Abstract
Objectives
Modifiable lifestyle, environmental, and infectious risk factors associated with cancer impact both cancer incidence and mortality at the population level. Most studies estimating this burden focus on cancer incidence. However, because these risk factors are associated with cancers of disparate mortality rates, the burden associated with cancer incidence could differ from cancer mortality. Therefore, estimating the cancer mortality attributable to these risk factors provides additional insight into cancer prevention. Here, we estimated future cancer deaths and the number of avoidable deaths in Canada due to modifiable risk factors.
Methods
The projected cancer mortality data came from OncoSim, a web-based microsimulation tool. These data were applied to the methodological framework that we previously used to estimate the population attributable risks and the potential impact fractions of modifiable risk factors on Canadian cancer incidence.
Results
We estimated that most cancer deaths will be attributed to tobacco smoking with an average of 27,900 deaths annually from 2024 to 2047. If Canada’s current trends in excess body weight continue, cancer deaths attributable to excess body weight would double from 2786 deaths in 2024 to 5604 deaths in 2047, becoming the second leading modifiable cause of cancer death. Applying targets to reduce these risk factors, up to 34,600 cancer deaths could be prevented from 2024 to 2047.
Conclusion
Our simulated results complement our previous findings on the cancer incidence burden since decreasing the overall burden of cancer will be accelerated through a combination of decreasing cancer incidence and improving survival outcomes through improved treatments.
Supplementary Information
The online version contains supplementary material available at 10.17269/s41997-020-00455-7.
Keywords: Cancer prevention, Cancer mortality, Risk factors, Microsimulation
Résumé
Objectifs
Les facteurs de risque modifiables associés au cancer (liés au mode de vie, à l’environnement, aux maladies infectieuses) ont des effets à la fois sur l’incidence du cancer et sur la mortalité par cancer à l’échelle de la population. La plupart des études qui estiment ce fardeau portent sur l’incidence du cancer. Cependant, comme les facteurs de risque susmentionnés sont associés à des cancers dont les taux de mortalité sont disparates, le fardeau associé à l’incidence du cancer pourrait différer de la mortalité par cancer. En conséquence, l’estimation de la mortalité par cancer imputable à ces facteurs de risque pourrait éclairer la prévention du cancer. Nous estimons ici les décès futurs par cancer et le nombre de décès évitables au Canada dus à des facteurs de risque modifiables.
Méthode
Les données projetées sur la mortalité par cancer proviennent d’OncoSim, un outil de microsimulation en ligne. Elles ont été appliquées au cadre méthodologique que nous avions déjà utilisé pour estimer les risques attribuables dans la population et les fractions de l’incidence potentielle des facteurs de risque modifiables sur l’incidence canadienne du cancer.
Résultats
Selon nos estimations, entre 2024 et 2047, la plupart des décès par cancer seront imputés au tabagisme, qui causera en moyenne 27 900 décès par année. Si les tendances actuelles au Canada en matière de surpoids se maintiennent, les décès par cancer attribuables au surpoids doubleraient, passant de 2 786 décès en 2024 à 5 604 en 2047, et le surpoids deviendrait la deuxième cause modifiable de décès par cancer. En appliquant des cibles de réduction de ces facteurs de risque, jusqu’à 34 600 décès par cancer pourraient être évités entre 2024 et 2047.
Conclusion
Les résultats de notre simulation confirment nos constatations antérieures sur le fardeau de l’incidence du cancer, car la diminution du fardeau global du cancer sera accélérée par une combinaison de la diminution de l’incidence du cancer et de l’amélioration des résultats de survie grâce à l’amélioration des traitements.
Mots-clés: Prévention du cancer, mortalité par cancer, facteurs de risque, microsimulation
Introduction
With an estimated 83,300 projected deaths due to cancer in Canada in 2020, cancer is the leading cause of death in Canada accounting for approximately 30% of all deaths among Canadians (Brenner et al. 2020). Cancer mortality rates vary across cancer sites, largely due to differences in cancer incidence and also in differences in cancer control activities such as screening, diagnosis, treatment, and follow-up care, and spatial differences in the many risk factors for cancer such as tobacco smoking, alcohol use, diet, sun exposure, body size, and physical activity (Canadian Cancer Society’s Advisory Committee 2017). With cancer a leading cause of mortality and morbidity, preventive efforts to reduce the cancer burden are a top priority globally (Bray and Soerjomataram 2018). Understanding the incidence and subsequent preventable mortality burden of cancer due to modifiable risk factors is an important step towards focusing preventive efforts and prevention resources. Reducing cancer mortality involves efforts to control the disease across the cancer continuum from incidence (primary prevention) to screening and treatment (secondary prevention) and survival (tertiary prevention).
Cancer etiologic research over the past six decades has elucidated risk factors for which there is moderate to strong evidence that they are associated with an elevated risk of different cancer sites. Systematic reviews and syntheses of this evidence have been conducted by the International Agency for Research on Cancer (IARC) and the World Cancer Research Fund (WCRF), as two of the leading organizations focused on cancer prevention (World Cancer Research Fund/American Institute for Cancer Research 2011, 2018a, 2018b). Some of the leading risk factors that have been identified through these evidence synthesis activities include factors that are both modifiable and non-modifiable (International Agency for Research on Cancer (IARC) 2004, 2010, 2016). In our previous work on the main modifiable risk factors for cancer incidence (Poirier et al. 2019a), we focused on tobacco smoking, inadequate physical activity, excess body weight, sedentary behaviour, alcohol consumption, low fruit and vegetable intake, red and processed meat consumption, air pollution, residential radon, ultraviolet radiation, Helicobacter pylori (H. pylori), human papillomavirus, Epstein-Barr virus, and hepatitis B and C viruses as key risk factors for which there were population-level exposure prevalence data available. Our study examined the current and future burden of cancer incidence that could be prevented if exposures to these modifiable risk factors were reduced or eliminated. The study was limited to examining only the burden of cancer associated with cancer incidence and did not consider the resulting decreased burden of cancer mortality that would ensue with decreases in cancer incidence, because we previously did not have reliable projections of cancer mortality data. We estimated that up to 70,000 (or 37%) of all cancers diagnosed in Canada in 2015 could be attributed to 16 modifiable risk factors, where this figure could rise to 102,000 cases in 2042. By achieving modest to aspirational intervention targets in all risk factors (such as 10% to 50% reduction in exposure prevalence), about 10,700 to 39,700 cancer cases could be prevented annually by 2042.
OncoSim, a suite of microsimulation models developed with Canadian population-level data by the Canadian Partnership Against Cancer and Statistics Canada, is a modeling analysis tool that can be used to project cancer incidence, mortality, and cancer management costs to inform policy and decision-making in regard to cancer care, treatment, and prevention (Gauvreau et al. 2017). The OncoSim framework supports cancer policies and decision-making across Canada because its suite of models can simulate the health and economic impacts of current, future, and past cancer control interventions in the Canadian population using Canadian health care costs (Ruan et al. 2021). Specifically, OncoSim has projected cancer deaths in Canada up to 2051 based on validated models (Gauvreau et al. 2017). We have also validated the OncoSim mortality projections against historical mortality data in Canada. These mortality projections present an opportunity to apply our methodological approach for estimating preventable incidence in estimating the number of future cancer deaths attributable to modifiable risk factors. The objective of these analyses was to estimate the attributable cancer mortality due to modifiable risk factors, and to estimate the preventable cancer mortality with various intervention targets in Canada.
Methods
Projected cancer mortality
The projected cancer mortality was retrieved from the OncoSim microsimulation model (version 3.2.7), which is based on death data from the Canadian Vital Statistics Death Database. The OncoSim model, previously known as the Cancer Risk Management Model (CRMM), is a publicly accessible, web-based tool consisting of four in-depth cancer models (lung, colorectal, cervical, and breast) and a generalized all-cancer model (an additional 27 other cancers). The development, validation, and application of the OncoSim model have been extensively documented (Coldman et al. 2018; Gauvreau et al. 2017; Popadiuk et al. 2016). In this study, we used the cancer mortality projections of 31 cancer types diagnosed among Canadians 35 years of age and over, from the years 2024 to 2047. This time window corresponds to the period of estimates for the population attributable risk (PAR) and potential impact fraction (PIF) from the Canadian Population Attributable Risk of Cancer study, or ComPARe (2019 to 2042). Here, we assumed a five-year latency between cancer incidence and death.
Risk factor selection and risk estimates
We included risk factors where we had previously estimated the future burden of their associated cancers and preventable fractions (Brenner et al. 2018, 2019). This included active and passive tobacco smoking exposure, excess body weight, physical inactivity, leisure-time sedentary behaviour, alcohol use, low fruit and vegetable consumption, red and processed meat consumption, ultraviolet radiation risk behaviours, air pollution (PM2.5), residential radon, H. pylori, and hepatitis B and C viruses. We applied the same relative risks for each exposure-cancer association that were used in the ComPARe study (Table S1) (Brenner et al. 2018, 2019). We assumed that the relative risk of cancer incidence for each exposure was approximately equal to the relative risk of cancer death for the same exposure. These risk estimates were extracted from the WCRF continuous update project, from high-quality systematic reviews and meta-analyses published up to August 2017, or through our own meta-analyses when a summary estimate of interest was not available (Brenner et al. 2018, 2019).
Exposure prevalence projection and intervention scenarios
The specific methods to project the future prevalence of exposures have been previously described (Brenner et al. 2018). Briefly, when historical repeated prevalence measures were available (i.e., active and passive smoking, body mass index (BMI), inadequate physical activity, leisure-time sedentary behaviour, alcohol use, low fruit and vegetable intake, air pollution, and hepatitis C virus (HCV)), we projected future prevalence up to 2032 (2036 for air pollution) based on the assumption that the observed past trend would continue into the future. For most risk factors, the time period of exposure up to 2032 corresponds to the projected mortality up to 2047, accounting for a 15-year latency period between exposure and death. When historical prevalence measures were limited (e.g., only one measure for ultraviolet radiation risk behaviours, radon, hepatitis B virus (HBV), H. pylori, and red and processed meat), we assumed constant future prevalence at the value of the past prevalence measure. We used the same intervention scenarios as in the ComPARe study, which are described in detail elsewhere (Poirier et al. 2019b) and summarized in Table 1. When evidence-based intervention targets were not available, we included “benchmark” targets for the risk factors, such as 10%, 25%, and 50% relative reductions in the exposure prevalence.
Table 1.
Risk factors, associated cancers, intervention targets or scenarios, and the method to estimate potential impact fractions
| Risk factor | Associated cancers | Intervention targets/scenarios | Method to estimate PIF |
|---|---|---|---|
| Active smoking | Lung, oral cavity, larynx, esophagus, stomach, liver, pancreas, colon and rectum, kidney, bladder, ureter, ovary, cervix, breast, acute myeloid leukemia |
10% reduction in smoking by 2032 25% reduction in smoking by 2032 50% reduction in smoking by 2032 Price increases reduced smoking by 3.7% in 2018 Reduce smoking prevalence to 5% by 2035 WHO 2020 goal: 30% relative reduction in current smoking by 2020 |
Proportions shift
|
| Passive smoking | Lung, colon and rectum, cervix, breast |
10% reduction in secondhand smoking by 2032 25% reduction in secondhand smoking by 2032 50% reduction in secondhand smoking by 2032 |
Proportions shift |
| Excess weight (as measured by body mass index) | Esophagus (ADC), stomach (cardia), liver, pancreas, colon, rectum, kidney, gallbladder, multiple myeloma, thyroid, breast (post-menopausal), ovary, endometrium, prostate (advanced) |
Population BMI held at 2018 level Population BMI reverted to 1994 distribution Population mean BMI reduced by 1.0 Prevalence of overweight and obesity reduced by 5% by 2032 Prevalence of overweight and obesity reduced by 10% by 2032 Prevalence of overweight and obesity reduced by 25% by 2032 |
Distribution shift
|
| Inadequate physical activity | Breast (post-menopausal), lung, endometrium, colon, rectum, kidney, bladder, esophagus (ADC), stomach, liver, small intestine, myeloid leukemia, multiple myeloma, head and neck, non-Hodgkin lymphoma |
10% reduction in inadequate physical activity by 2032 25% reduction in inadequate physical activity by 2032 50% reduction in inadequate physical activity by 2032 |
Proportions shift |
| Sedentary behaviour | Breast, colon and rectum, endometrium, ovary |
10% reduction in sedentary time by 2032 25% reduction in sedentary time by 2032 50% reduction in sedentary time by 2032 |
Proportions shift |
| Alcohol | Breast, colon and rectum, oral cavity, larynx, liver, esophagus (SCC), pancreas, stomach |
Alcohol drinking prevalence decreases by 10% by 2032 Alcohol drinking prevalence decreases by 25% by 2032 Alcohol drinking prevalence decreases by 50% by 2032 Everyone who drinks > 1/day reduces 1 drink each day WCRF guideline Canadian guideline |
Proportions shift Relative risk shift
|
| Low fruit | Pancreas, bladder, lung, colon and rectum, breast, stomach, esophagus (SCC) |
Everyone increases fruit intake by 1 serving/day Everyone increases fruit intake by 2 servings/day A relative 50% reduction in the prevalence of low fruit intake by 2032 |
Relative risk shift Proportions shift |
| Low vegetable | Pancreas, bladder, lung, colon and rectum, head and neck, ovary, liver, esophagus (ADC) |
Everyone increases vegetable intake by 1 serving/day Everyone increases vegetable intake by 2 servings/day A relative 50% reduction in the prevalence of low vegetable intake by 2032 |
Relative risk shift Proportions shift |
| Processed meat | Colon and rectum, pancreas, stomach (non-cardia), esophagus (SCC) |
Everyone reduces processed meat by 0.2 servings/week Everyone reduces processed meat by 0.5 servings/week Everyone reduces processed meat by 1 serving/week |
Relative risk shift |
| Red meat | Colon and rectum, pancreas, stomach |
Everyone reduces red meat by 0.5 servings/week Everyone reduces red meat by 1 serving/week Everyone reduces red meat by 2 servings/week |
Relative risk shift |
| Ultraviolet radiation risk behaviours (indoor tanning, sunburn, sunbathing) | Melanoma |
10% reduction in UVR risk behaviours by 2037 25% reduction in UVR risk behaviours by 2037 50% reduction in UVR risk behaviours by 2037 |
Proportions shift |
| PM2.5 | Lung |
Declining trend 50% reduction off stabilized levels by 2036 |
Distribution shift |
| Radon | Lung |
Radon mitigation of above 100 Bq/m3 to 50 Bq/m3 by 2036 Radon mitigation of above 200 Bq/m3 to 50 Bq/m3 by 2036 |
Distribution shift |
| Hepatitis B virus | Hepatocellular carcinoma |
10% reduction in the prevalence of HBV infection by 2027 25% reduction in the prevalence of HBV infection by 2027 50% reduction in the prevalence of HBV infection by 2027 |
Proportions shift |
| Hepatitis C virus | Hepatocellular carcinoma, non-Hodgkin lymphoma |
10% reduction in the prevalence of HCV infection by 2027 25% reduction in the prevalence of HCV infection by 2027 50% reduction in the prevalence of HCV infection by 2027 |
Proportions shift |
| Helicobacter pylori | Stomach (non-cardia) |
10% reduction in the prevalence of H. pylori infection by 2027 25% reduction in the prevalence of H. pylori infection by 2027 50% reduction in the prevalence of H. pylori infection by 2027 |
Proportions shift |
ADC, adenocarcinoma; Bq/m3, becquerels per cubic metre; HBV, hepatitis B virus; HCV, hepatitis C virus; H. pylori, Helicobacter pylori; P, prevalence of risk factor; PIF, potential impact fraction; PM2.5, particulate matter with a diameter of less than 2.5 μm; RR, relative risk; SCC, squamous cell carcinoma; UVR, ultraviolet radiation; WCRF, World Cancer Research Fund; WHO, World Health Organization
Estimation of preventable future cancer deaths
We used the same PIF framework employed in the ComPARe study to estimate the number of preventable cancer deaths in Canada up to 2047 based on the intervention scenarios. First, we calculated the PIF (the fraction of cancer deaths that could be prevented) of each scenario using the specific approach for each given exposure (Table 1). We then estimated the number of avoidable cancer deaths by multiplying the projected future cancer deaths with the PIF for each exposure-cancer association. We assumed a 15-year latency period between the recorded exposure to lifestyle and environmental risk factors (2009–2032) and cancer death (2024–2047), and a 20-year latency period for the infectious agents (HBV, HCV, and H. pylori) (2004–2027).
Results
Projected cancer mortality
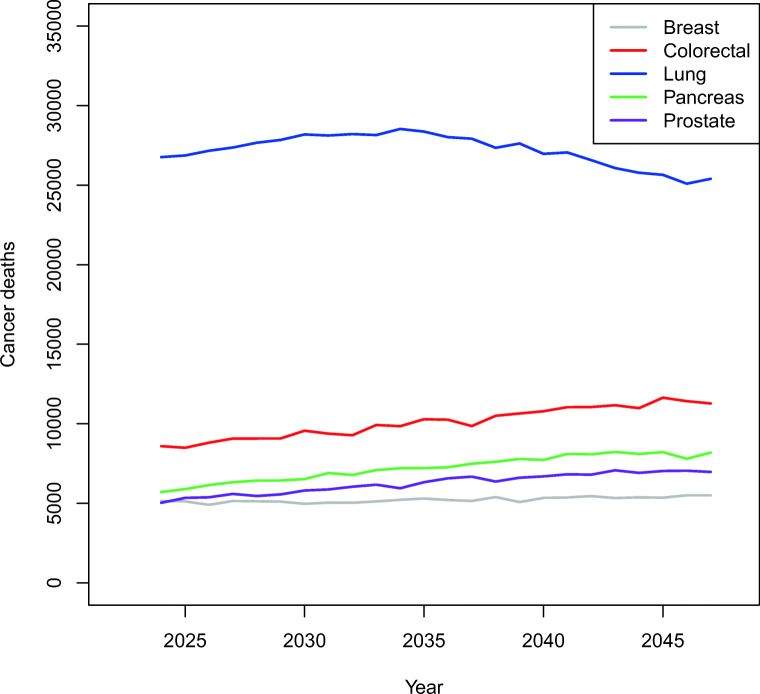
From 2024 to 2047, we projected that the deaths from all cancers among Canadians aged 35 and over would rise from around 89,850 in 2024 to 112,920 in 2047. In 2047, the five highest projected mortality cancers were lung (25,400), colorectal (11,280), pancreas (8190), prostate (6970), and breast (5510) (Fig. 1). Colorectal, pancreatic, prostate, and breast cancers all showed a slight increase in the number of deaths over time, while lung cancer peaked at 28,530 deaths in 2034 and declined thereafter (Fig. 1).
Fig. 1.
Projected breast (females), colorectal, lung, pancreatic, and prostate (males) cancer deaths in Canadians 35 years of age and over from 2024
More cancer deaths were projected in males than in females (average of years 2024–2047, males: 54,500/year, females: 49,800/year). Among males, lung remained as the cancer with the highest mortality; however, annual lung cancer deaths started declining after 2034 (Fig. S1). The second to fifth highest mortality cancers in males were prostate, colorectal, pancreatic, and bladder. Similarly for females, lung cancer also had the highest mortality, with a peak number of annual deaths reaching 15,380 in 2034 that is projected to decrease to 14,180 in 2047. For females, the next leading cancers with the highest mortality were breast, colorectal, pancreatic, and ovarian. The number of deaths due to breast cancer remained steady during this projected time period, while a slight increase in deaths from colorectal, pancreatic, and ovarian cancers was estimated to occur over the modeling period.
Attributable cancer mortality
We projected the attributable cancer deaths due to 10 lifestyle, three environmental, and three infectious agent risk factors (Table S2). Active tobacco smoking was associated with the most cancer deaths between 2024 and 2047, leading to an average of 27,900 cancer deaths each year. Excessive body weight would be attributed to a projected 2790 cancer deaths in 2024, which is estimated to double to 5600 deaths by 2047, the largest increase among all the risk factors under study.
Preventable cancer mortality
We further projected the number of cancer deaths that could be prevented if certain intervention targets could be achieved. Reducing the prevalence of active smoking by 10%, 25%, or 50% by 2032 could potentially prevent 6370, 15,920, or 31,870 cancer deaths from 2024 to 2047, respectively (Table 2). If we were able to achieve the World Health Organization (World Health Organization 2013) goal of reducing smoking by 30% by 2020 (World Health Organization 2012), as many as 34,601 cancer deaths from 2024 to 2047 would be prevented. In addition to the preventable deaths due to active tobacco smoking, 10%, 25%, or 50% reductions in the exposure to passive smoking by 2032 could additionally prevent 280, 690, or 1380 cancer deaths, respectively. Lung cancer deaths would be the most preventable cancer deaths from smoking intervention. A 10%, 25%, or 50% reduction in both active and passive smoking could prevent a cumulative 4950, 12,370, or 24,740 lung cancer deaths, respectively (Tables S3–S4).
Table 2.
Projected cancer deaths, attributable or preventable cancer deaths, and cumulative preventable cancer deaths of selected lifestyle factors that are associated with cancers in Canada from 2024 to 2047 estimated using the OncoSim all-cancer model
| Exposure | Intervention targets/scenarios | Projected cancer deaths* | Preventable/attributable deaths** | Cumulative preventable deaths† |
|---|---|---|---|---|
| Active smoking | Base | 72,934 | 26,446 | |
| 10% reduction in smoking by 2032 | 72,200 | 734 | 6373 | |
| 25% reduction in smoking by 2032 | 71,098 | 1836 | 15,934 | |
| 50% reduction in smoking by 2032 | 69,262 | 3672 | 31,869 | |
| Price increases reduced smoking by 3.7% in 2018 | 71,016 | 1918 | 28,461 | |
| Reduce smoking prevalence to 5% by 2035 | 69,103 | 3831 | 33,158 | |
| WHO 2020 goal: 30% relative reduction in current smoking by 2020 | 70,731 | 2203 | 34,601 | |
| Passive smoking | Base | 23,453 | 278 | |
| 10% reduction in secondhand smoking by 2032 | 23,425 | 28 | 276 | |
| 25% reduction in secondhand smoking by 2032 | 23,384 | 70 | 690 | |
| 50% reduction in secondhand smoking by 2032 | 23,314 | 139 | 1379 | |
| Alcohol | Base | 34,405 | 2809 | |
| Alcohol drinking prevalence decreases by 10% by 2032 | 34,124 | 281 | 4312 | |
| Alcohol drinking prevalence decreases by 25% by 2032 | 33,703 | 702 | 10,780 | |
| Alcohol drinking prevalence decreases by 50% by 2032 | 33,000 | 1405 | 21,557 | |
| Everyone who drinks > 1/day reduces 1 drink | 33,062 | 1343 | 21,526 | |
| WCRF guideline | 33,584 | 822 | 13,240 | |
| Canadian guideline | 34,060 | 345 | 5756 | |
| Excess body weight | Base | 49,241 | 5604 | |
| Population BMI held at 2018 level | 47,978 | 1264 | 9030 | |
| Population BMI reverted to 1994 distribution | 46,199 | 3042 | 22,495 | |
| Population mean BMI reduced by 1.0 | 47,333 | 1909 | 13,792 | |
| Prevalence of overweight and obesity reduced by 5% by 2032 | 48,044 | 1198 | 8561 | |
| Prevalence of overweight and obesity reduced by 10% by 2032 | 47,180 | 2061 | 14,951 | |
| Prevalence of overweight and obesity reduced by 25% by 2032 | 45,612 | 3630 | 27,234 | |
| Inadequate physical activity | Base | 53,266 | 4898 | |
| 10% reduction in inadequate physical activity by 2032 | 52,862 | 405 | 3309 | |
| 25% reduction in inadequate physical activity by 2032 | 52,255 | 1012 | 8271 | |
| 50% reduction in inadequate physical activity by 2032 | 51,243 | 2023 | 16,543 | |
| Sedentary behaviour | Base | 21,114 | 1975 | |
| 10% reduction in sedentary time by 2032 | 20,926 | 188 | 1438 | |
| 25% reduction in sedentary time by 2032 | 20,643 | 471 | 3595 | |
| 50% reduction in sedentary time by 2032 | 20,172 | 942 | 7191 | |
| Low fruit consumption | Base | 58,821 | 5647 | |
| Everyone increases fruit intake by 1 serving/day | 56,594 | 2227 | 43,500 | |
| Everyone increases fruit intake by 2 servings/day | 54,849 | 3973 | 78,740 | |
| A relative 50% reduction in the prevalence of low fruit by 2032 | 55,998 | 2823 | 22,692 | |
| Low vegetable consumption | Base | 59,410 | 4297 | |
| Everyone increases vegetable intake by 1 serving/day | 57,555 | 1855 | 37,012 | |
| Everyone increases vegetable intake by 2 servings/day | 56,217 | 3192 | 65,959 | |
| A relative 50% reduction in the prevalence of low vegetable by 2032 | 57,262 | 2148 | 17,824 | |
| Processed meat | Base | 22,397 | 1094 | |
| Everyone reduces processed meat by 0.2 servings/week | 22,226 | 170 | 3068 | |
| Everyone reduces processed meat by 0.5 servings/week | 21,974 | 423 | 7629 | |
| Everyone reduces processed meat by 1 serving/week | 21,563 | 834 | 15,065 | |
| Red meat | Base | 23,288 | 1849 | |
| Everyone reduces red meat by 0.5 servings/week | 23,006 | 282 | 5012 | |
| Everyone reduces red meat by 1 serving/week | 22,728 | 560 | 9958 | |
| Everyone reduces red meat by 2 servings/week | 22,184 | 1104 | 19,655 |
BMI, body mass index; WCRF, World Cancer Research Fund; WHO, World Health Organization
*Projected cancer deaths from all cancer sites associated with the risk factor in 2047
**Attributable cancer deaths of base scenario in 2047, or preventable cancer deaths of interventions in 2047
†Cumulative preventable deaths from 2024 to 2047
In 2047, 2648 cancer deaths were attributed to alcohol consumption. If the drinking prevalence was decreased by 10%, 25%, or 50%, a cumulative 4310, 10,780, or 21,560 cancer deaths could be prevented from 2024 to 2047, respectively. In addition, if all Canadians were to drink within the WCRF or Canadian drinking guideline (WCRF: no more than 2 drinks a day for males and 1 drink a day for females; Canada: no more than 3 drinks a day for males and 2 drinks a day for females) (Butt et al. 2011; World Cancer Research Fund/American Institute for Cancer Research 2007), a cumulative 13,240 or 5760 cancer deaths could be prevented (Table 2). More than half of these preventable cancer deaths were from colorectal and oral cancers. For example, there were 4140 colorectal cancers and 3106 oral cancers among the 13,240 preventable cancer deaths due to not meeting the WCRF guideline (Table S5).
If we were able to halt the increasing trend of excess weight at the population level by holding the population BMI distribution at 2018 levels, 9030 cancer deaths could be prevented (Table 2). More aspirational intervention targets, such as reverting the population BMI to its distribution as of 1994, or reducing mean BMI by 1.0, would prevent 22,500 or 13,790 cancer deaths, respectively. Finally, a reduction in the prevalence of Canadians with a BMI over 25.0 by 5%, 10%, or 25% would prevent 8560, 14,950, or 27,230 cancer deaths, respectively (Table 2, Table S6). A 10%, 25%, or 50% reduction in the prevalence of inadequate physical activity could prevent 3310, 8270, or 16,540 cancer deaths. Likewise, the same percent reductions in the percentage of people classified as sedentary could prevent 1440, 3600, or 7190 cancer deaths (Table 2, Tables S7–S8).
We found that increasing fruit or vegetable consumption by one serving per day could prevent 5350 or 2638 colorectal cancer deaths by 2047 (Tables S9–S10). If all convincing, probable, and suggestive associations between cancers and low fruit or vegetable consumption were considered, a total of 43,500 (fruit) or 37,010 (vegetable) cancer deaths could be prevented by 2047 (Table 2). Reducing processed meat or red meat consumption by one serving per week could prevent 7010 or 3370 colorectal cancer deaths, respectively (Tables S11–S12). If the suggestive association with pancreatic and stomach cancers were included, reducing processed or red meat consumption by one serving per week could prevent 15,070 or 9960 cancer deaths by 2047, respectively (Table 2).
We examined the preventable lung cancer deaths due to outdoor air pollution (PM2.5) and residential radon exposure and the melanoma deaths due to UVR risk behaviours (Table 3, Table S13). Achieving a 50% reduction off the stabilized PM2.5 levels by 2036 could prevent 2920 lung cancer deaths by 2047. Mitigation of radon exposure in homes above 100 becquerels per cubic metre (Bq/m3; WHO guideline) or 200 Bq/m3 (Canadian guideline) to 50 Bq/m3, 2830 or 1840 lung cancer deaths could be prevented by 2047, respectively. Reduction in UVR risk behaviours such as sunbathing, sunburn, and indoor tanning by 10%, 25%, or 50% would prevent 410, 1050, or 2230 melanoma deaths by 2047, respectively.
Table 3.
Projected cancer deaths, attributable or preventable cancer deaths, and cumulative preventable cancer deaths of selected environmental factors and infections that are associated with cancers in Canada from 2024 to 2047 estimated using the OncoSim all-cancer model
| Exposure | Intervention targets/scenarios | Projected cancer deaths* | Preventable/attributable deaths** | Cumulative preventable deaths† |
|---|---|---|---|---|
| Air pollution (PM2.5) | Base | 25,395 | 1360 | |
| Declining trend | 25,145 | 250 | 2288 | |
| 50% reduction off stabilized levels by 2036 | 25,032 | 363 | 2916 | |
| Radon | Base | 25,395 | 1735 | |
| Radon mitigation of above 100 Bq/m3 to 50 Bq/m3 by 2036 | 25,064 | 331 | 2832 | |
| Radon mitigation of above 200 Bq/m3 to 50 Bq/m3 by 2036 | 25,175 | 220 | 1841 | |
| UVR risk behaviours | Base | 2028 | 1190 | |
| UVR risk behaviours | 10% reduction in UVR risk behaviours by 2037 | 1985 | 43 | 408 |
| UVR risk behaviours | 25% reduction in UVR risk behaviours by 2037 | 1916 | 112 | 1053 |
| UVR risk behaviours | 50% reduction in UVR risk behaviours by 2037 | 1784 | 244 | 2232 |
| HBV | Base | 2484 | 219 | |
| HBV | 10% reduction in the prevalence of HBV infection by 2027 | 2462 | 22 | 101 |
| HBV | 25% reduction in the prevalence of HBV infection by 2027 | 2429 | 55 | 253 |
| HBV | 50% reduction in the prevalence of HBV infection by 2027 | 2374 | 110 | 507 |
| HCV | Base | 7223 | 385 | |
| HCV | 10% reduction in the prevalence of HCV infection by 2027 | 7184 | 38 | 182 |
| HCV | 25% reduction in the prevalence of HCV infection by 2027 | 7127 | 96 | 453 |
| HCV | 50% reduction in the prevalence of HCV infection by 2027 | 7031 | 192 | 907 |
| H. pylori | Base | 2361 | 1406 | |
| H. pylori | 10% reduction in the prevalence of H. pylori infection by 2027 | 2221 | 141 | 611 |
| H. pylori | 25% reduction in the prevalence of H. pylori infection by 2027 | 2010 | 352 | 1528 |
| H. pylori | 50% reduction in the prevalence of H. pylori infection by 2027 | 1658 | 703 | 3056 |
Bq/m3, becquerels per cubic metre; HBV, hepatitis B virus; HCV, hepatitis C virus; H. pylori, Helicobacter pylori; PM2.5, particulate matter with a diameter of less than 2.5 μm; UVR, ultraviolet radiation
*Projected cancer deaths from all cancer sites associated with the risk factor in 2047
**Attributable cancer deaths of base scenario in 2047, or preventable cancer deaths of interventions in 2047
†Cumulative preventable deaths from 2024 to 2047
Finally, we estimated the preventable cancer deaths due to infectious agents including HBV, HCV, and H. pylori (Table 3, Tables S14–S16). A 50% reduction in HBV or HCV infection could prevent 510 or 910 cancer deaths by 2047, respectively. Reducing H. pylori infection prevalence by 10%, 25%, or 50% by 2027 would prevent 610, 1530, or 3060 non-cardia stomach cancer deaths by 2047, respectively.
Discussion
We previously estimated the current and future burden of incident cancers in Canada due to modifiable risk factors as part of the ComPARe study (Brenner et al. 2019; Poirier et al. 2019c). The ComPARe study results on cancer incidence were incorporated within the OncoSim framework to estimate future cancer mortality and estimate preventable cancer mortality from years 2024 to 2047. Using validated projections of cancer mortality from OncoSim, we estimated the future burden of cancer deaths due to a selection of lifestyle, environmental and infectious agent risk factors, based on the methodological framework and hierarchy of evidence developed for the ComPARe study. Our projections suggest that deaths from all cancers in those aged 35 and over would increase by approximately 23,000 cases from 2024 to 2047 in Canada. At the end of this time period that we used, the OncoSim model projected that the greatest number of cancer deaths in 2047 would be for lung, colorectal, and pancreas cancers as well as prostate cancer in men and breast cancer in women. The model also projected that cases would be higher among males than among females. This projected ranking is similar to the current situation in Canada, except that there are more breast cancer deaths than prostate cancer deaths (Brenner et al. 2020).
Depending on the intervention scenario used in these analyses, between 6370 and 34,600 cancer deaths could be prevented by 2047 with reductions in active smoking prevalence. By reducing the prevalence of overweight and obesity, between 8560 and 27,230 cancer deaths could be prevented by 2047, and with reductions to alcohol intake, between 4310 and 21,560 deaths could be prevented. Increasing the prevalence of leisure-time physical activity could prevent between 3310 and 16,540 cancer deaths by 2047. Population-level modifications to diet, UVR exposure, air pollution levels, radon exposure, and infections could also reduce thousands of cancer deaths. Tens of thousands of cancer deaths could be prevented if various population-level changes to modifiable cancer risk behaviours were made, highlighting the importance of prioritizing the development and implementation of cancer prevention strategies at the population level.
We used a similar methodological framework as the ComPARe study (Brenner et al. 2018). We obtained the same PARs and PIFs for individual risk factor-cancer associations and, therefore, the findings that we estimated for cancer mortality were generally similar to those found for cancer incidence. Nevertheless, there were some differences in the findings for all associated cancers. For example, we previously reported that in 2042, active smoking will be attributed to 31.8% and 20.1% of associated incident cancer cases in males and females, respectively (Poirier et al. 2019a). For cancer mortality, we estimated here that 40% and 32% of associated cancer deaths in males and females were attributable to active tobacco smoking, respectively, in 2047 (Table S2). This difference in percentages of cancers that can be attributed to active smoking for cancer incidence versus cancer mortality can likely be explained by the low survival rates for smoking-associated cancers (e.g., lung, esophageal, pancreatic, and liver cancer) (Canadian Cancer Society’s Advisory Committee 2019). This observation reinforces the urgency of taking immediate actions on tobacco smoking.
Our study had notable strengths as we were able to conduct a comprehensive assessment of the attributable and preventable cancer deaths associated with several lifestyle, environmental, and infectious agent risk factors using novel methods. Specifically, we demonstrated an approach to incorporating the information from two sources, i.e., the PAR and PIF results from the ComPARe study and the cancer mortality data from the OncoSim platform, and how such collaborations can lead to new findings that provide more evidence on cancer prevention. Both the ComPARe data and the OncoSim platform are very accessible because they are freely available to the public and consequently can be accessed to investigate any number of related questions. As with any projection or simulation modeling framework, OncoSim has limitations. OncoSim requires ongoing updates with newer input data (incidence and mortality) and calibration for accuracy. As access and ongoing updates to more recent data are needed, there is a time lag for incorporating the latest data into the tool. There were also limitations specific to this study. First, we assumed that the relative risk of cancer incidence and that of cancer death for each exposure were the same. This assumption only holds when the cancer prognosis is independent of the past exposure to a risk factor. Although it is reasonable for most risk factors, the prognosis could be affected by certain other risk factors. For example, post-menopausal breast cancers attributable to excess body weight were shown to be associated with poorer prognosis (Sun et al. 2018). Ideally, our study should have used risk estimates for cancer deaths due to the specific exposures. However, risk estimates on cancer deaths are not consistently available for most exposures as they are for risk and are subject to substantial heterogeneity related to study design and case heterogeneity. Second, we used a five-year average latency period between cancer diagnosis and cancer death. This is an oversimplification, as in reality, the median survival of cancers varies by cancer type. We carried out a sensitivity analysis with different latency periods from 0 to 10 years (Table S17). The influence on the number of attributable cancer deaths by the length of latency varies by risk factors. The variation is determined by the projected trend of risk factor prevalence. Latency period has no influence on the attributable cancer death if risk prevalence is constant. In contrast, larger annual changes in risk factor prevalence lead to higher sensitivity to the latency period. Finally, because this study was an extension of the ComPARe study, most of the ComPARe study limitations (Brenner et al. 2018) were also present in this study. For example, the prevalence data of all lifestyle risk factors were from self-reported surveys that are subjected to measurement errors and self-report bias. Some risk estimates, although from high-quality meta-analyses, might not be adequately adjusted for confounding factors. In addition, we did not include any cancer risk factors that were not included in the ComPARe study such as hormone therapy and occupational exposures.
This study is a supplement to the ComPARe study, in which we outlined the most impactful interventions by risk factor that would contribute to reductions in Canadian cancer mortality (Poirier et al. 2019b). The interventions examined in this study were either evidence-based scenarios or aspirational targets that were taken into consideration for the Canadian population (Brenner et al. 2018, 2019). Our results are aimed to inform health policies and decision-makers regarding the potential effectiveness of these interventions in reducing population exposures to various cancer risk factors, and in lowering cancer incidence and mortality. For active smoking in Canada, we previously suggested a hypothetical scenario of increasing cigarette prices to reduce smoking prevalence (Poirier et al. 2019b). Past systematic reviews concluded that a price increase on tobacco products should be prioritized as a method to reduce the prevalence of smoking (Hoffman and Tan 2015). For excess body weight, physical inactivity, and sedentary behaviour, our future estimates relied on population reductions to all three risk factors. Achieving those reduction targets through public health interventions may come from overlapping interventions. For example, systematic reviews on interventions for reducing adult obesity showed that targeting physical activity and the built environment had reduced overall weight and BMI (Tseng et al. 2018). Technological interventions delivered through multiple information channels have also demonstrated greater weight loss compared to control groups (Hutchesson et al. 2015). Interventions that target obesity and excess body weight often also impact physical inactivity and sedentary behaviour. Similar effects have also been observed in randomized control trials (RCTs) of nutrition and diet, which led to increased vegetable and fruit consumption and decreased meat intake (Hawkes et al. 2012; Sacerdote et al. 2006). Feasibility studies which can have several areas of focus, such as acceptability, adaptation, integration, practicality, and efficacy, can be used to evaluate health intervention programs (Bowen et al. 2009). RCTs of health interventions have also been used to determine the efficacy and effectiveness of health interventions (Jelsma et al. 2019; Song and Baicker 2019). Both feasibility studies and RCTs have found some low-cost work-place, school-based, and home-based interventions to be effective in changing unhealthy behaviours (Brown et al. 2018; Djuric et al. 2010; Eather et al. 2019; Jelsma et al. 2019; Song and Baicker 2019). As with any implementation of an intervention, even evidence-based approaches may prove less effective in real-world settings. The scenarios included in this manuscript are presented as a spectrum of potential interventions ranging in cost and feasibility. To make meaningful progress in cancer prevention, considerable up-front investment will be required, preferably starting with those interventions that are most feasible with the shortest intervention window, e.g., vaccination and exposure remediation, progressing to those that require long-term effort and investment.
Conclusion
We utilized the OncoSim modeling and projection framework to extend our previous analyses of modifiable cancer incidence to include cancer mortality. The findings of relative cancer burden were similar between OncoSim and the ComPARe study. With the OncoSim model, we projected that deaths from all cancers among those aged 35 and over would increase by approximately 23,000 cases from 2024 to 2047 in Canada. The highest number of preventable cancer deaths was estimated to be attributable to active tobacco smoking. We also projected preventable cancer deaths related to various cancer intervention targets, which provided additional evidence in support of intervention programs for cancer control. The results from our microsimulation modeling echo the previous findings on the modifiable cancer incidence burden from the ComPARe study (Poirier et al. 2019c) that over 100,000 cancer deaths could potentially be avoided through ambitious cancer prevention targets. These results provide additional data to inform cancer prevention strategies for governments, policymakers, and health promotion programs.
Contributions to knowledge
What does this study add to existing knowledge?
Models used in this study suggest that by 2047 the highest cancer deaths in Canada would be from lung, colorectal, pancreas, prostate and breast cancers.
By 2047, active tobacco smoking and excess body weight are projected to be the leading modifiable risk factors for preventable cancer mortality.
About 34,600 cancer deaths associated with active tobacco smoking could be prevented from 2024 to 2047 if a 30% relative reduction in smoking prevalence was achieved by 2020.
About 27,200 cancer deaths associated with excess body weight could be prevented from 2024 to 2047 if a 25% reduction in the prevalence of overweight and obesity is achieved by 2032.
What are the key implications for public health interventions, practice or policy?
The results of our study provide additional information to support cancer prevention strategies through intervention targets for governments, policy makers and health promotion programs.
Our results projected preventable cancer deaths related to various cancer intervention targets, which provides further evidence in support of intervention programs for cancer control.
Noteworthy interventions from our study included meeting reduction targets for smoking, obesity, and inadequate physical activity and increasing vegetable and fruit intake to prevent cancer mortality in Canada.
Supplementary information
(A) Projected bladder, colorectal, lung, pancreatic and prostate cancer deaths in Canadian males 35 years of age and older from 2024 (B) Projected breast, colorectal, lung, ovarian and pancreatic cancer deaths in Canadian females 35 years of age and older from 2024 (PDF 6 kb)
(DOCX 220 kb)
Acknowledgements
We would like to give our thanks and acknowledge the support from the CPAC OncoSim team. We would also like to acknowledge Claude Nadeau from Statistics Canada for his programming advice and support.
Funding
OncoSim is led and supported by the Canadian Partnership Against Cancer, with model development by Statistics Canada, and is made possible through funding from Health Canada.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joy Pader and Yibing Ruan contributed equally to this work.
References
- Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, Bakken S, Kaplan CP, Squiers L, Fabrizio C, Fernandez M. How we design feasibility studies. American Journal of Preventive Medicine. 2009;36(5):452–457. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Soerjomataram I. Population attributable fractions continue to unmask the power of prevention. British Journal of Cancer. 2018;118(8):1031–1032. doi: 10.1038/s41416-018-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DR, Poirier AE, Walter SD, King WD, Franco EL, Demers PA, Villeneuve PJ, Ruan Y, Khandwala F, Grevers X, Nuttall R, Smith L, De P, Volesky K, O’Sullivan D, Hystad P, Friedenreich CM, Com PSG. Estimating the current and future cancer burden in Canada: methodological framework of the Canadian population attributable risk of cancer (ComPARe) study. BMJ Open. 2018;8(7):e022378. doi: 10.1136/bmjopen-2018-022378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DR, Friedenreich CM, Ruan Y, Poirier AE, Walter SD, King WD, Franco EL, Demers PA, Villeneuve PJ, Grevers X, Nuttall R, Smith LM, Volesky KD, O’Sullivan DE, De P. The burden of cancer attributable to modifiable risk factors in Canada: methods overview. Preventive Medicine. 2019;122:3–8. doi: 10.1016/j.ypmed.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Brenner DR, Weir HK, Demers AA, Ellison LF, Louzado C, Shaw A, Turner D, Woods RR, Smith LM. Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192(9):E199–e205. doi: 10.1503/cmaj.191292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B, Harris KJ, Heil D, Tryon M, Cooksley A, Semmens E, Davis J, Gandhi K. Feasibility and outcomes of an out-of-school and home-based obesity prevention pilot study for rural children on an American Indian reservation. Pilot and Feasibility Studies. 2018;4:129–129. doi: 10.1186/s40814-018-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt P, Beirness D, Gliksman L, Paradis C, Stockwell T. Alcohol and health in Canada: a summary of evidence and guidelines for low-risk drinking. Ottawa: Canadian Centre on Substance Abuse; 2011. [Google Scholar]
- Canadian Cancer Society’s Advisory Committee. (2017). Canadian Cancer Statistics 2017. http://www.cancer.ca/Canadian-Cancer-Statistics-2017-EN.pdf. Accessed 12 Mar 2020.
- Canadian Cancer Society’s Advisory Committee. (2019). Canadian Cancer Statistics 2019. https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2019-EN.pdf?la=en. Accessed 12 Mar 2020.
- Coldman A, Pader J, Gauvreau C, Memon S, Fitzgerald N, Flanagan W, Nadeau C, Earle C, Wolfson M, Miller A, Lacombe J. Simulating results from trials of sigmoidoscopy screening using the OncoSim microsimulation model. Journal of Cancer Policy. 2018;15:52–58. doi: 10.1016/j.jcpo.2017.12.006. [DOI] [Google Scholar]
- Djuric Z, Ellsworth JS, Ren J, Sen A, Ruffin MTT. A randomized feasibility trial of brief telephone counseling to increase fruit and vegetable intakes. Preventive Medicine. 2010;50(5–6):265–271. doi: 10.1016/j.ypmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eather N, Riley N, Miller A, Smith V, Poole A, Vincze L, Morgan PJ, Lubans DR. Efficacy and feasibility of HIIT training for university students: the Uni-HIIT RCT. Journal of Science and Medicine in Sport. 2019;22(5):596–601. doi: 10.1016/j.jsams.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Gauvreau CL, Fitzgerald NR, Memon S, Flanagan WM, Nadeau C, Asakawa K, Garner R, Miller AB, Evans WK, Popadiuk CM, Wolfson M, Coldman AJ. The OncoSim model: development and use for better decision-making in Canadian cancer control. Current oncology (Toronto, Ont.) 2017;24(6):401–406. doi: 10.3747/co.24.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes AL, Patrao TA, Green A, Aitken JF. CanPrevent: a telephone-delivered intervention to reduce multiple behavioural risk factors for colorectal cancer. BMC Cancer. 2012;12:560. doi: 10.1186/1471-2407-12-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman SJ, Tan C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health. 2015;15:744. doi: 10.1186/s12889-015-2041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchesson MJ, Rollo ME, Krukowski R, Ells L, Harvey J, Morgan PJ, Callister R, Plotnikoff R, Collins CE. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obesity Reviews. 2015;16(5):376–392. doi: 10.1111/obr.12268. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). (2004). IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum, 83, 1–1438. [PMC free article] [PubMed]
- International Agency for Research on Cancer (IARC). (2010). IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum, 96, 3–1383. [PMC free article] [PubMed]
- International Agency for Research on Cancer (IARC). (2016). IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Outdoor air pollution. IARC Monogr Eval Carcinog Risks Hum, 109 . https://www.ncbi.nlm.nih.gov/books/NBK368024/. Accessed 1 Dec 2020.
- Jelsma JGM, Renaud LR, Huysmans MA, Coffeng JK, Loyen A, van Nassau F, Bosmans JE, Speklé EM, van der Beek AJ, van der Ploeg HP. The Dynamic Work study: study protocol of a cluster randomized controlled trial of an occupational health intervention aimed at reducing sitting time in office workers. BMC Public Health. 2019;19(1):188–188. doi: 10.1186/s12889-019-6467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier AE, Ruan Y, Grevers X, Walter S, Villeneuve P, Friedenreich C, Brenner D. Estimates of the current and future burden of cancer attributable to active and passive tobacco smoking in Canada. Preventive Medicine. 2019;122:9–19. doi: 10.1016/j.ypmed.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Poirier AE, Ruan Y, Volesky KD, King WD, O’Sullivan DE, Gogna P, Walter SD, Villeneuve PJ, Friedenreich CM, Brenner DR. The current and future burden of cancer attributable to modifiable risk factors in Canada: Summary of results. Preventive Medicine. 2019;122:140–147. doi: 10.1016/j.ypmed.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Popadiuk C, Gauvreau CL, Bhavsar M, Nadeau C, Asakawa K, Flanagan WM, Wolfson MC, Coldman AJ, Memon S, Fitzgerald N, Lacombe J, Miller AB. Using the Cancer Risk Management Model to evaluate the health and economic impacts of cytology compared with human papillomavirus DNA testing for primary cervical cancer screening in Canada. Current Oncology. 2016;23(Suppl 1):S56–S63. doi: 10.3747/co.23.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, Y., Poirier, A. E., Pader, J., Asakawa, K., Lu, C., Memon, S., Miller, A., Walter, S. D., Villeneuve, P. J., King, W. D., Volesky, K. D., Smith, L., De, P., Friedenreich, C. M., & Brenner, D. R. (2021). Estimating the future cancer management costs attributable to modifiable risk factors in Canada. Can J Public Health, 112. 10.17269/s41997-021-00502-x. [DOI] [PMC free article] [PubMed]
- Sacerdote C, Fiorini L, Rosato R, Audenino M, Valpreda M, Vineis P. Randomized controlled trial: effect of nutritional counselling in general practice. International Journal of Epidemiology. 2006;35(2):409–415. doi: 10.1093/ije/dyi170. [DOI] [PubMed] [Google Scholar]
- Song Z, Baicker K. Effect of a workplace wellness program on employee health and economic outcomes: a randomized clinical trial. JAMA. 2019;321(15):1491–1501. doi: 10.1001/jama.2019.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zhu Y, Qian Q, Tang L. Body mass index and prognosis of breast cancer: an analysis by menstruation status when breast cancer diagnosis. Medicine. 2018;97(26):e11220. doi: 10.1097/MD.0000000000011220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng E, Zhang A, Shogbesan O, Gudzune KA, Wilson RF, Kharrazi H, Cheskin LJ, Bass EB, Bennett WL. Effectiveness of policies and programs to combat adult obesity: a systematic review. Journal of General Internal Medicine. 2018;33(11):1990–2001. doi: 10.1007/s11606-018-4619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund/ American Institute for Cancer Research. (2011). Food, nutrition, physical activity, and the prevention of colorectal cancer. https://www.wcrf.org/sites/default/files/Colorectal-Cancer-2011-Report.pdf. Accessed 2 Apr 2020.
- World Cancer Research Fund/ American Institute for Cancer Research. (2018a). Body fatness and weight gain and the risk of cancer. Continuous Update Project, Issue. https://www.wcrf.org/dietandcancer/exposures/body-fatness. Accessed 2 Apr 2020.
- World Cancer Research Fund/ American Institute for Cancer Research. (2018b). Diet, nutrition, physical activity and cancer: a global perspective 3rd edition. Continuous Update Project, Issue. https://www.wcrf.org/dietandcancer/about. Accessed 2 Apr 2020.
- World Cancer Research Fund/ American Institute for Cancer Research. (2007). Food, nutrition, physical activity, and the prevention of cancer: a global perspective. WCRF/AICR Expert Report, Issue.
- World Health Organization. (2012). Target 5: reduce tobacco use. https://www.who.int/nmh/ncd-tools/target5/en/. Accessed 10 Jun 2020.
- World Health Organization. (2013). Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013-2020. Geneva: World Health Organization.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Projected bladder, colorectal, lung, pancreatic and prostate cancer deaths in Canadian males 35 years of age and older from 2024 (B) Projected breast, colorectal, lung, ovarian and pancreatic cancer deaths in Canadian females 35 years of age and older from 2024 (PDF 6 kb)
(DOCX 220 kb)