) | PCMC (CAD in millions) | ACC | PCC | CMC (CAD in millions) | PCMC (CAD in millions) | ACC | PCC | CMC (CAD in millions) | PCMC (CAD in millions) | ||
| Active smoking | Base | 249,755 | 19,766.6 | 310,267 | 24,595.0 | 560,022 | 44,361.5 | ||||||
| 10% reduction by 2032 | 246,680 | 3074 | 19,501.4 | 265.1 | 305,682 | 4585 | 24,226.4 | 368.6 | 552,362 | 7659 | 43,727.9 | 633.7 | |
| 25% reduction by 2032 | 242,069 | 7686 | 19,103.8 | 662.8 | 298,805 | 11,462 | 23,673.6 | 921.4 | 540,874 | 19,148 | 42,777.4 | 1,584.2 | |
| 50% reduction by 2032 | 234,383 | 15,371 | 18,441.0 | 1,325.5 | 287,343 | 22,924 | 22,752.2 | 1,842.8 | 521,726 | 38,295 | 41,193.2 | 3,168.3 | |
| 30% reduction by 2022 | 233,796 | 15,959 | 18,399.6 | 1,366.9 | 286,506 | 23,761 | 22,694.5 | 1,900.4 | 520,302 | 39,720 | 41,094.2 | 3,267.4 | |
| Passive smoking | Base | 9025 | 553.6 | 4753 | 341.3 | 13,778 | 894.9 | ||||||
| 10% reduction by 2032 | 8766 | 259 | 537.6 | 16.0 | 4620 | 132 | 331.8 | 9.5 | 13,387 | 391 | 869.3 | 25.6 | |
| 25% reduction by 2032 | 8378 | 647 | 513.5 | 40.1 | 4422 | 331 | 317.5 | 23.8 | 12,800 | 978 | 831.0 | 63.9 | |
| 50% reduction by 2032 | 7732 | 1293 | 473.4 | 80.2 | 4091 | 662 | 293.7 | 47.6 | 11,822 | 1955 | 767.1 | 127.8 | |
| Alcohol | Base | 20,547 | 1,869.8 | 42,291 | 3,848.7 | 62,838 | 5,718.5 | ||||||
| 10% reduction by 2032 | 19,102 | 1445 | 1,737.7 | 132.1 | 39,609 | 2683 | 3,604.7 | 243.9 | 58,710 | 4128 | 5,342.5 | 376.0 | |
| 25% reduction by 2032 | 16,934 | 3613 | 1,539.6 | 330.2 | 35,585 | 6707 | 3,238.8 | 609.9 | 52,519 | 10,319 | 4,778.4 | 940.0 | |
| 50% reduction by 2032 | 13,322 | 7225 | 1,209.5 | 660.3 | 28,878 | 13,413 | 2,628.9 | 1,219.7 | 42,199 | 20,639 | 3,838.4 | 1,880.0 | |
| WCRF Guideline | 16,888 | 3659 | 1,499.9 | 369.9 | 36,263 | 6028 | 3,282.6 | 566.0 | 53,151 | 9687 | 4,782.6 | 935.9 | |
| CDN Guideline | 18,981 | 1566 | 1,702.5 | 167.3 | 39,204 | 3088 | 3,555.4 | 293.3 | 58,184 | 4654 | 5,257.9 | 460.5 | |
| Excess body fat | Base | 63,311 | 5,070.5 | 51,256 | 4,713.3 | 114,567 | 9,783.9 | ||||||
| 10% reduction by 2032 | 59,255 | 4056 | 4,742.8 | 327.7 | 47,966 | 3290 | 4,409.8 | 303.5 | 107,221 | 7346 | 9,152.6 | 631.3 | |
| 25% reduction by 2032 | 53,172 | 10,139 | 4,251.3 | 819.3 | 43,030 | 8226 | 3,954.5 | 758.9 | 96,202 | 18,365 | 8,205.7 | 1,578.1 | |
| 50% reduction by 2032 | 43,033 | 20,278 | 3,432.0 | 1,638.6 | 34,804 | 16,451 | 3,195.6 | 1,517.7 | 77,837 | 36,730 | 6,627.6 | 3,156.3 | |
| Inadequate physical activity | Base | 91,738 | 6,467.7 | 57,382 | 4,247.3 | 149,120 | 10,715.0 | ||||||
| 10% reduction by 2032 | 87,215 | 4523 | 6,146.9 | 320.8 | 54,518 | 2865 | 4,034.4 | 212.9 | 141,733 | 7387 | 10,181.3 | 533.6 | |
| 25% reduction by 2032 | 80,431 | 11,307 | 5,665.8 | 801.9 | 50,221 | 7162 | 3,715.1 | 532.2 | 130,651 | 18,469 | 9,380.9 | 1,334.1 | |
| 50% reduction by 2032 | 69,124 | 22,614 | 4,863.9 | 1,603.8 | 43,059 | 14,323 | 3,182.9 | 1,064.4 | 112,183 | 36,937 | 8,046.8 | 2,668.2 | |
| Sedentary behaviour | Base | 48,858 | 3,517.5 | 17,057 | 1,227.8 | 65,916 | 4,745.3 | ||||||
| 10% reduction by 2032 | 45,769 | 3089 | 3,294.2 | 223.3 | 16,002 | 1055 | 1,151.8 | 76.0 | 61,772 | 4144 | 4,446.0 | 299.3 | |
| 25% reduction by 2032 | 41,136 | 7723 | 2,959.2 | 558.3 | 14,420 | 2637 | 1,037.9 | 189.9 | 55,556 | 10,360 | 3,997.1 | 748.2 | |
| 50% reduction by 2032 | 33,413 | 15,445 | 2,400.9 | 1,116.6 | 11,783 | 5274 | 848.0 | 379.8 | 45,196 | 20,720 | 3,248.8 | 1,496.5 | |
| Low fruit | Base | 65,596 | 4,565.2 | 52,460 | 4,014.2 | 118,056 | 8,579.3 | ||||||
| 10% reduction by 2032 | 61,607 | 3989 | 4,297.4 | 267.8 | 49,354 | 3106 | 3,782.5 | 231.7 | 110,961 | 7094 | 8,079.9 | 499.4 | |
| 25% reduction by 2032 | 55,623 | 9972 | 3,895.7 | 669.5 | 44,697 | 7763 | 3,435.0 | 579.1 | 100,320 | 17,735 | 7,330.7 | 1,248.6 | |
| 50% reduction by 2032 | 45,651 | 19,945 | 3,226.2 | 1,339.0 | 36,934 | 15,526 | 2,855.9 | 1,158.3 | 82,585 | 35,471 | 6,082.1 | 2,497.2 | |
| Low vegetable | Base | 33,099 | 2,978.8 | 57,169 | 4,846.5 | 90,268 | 7,825.4 | ||||||
| 10% reduction by 2032 | 31,548 | 1551 | 2,838.2 | 140.6 | 54,195 | 2974 | 4,593.0 | 253.6 | 85,742 | 4526 | 7,431.2 | 394.2 | |
| 25% reduction by 2032 | 29,220 | 3879 | 2,627.2 | 351.6 | 49,733 | 7435 | 4,212.6 | 633.9 | 78,954 | 11,314 | 6,839.9 | 985.5 | |
| 50% reduction by 2032 | 25,342 | 7757 | 2,275.7 | 703.2 | 42,298 | 14,871 | 3,578.7 | 1,267.8 | 67,640 | 22,628 | 5,854.4 | 1,971.0 | |
| Red meat | Base | 8411 | 618.1 | 19,054 | 1,596.8 | 27,465 | 2,214.9 | ||||||
| Reduce 0.5 servings/week | 7924 | 487 | 582.2 | 35.9 | 18,121 | 934 | 1,518.2 | 78.6 | 26,044 | 1421 | 2,100.4 | 114.4 | |
| Reduce 1 serving/week | 7441 | 970 | 546.6 | 71.5 | 17,198 | 1857 | 1,440.6 | 156.1 | 24,639 | 2826 | 1,987.3 | 227.6 | |
| Processed meat | Base | 5865 | 435.0 | 13,674 | 1,109.6 | 19,539 | 1,544.6 | ||||||
| Reduce 0.2 servings/week | 5447 | 418 | 403.6 | 31.4 | 13,018 | 656 | 1,055.9 | 53.7 | 18,465 | 1074 | 1,459.5 | 85.1 | |
| Reduce 0.5 servings/week | 4825 | 1040 | 356.9 | 78.0 | 12,044 | 1630 | 976.2 | 133.5 | 16,869 | 2670 | 1,333.1 | 211.5 | |
| UV risk behaviours | Base | 4861 | 429.4 | 2951 | 202.0 | 7812 | 631.3 | ||||||
| 10% reduction by 2032 | 4594 | 267 | 405.5 | 23.9 | 2787 | 164 | 190.6 | 11.3 | 7381 | 431 | 596.1 | 35.2 | |
| 25% reduction by 2032 | 4197 | 664 | 369.6 | 59.8 | 2543 | 408 | 173.6 | 28.3 | 6740 | 1072 | 543.2 | 88.1 | |
| 50% reduction by 2032 | 3532 | 1329 | 309.8 | 119.6 | 2132 | 819 | 145.3 | 56.7 | 5664 | 2148 | 455.1 | 176.2 | |
| Air pollution | Base | 14,711 | 1,040.3 | 10,645 | 762.1 | 25,356 | 1,802.4 | ||||||
| Declining trend | 12,889 | 1822 | 911.2 | 129.1 | 9367 | 1279 | 670.4 | 91.7 | 22,255 | 3101 | 1,581.6 | 220.8 | |
| 50% reduction by 2032 | 11,284 | 3427 | 797.4 | 242.9 | 8240 | 2405 | 589.7 | 172.4 | 19,524 | 5832 | 1,387.1 | 415.3 | |
| H. pylori | Base | 14,950 | 1,439.1 | 19,548 | 1,971.6 | 34,498 | 3,410.7 | ||||||
| 10% reduction by 2032 | 14,250 | 700 | 1,374.6 | 64.5 | 18,615 | 933 | 1,880.0 | 91.6 | 32,865 | 1633 | 3,254.6 | 156.1 | |
| 25% reduction by 2032 | 13,202 | 1748 | 1,277.8 | 161.3 | 17,217 | 2331 | 1,742.6 | 229.0 | 30,419 | 4079 | 3,020.5 | 390.2 | |
| 50% reduction by 2032 | 11,456 | 3494 | 1,116.6 | 322.6 | 14,889 | 4659 | 1,513.6 | 457.9 | 26,345 | 8153 | 2,630.2 | 780.5 | |
| HBV | Base | 421 | 27.4 | 2532 | 299.5 | 2953 | 326.9 | ||||||
| 10% reduction by 2032 | 400 | 21 | 25.9 | 1.5 | 2395 | 137 | 283.0 | 16.5 | 2795 | 158 | 308.9 | 18.0 | |
| 25% reduction by 2032 | 363 | 58 | 23.7 | 3.7 | 2187 | 345 | 258.3 | 41.2 | 2550 | 403 | 281.9 | 44.9 | |
| 50% reduction by 2032 | 308 | 113 | 20.0 | 7.4 | 1846 | 686 | 217.1 | 82.4 | 2154 | 799 | 237.0 | 89.8 | |
| HCV | Base | 1268 | 91.2 | 5360 | 608.1 | 6628 | 699.4 | ||||||
| 10% reduction by 2032 | 1208 | 60 | 86.9 | 4.3 | 5112 | 248 | 578.6 | 29.6 | 6320 | 308 | 665.5 | 33.8 | |
| 25% reduction by 2032 | 1118 | 150 | 80.5 | 10.7 | 4742 | 618 | 534.2 | 73.9 | 5860 | 768 | 614.8 | 84.6 | |
| 50% reduction by 2032 | 974 | 294 | 69.8 | 21.4 | 4127 | 1233 | 460.3 | 147.8 | 5101 | 1527 | 530.1 | 169.2 | |
All numbers are cumulative. ACC, attributable cancer cases; CAD, Canadian dollars; CDN, Canadian; CMC, cancer management costs; PCC, preventable cancer cases; PCMC, preventable cancer management costs; WCRF, World Cancer Research Fund
Attributable and preventable cancer incidence and management costs of lifestyle risk factors
In the status quo scenarios for lifestyle risk factors, the OncoSim model projects that over 560,000 cancer cases (15.7% of all projected cancers) will be attributable to active smoking from 2032 to 2044 in Canada. The projected attributable cancer incidence is higher in men than in women (310,300 versus 249,800 cases). The estimated cost of cancer management attributable to active smoking over this period is CAD $44.4 billion. Under the most ambitious intervention scenarios (30% relative reduction by 2022), the model estimates that such reduction in active smoking results in 39,700 cases prevented between 2032 and 2044. This reduction in smoking-related cancers is associated with a potentially avoidable CAD $3.3 billion in cancer management costs.
After active smoking, the next highest cost estimated from this model is for inadequate physical activity, for which a cost of CAD $10.7 billion is estimated from a cumulative 149,100 cases (4.2% of all cancers) between 2032 and 2044, when all suggestive sites are included. Including all suggestive cancer sites, interventions on inadequate physical activity and low fruit and vegetable consumption could prevent up to 36,900, 35,500 and 22,600 cases, respectively, and save CAD $2.7, $2.5 and $2.0 billion in cancer management costs.
During the same period, excess body weight accounts for 114,600 cases, resulting in an estimate of CAD $9.8 billion in cancer management costs. Reductions in excess body weight would lead to slightly fewer preventable cases and slightly lower cancer management costs than would similar reductions in smoking and inadequate physical activity, with 36,700 cases prevented and CAD $3.2 billion avoidable.
For alcohol consumption, we also examined the WCRF and Canadian drinking guidelines and intervention targets. If all Canadians were to abide by the WCRF guidelines, a total of 9700 cases and CAD $936 million in cancer management costs could potentially be avoided. This is similar to a 25% reduction in drinking prevalence (i.e., 25% of drinkers (0–4 or more drinks/day) become non-drinkers), which would prevent 10,300 cases and save CAD $940 million. The scenario in which all Canadians abide by the Canadian guidelines would have a prevention effect of 4700 cases and CAD $461 million in costs, which is slightly better than a 10% reduction in drinking prevalence (4100 cases and CAD $376 million in costs).
By reducing red meat consumption by one serving per week, the model estimates that 2800 cancer cases could be prevented, and CAD $228 million could be saved. Reducing red meat consumption by 0.5 servings per week could prevent 1400 cancer cases and save CAD $114 million. In comparison, reducing the consumption of processed meat by 0.2 or 0.5 servings per week, the model estimates that 1100 or 2700 cancer cases could be prevented, and CAD $85 or $212 million could be saved, respectively.
Attributable and preventable cancer incidence and management costs of environmental risk factors
An estimated 7800 skin melanoma cases from 2025 to 2037 will be attributable to UV risk behaviours (sun bathing, sun tanning, and indoor tanning), resulting in an estimated cancer management cost of CAD $631 million. More cases and higher costs are expected for women (4900 cases, CAD $429 million) than for men (2900 cases, CAD $202 million). Reducing the proportion of people who engage in such behaviours by 50% could prevent up to 2100 melanoma cases and save up to CAD $176 million.
Another environmental risk factor, PM2.5, is projected to cause 25,000 lung cancer cases from 2032 to 2044 and lead to CAD $1.8 billion in cancer management costs. We applied a declining trend and a 50% reduction in PM2.5 levels by 2032. PM2.5 level would decrease from 8.3 μg/m3 in 2015 to 5.2 μg/m3 in 2032 with the current declining trend or 4.7 μg/m3 with a 50% reduction by 2032. Such reductions are projected to prevent 3100 (declining trend) to 5800 (50% reduction) lung cancers from 2032 to 2044, with cancer management cost savings of CAD $221 to $415 million, respectively.
Attributable and preventable cancer incidence and management costs of infectious agents
Among the infectious agents that were modelled, H. pylori was estimated to lead to 34,500 stomach cancer cases from 2035 to 2047, leading to CAD $3.4 billion in potential cancer management costs. Due to a lack of data on gastric mucosa-associated lymphoid tissue in the OncoSim models, this estimate excludes the incidence and cost of this specific cancer type, which was estimated to have 320 cases attributable to H. pylori infection in 2015 (Volesky et al. 2019a). Consistent with their high attributable cases and costs, interventions for H. pylori are projected to prevent up to 8200 stomach cancer cases and save up to CAD $781 million from 2035 to 2047 with a 50% reduction in prevalence of the infection.
HBV and HCV are estimated to produce 3000 and 6600 cases during the same period, respectively, with cancer management costs of CAD $327 and $699 million. Reducing HBV and HCV prevalence will prevent up to 800 and 1500 cancer cases, respectively, and save CAD $90 and $169 million, respectively. Since more cumulative attributable cases are estimated for men than for women between 2035 and 2047 (HBV: 2500 versus 400 cases; HCV: 5400 versus 1300 cases), the resulting cancer management costs for men are greater (HBV: CAD $300 versus $27 million; HCV: CAD $608 versus $91 million).
Discussion
Our projections were based on simulations from the OncoSim platform, following the integration of the results from the ComPARe study into the OncoSim tool. This integration was the collaborative work of the ComPARe study team, the CPAC and Statistics Canada. Through this collaboration, we estimated and projected the attributable economic burden of the modifiable risk factors, as well as the costs avoided by achieving intervention targets. This tool is now available to all interested researchers. For example, it could guide health promotion practitioners in designing intervention programs and simulating the outcomes, such as the potential cancer incidence prevented, and cancer management costs saved. More importantly, this tool is now available to all interested researchers. For example, it could guide health promotion practitioners in designing intervention programs and simulating the outcomes, such as the potential cancer incidence prevented and cancer management costs saved.
Here we reported the projected economic burden of cancers attributable to 15 lifestyle, environmental, and infectious risk factors, and the cancer management costs that could be avoided if interventions on these risk factors were implemented. We showed that interventions on modifiable risk factors could provide considerable savings in the Canadian cancer control budget. These findings provide evidence that can be used by policy and decision makers to prioritize cancer control efforts and manage budget constraints in the face of increasing cancer incidence.
Estimations of the attributable economic burden of cancer due to certain risk factors have been conducted previously in Canada (Krueger et al. 2016). Krueger et al. estimated the attributable direct and indirect economic burden in Canada in 2013 due to smoking, alcohol, excess body weight, and physical inactivity. They estimated that the direct cost attributable to the aforementioned risk factors were CAD $794, $293, $341 and $238 million, respectively. In contrast, our simulations estimated an attributable cost of $2,695, $234, $355 and $633 million for these risk factors in 2013 (data not shown). These large discrepancies in the results for smoking and physical inactivity could partially be explained by the inclusion cancer sites. Since the time of Krueger and colleagues’ publication, several additional cancer sites have been consistently associated with exposure to these modifiable risk factors, and therefore we were able to include them in these updated analyses. For example, Krueger et al. estimated that about 26,000 and 6000 cancers were attributable to smoking and physical inactivity, respectively, whereas we estimated 35,600 and 9300 cancers correspondingly. In addition, Krueger’s study used 2008 currency year, while our data were based on a more recent Canadian cancer management costs estimate from de Oliveira et al. (2018) and used the 2019 currency year.
According to our projections in our research, smoking will be the largest contributor to cancer management costs, with an average cost of over CAD $3.4 billion each year from 2032 to 2044. A large economic burden is also attributable to excess body weight and inadequate physical activity, incurring an average annual cost of CAD $750 and $820 million, respectively, over this period. About $3.3 and $2.7 billion could be saved from a 50% reduction in active smoking and inadequate physical activity, respectively. Over CAD $3 billion could be saved from 2032 to 2044, with a 50% reduction in the prevalence of excess body weight. Among the infectious factors examined, we projected that H. pylori infection would be attributed to the largest economic burden of $260 million each year. We showed that interventions on modifiable risk factors could provide considerable savings in the Canadian cancer control budget. While modifiable, it is important to note that interventions for some of these risk factors depend on government policies and individual-level support for these policies.
Although our analyses focused specifically on the burden of cancer, the majority of the modifiable cancer risk factors we included in our analyses are also associated with other chronic diseases such as cardiovascular disease and diabetes (The Lancet 2020). Therefore, the avoidable health costs associated with the interventions we considered are likely much higher than presented here if we were to look at the impact on other health conditions associated with the same risk factors. Interventions for some of these cancer and chronic disease risk factors depend greatly on government policies and individual-level support with the policies.
Our study has several limitations. First, our model relies on historical data and does not account for changes in risk factor prevalence or costs of cancer care over the time period that was examined. For example, we were only able to include in the OncoSim model the new classes of cancer treatments for lung cancer, such as targeted small molecule inhibitors and immunotherapies, which are more costly than the more commonly used therapies. The treatment cost of other cancers may also be underestimated. Second, this model only simulates the outcome of direct cancer management costs. Indirect costs, such as short-term and long-term disability, and premature mortality, are not included in our model, yet the indirect costs could be a larger burden than the direct costs. Therefore, our estimates should be considered as lower-end cost estimates, particularly in light of the additional benefit from the reduction of these cancer risk factors as they apply to other chronic diseases. For instance, Krueger et al. estimated a $9.6 billion attributable burden of cancer due to smoking, alcohol, excess body weight, and physical inactivity altogether, in which $7.9 billion were the indirect costs. Third, although the in-depth modules of OncoSim can project health-adjusted life-years (HALYs), which is an important parameter in estimating the cost-effectiveness of intervention, screening, or treatment, the OncoSim PAR/PIF module was on the population level, which does not support the estimation of HALYs.
Conclusion
Using the OncoSim tool, we showed that a substantial amount of cancer care costs and the number of new cancer cases could be prevented with effective interventions on a variety of risk factors. With the OncoSim model, we projected that interventions to reduce smoking-related cancers could save up to CAD $3.3 billion in cancer management costs and 39,700 cases prevented between 2032 and 2044. In addition, interventions to reduce the prevalence of excess body weight could prevent 36,100 cancer cases and save CAD $3.2 billon. With rising costs in cancer care and limited budgets, our results are valuable for researchers and policymakers to estimate the cancer and economic burdens attributable to modifiable risk factors. Policymakers and health promotion practitioners can use the OncoSim PAR/PIF module to evaluate the potential effects of intervention programs, particularly on the number of cancers prevented and the cancer care costs saved.
Contributions to knowledge
What does this study add to existing knowledge?
This study provides a projection of the future cancer management costs in Canada that are attributable to the most important modifiable cancer risk factors.
Active smoking, inadequate physical activity and excess body weight are projected to lead to CAD $44.4, $10.7 and $9.8 billion in cancer management costs from 2032 to 2044, respectively.
A 50% reduction in the prevalence of active smoking, excess body weight and inadequate physical activity could lead to about CAD $3.3, $3.0 and $2.7 billion in savings, respectively, from 2032 to 2044.
What are the key implications for public health interventions, practice or policy?
Interventions to reduce the prevalence of excess body weight could prevent 36,100 cancer cases and save CAD $3.2 billion in cancer management costs.
Interventions targeting inadequate physical activity and low fruit and vegetable consumption could prevent up to 36,900, 32,800 and 22,300 cancer cases, respectively, and avoid cancer management costs totaling an estimated CAD $2.7 (25%), $2.5 (29%) and $2.0 (25%) billion, respectively.
Supplementary Information
(XLSX 149 kb)
Acknowledgements
This analysis is based on the Canadian Partnership Against Cancer’s OncoSim model. The assumptions and calculations underlying the simulation results were prepared by the authors, and the responsibility for the use and interpretation of these data and their reporting is entirely that of the authors.
Code availability
This study did not involve the use of codes. The OncoSim model used in the study is available at https://www.partnershipagainstcancer.ca/tools/oncosim. Access and use are available upon request.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analyses were performed by Yibing Ruan, Abbey E. Poirier and Joy Pader. All authors contributed to, read and approved the final manuscript.
Funding
This research is supported by a Canadian Cancer Society Partner Prevention Research Grant (grant #703106). OncoSim is led and supported by the Canadian Partnership Against Cancer, with model development by Statistics Canada, and is made possible through funding from Health Canada.
Data availability
The OncoSim suite of models is available at https://www.partnershipagainstcancer.ca/tools/oncosim/. Access and use are available upon request.
Declarations
Ethics approval
This study did not require ethics approval as it did not involve human participants.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Brenner, D. R., Friedenreich, C. M., Ruan, Y., Poirier, A. E., Walter, S. D., King, W. D., Franco, E. L., Demers, P. A., Villeneuve, P. J., Grevers, X., Nuttall, R., Smith, L. M., Volesky, K. D., O’Sullivan, D. E., & De, P. (2019). The burden of cancer attributable to modifiable risk factors in Canada: Methods overview. Preventive Medicine, 122, 3–8. 10.1016/j.ypmed.2019.03.007. [DOI] [PubMed]
- Brenner, D. R., Weir, H. K., Demers, A. A., Ellison, L. F., Louzado, C., Shaw, A., Turner, D., Woods, R. R., & Smith, L. M. (2020). Projected estimates of cancer in Canada in 2020. CMAJ, 192(9), E199–e205. 10.1503/cmaj.191292. [DOI] [PMC free article] [PubMed]
- Brenner, D. R., Weir, H. K., Demers, A. A., Ellison, L. F., Louzado, C., Shaw, A., Turner, D., Woods, R. R., & Smith, L. M. (2020). Projected estimates of cancer in Canada in 2020. CMAJ, 192(9), E199–e205. 10.1503/cmaj.191292. [DOI] [PMC free article] [PubMed]
- Çağlayan Ç, Terawaki H, Chen Q, Rai A, Ayer T, Flowers CR. Microsimulation modeling in oncology. JCO Clinical Cancer Informatics. 2018;2:1–11. doi: 10.1200/CCI.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira C, Pataky R, Bremner KE, Rangrej J, Chan KKW, Cheung WY, Hoch JS, Peacock S, Krahn MD. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer. 2016;16(1):809. doi: 10.1186/s12885-016-2835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira C, Weir S, Rangrej J, Krahn MD, Mittmann N, Hoch JS, Chan KKW, Peacock S. The economic burden of cancer care in Canada: a population-based cost study. CMAJ Open. 2018;6(1):E1–E10. doi: 10.9778/cmajo.20170144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau CL, Fitzgerald NR, Memon S, Flanagan WM, Nadeau C, Asakawa K, Garner R, Miller AB, Evans WK, Popadiuk CM, Wolfson M, Coldman AJ. The OncoSim model: development and use for better decision-making in Canadian cancer control. Current Oncology. 2017;24(6):401–406. doi: 10.3747/co.24.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). (2018). IARC monographs on the identification of carcinogenic hazards to humans. World Health Organization. https://monographs.iarc.fr/monographs-and-supplements-available-online/.
- Krueger H, Andres EN, Koot JM, Reilly BD. The economic burden of cancers attributable to tobacco smoking, excess weight, alcohol use, and physical inactivity in Canada. Current Oncology. 2016;23(4):241–249. doi: 10.3747/co.23.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan, D. E., Brenner, D. R., Villeneuve, P. J., Walter, S. D., Demers, P. A., Friedenreich, C. M., & King, W. D. (2019). Estimates of the current and future burden of melanoma attributable to ultraviolet radiation in Canada. Preventive Medicine, 122, 81–90. 10.1016/j.ypmed.2019.03.012. [DOI] [PubMed]
- Pader, J., Ruan, Y., Poirier, A. E., Asakawa, K., Lu, C., Memon, S., Miller, A., Walter, S., Villeneuve, P. J., King, W. D., Volesky, K. D., Smith, L., De, P., Friedenreich, C. M., & Brenner, D. R. (2021). Estimates of future cancer mortality attributable to modifiable risk factors in Canada. Can J Public Health, 112. 10.17269/s41997-020-00455-7. [DOI] [PMC free article] [PubMed]
- Poirier AE, Ruan Y, Volesky KD, King WD, O’Sullivan DE, Gogna P, Walter SD, Villeneuve PJ, Friedenreich CM, Brenner DR. The current and future burden of cancer attributable to modifiable risk factors in Canada: summary of results. Preventive Medicine. 2019;122:140–147. doi: 10.1016/j.ypmed.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Public Health Agency of Canada. (2019, Dec 9, 2019). Fact sheet: cancer in Canada. Statistics Canada. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/fact-sheet-cancer-canada.html. Accessed 9 June 2020.
- Smith A, Baines N, Memon S, Fitzgerald N, Chadder J, Politis C, Nicholson E, Earle C, Bryant H. Moving toward the elimination of cervical cancer: modelling the health and economic benefits of increasing uptake of human papillomavirus vaccines. Current Oncology. 2019;26(2):80–84. doi: 10.3747/co.26.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet. (2020). Global burden of disease. Elsevier. https://www.thelancet.com/gbd. Accessed 9 June 2020
- Volesky KD, El-Zein M, Franco EL, Brenner DR, Friedenreich CM, Ruan Y. Cancers attributable to infections in Canada. Preventive Medicine. 2019;122:109–117. doi: 10.1016/j.ypmed.2019.03.035. [DOI] [PubMed] [Google Scholar]
- Volesky KD, El-Zein M, Franco EL, Brenner DR, Friedenreich CM, Ruan Y. Estimates of the future burden of cancer attributable to infections in Canada. Preventive Medicine. 2019;122:118–127. doi: 10.1016/j.ypmed.2019.04.006. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. (2018). Diet, nutrition, physical activity and cancer: a global perspective 3rd edition (Continuous Update Project, Issue. https://www.wcrf.org/dietandcancer/about.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 149 kb)
Data Availability Statement
The OncoSim suite of models is available at https://www.partnershipagainstcancer.ca/tools/oncosim/. Access and use are available upon request.