SUMMARY
Background
Post-mastectomy pain syndrome (PMPS) is a surgical complication of breast surgery characterized by chronic neuropathic pain. The development of PMPS is multifactorial and research on its prevention is limited. The objective of this systematic review is to synthesize the existing evidence on interventions for lowering the incidence of persistent neuropathic pain after breast surgery.
Methods
Using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we performed a comprehensive search of the electronic databases of MEDLINE, Cochrane Library, Embase, CINAHL, PsycINFO, Web of Science, and ClinicalTrials.gov using a combination of database-specific controlled vocabulary and keyword searches. Two reviewers independently screened all unique records. Publications on chronic (>3-month duration) pain after breast cancer-related surgery were included. Studies were classified by modality.
Results
Our literature search yielded 7092 articles after deduplication. We identified 45 studies that met final inclusion criteria for analysis, including 37 randomized-controlled trials. These studies revealed seven major intervention modalities for prevention of PMPS: physical therapy, mindfulness-based cognitive therapy, oral medications, surgical intervention, anesthesia, nerve blocks, and topical medication therapy.
Conclusion
High-quality data on preventative techniques for PMPS are required to inform decisions for breast cancer survivors. We present a comprehensive assessment of the modalities available that can help guide breast and reconstructive surgeons employ effective strategies to lower the incidence and severity of PMPS. Our review supports the use of multimodal care involving both a peripherally targeted treatment and centrally acting medication to prevent the development of PMPS.
Keywords: post-mastectomy pain syndrome, prevention, neuropathic pain, systematic review
BACKGROUND
Persistent pain is a postoperative complication estimated to affect between 20% and 50% of mastectomy patients with considerable negative influence on quality of life.1 Post-mastectomy pain syndrome (PMPS) is a neuropathic condition defined as pain located in the anterior surface of the chest axilla, shoulder or upper half of the arm that persists for longer than 3 months after surgery.2,3 As treatment and diagnostic strategies advance, more women are expected to survive breast cancer, growing the population at risk for developing PMPS. Interventions are needed to prevent the development of this long-term, costly, and painful complication of breast cancer procedures.
The pathophysiology of PMPS involves unrelieved inflammation and neuropathic pain. Possible contributors include sensitization of peripheral nociceptors and their primary afferent neurons, growth of neuromas on pain-sensing fibers, and sensitization of nerve cells in the brain.4 Further, severe acute postoperative pain results in increased incidence of persistent postoperative pain.5,6 Consequently, preventive and perioperative forms of analgesia and pharmacological agents may reduce the development of, or transition to, PMPS.
Previous studies have reviewed associated risk factors for PMPS, including preoperative pain, axillary lymph node dissection (ALND), anxiety, younger age, and radiation therapy.7,8 Pharmacotherapy for prevention of general chronic pain after surgery has also been a focus of literature.4 However, a comprehensive review of interventions for the prevention of post-mastectomy neuropathic pain is lacking. In this study, we review the available modalities for prevention of the development of PMPS and assess their outcomes in order to guide optimal care for breast cancer patients.
MATERIALS AND METHODS
Our systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines as a framework to assess quality of evidence and risks of bias in the included studies.9 The protocol was registered with the National Institutes of Health Research database PROSPERO (ID = CRD42020167225).
Literature search and Study Appraisal
A research librarian (ABW) worked in collaboration with the review authors (SY, AC) to create a comprehensive search for identifying literature related to the neuropathic pain associated with breast cancer surgery. A search was developed for MEDLINE (PubMed), which was then translated for Cochrane Library databases (Wiley), Embase (Elsevier), CINAHL (Ebsco), PsycINFO (Ebsco), Web of Science (Clarivate), and ClinicalTrials.gov. All searches were conducted using a combination of database-specific controlled vocabulary and keyword searches without limits for date or publication type. English language filters were applied. Results were exported to a citation management software (EndNote) for deduplication. An example of the search strategy for PubMed is provided in Appendix A. References of eligible studies and review articles were reviewed to include studies not previously captured by the original search terms. To avoid selection bias, inclusion and exclusion criteria were agreed upon before data extraction and analysis (Table 1). Definition of the terms “chronic pain,” and “breast cancer-related procedures” was critical in creating the selection criteria (Appendix B). All unique records were uploaded into an online screening platform (Rayyan) for blinded independent screening by two reviewers, first through title and abstract screen and then through full-text review10; disagreements were resolved by the senior author (MFE).
Table 1.
Inclusion and Exclusion Criteria for Abstract and Full-Text Screening
|
Inclusion criteria Full-text, original articles Written in the English language Reporting outcomes for interventions to prevent chronic pain after breast cancer-related surgery Observational studies (case-control studies and cohort studies) Intervention studies, including RCTs |
|
Exclusion criteria Review articles Case reports, case series, conference abstracts, book chapters, and letters to editor Animal studies, cell studies, and cadaver studies Articles with follow-up <3 months Articles regarding only treatment of chronic postoperative pain |
RCTs – randomized-controlled trials
Data Extraction
Types of data extracted from each study included: (i) pain prevention modality, (ii) study design, (iii) number of patients, (iv) follow-up time, (v) outcome metrics, and (vii) major outcomes of intervention. Primary outcomes were limited to patient-reported pain characteristics, both quantitative and qualitative, and quality of life scores. Studies were organized based on thematic analysis of each pain prevention modality.
RESULTS
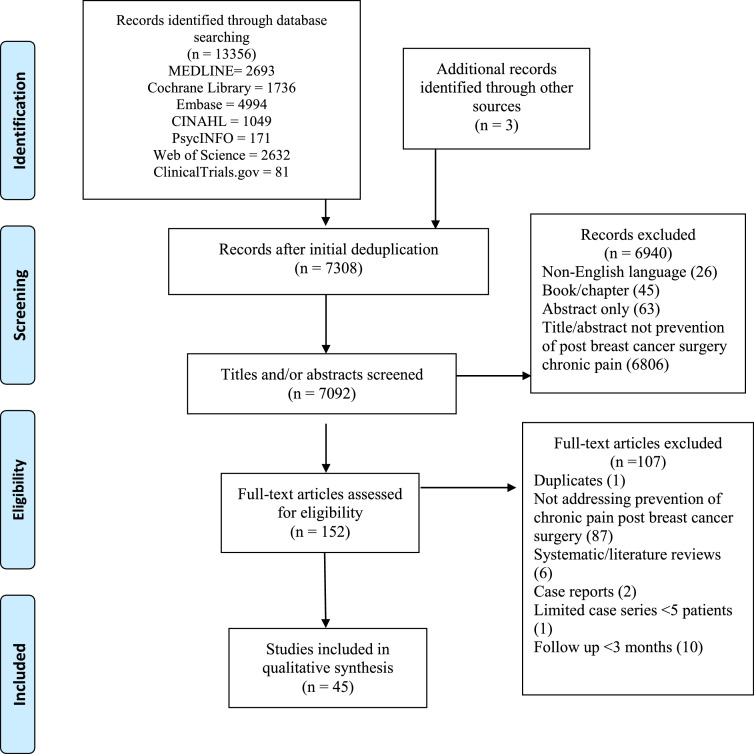
The initial literature search in the seven electronic databases yielded 7092 articles from the years 1946–2020 after deduplication. Following two rounds of screening, 45 studies fulfilled the eligibility criteria for inclusion, of which 37 were randomized-controlled trials (RCTs). Seven unique intervention modality groups were identified, with a range of reported outcomes (Table 2).
Table 2.
Study characteristics of all included studies
| Treatment Modality | Reference | Design, N (# of patients) | Intervention |
|---|---|---|---|
| Physical Therapy | Ammitzbøll et al., 202011 | Double-blinded RCT, 158 | 1-year progressive resistance training |
| De Groef et al., 201012 | Double-blinded RCT, 143 | 8 sessions of myofascial therapy | |
| Cognitive Therapy | Lacroix et al., 201913 | Prospective non-randomized study, 42 | Perioperative hypnosedation |
| Garssen et al., 201014 | RCT, 70 | Pre-surgical stress management training | |
| Nerve Blocks | Besch et al., 201019 | Retrospective comparative study, 191 | GA with pectoral nerve block |
| Fujii et al., 201820 | Double-blinded RCT, 80 | PECS II Block | |
| De Cassai et al., 201918 | Prospective observational comparative study, 132 | PECS II block | |
| Strazisar et al., 201415 | Prospective randomized study, 60 | Wound catheter with continuous infusion of local anesthetic | |
| Mohamed et al., 201817 | Double-blinded RCT, 90 | Wound irrigation with bupivacaine plus 5, 10, or 15 mg morphine | |
| Albi-Feldzer et al., 200016 | Double-blinded RCT, 236 | Wound infiltration with repeated injections of local anesthetic | |
| Shimizu et al., 201523 | Retrospective cohort study, 46 | Perioperative ultrasound-guided TPVB | |
| Karmakar et al., 201724 | Double-blinded RCT, 177 | Single injection or continuous infusion with catheter of TPVB | |
| Kamal et al., 201925 | Double-blinded RCT, 80 | TPVB of bupivacaine with 0.5 mg/kg or 1 mg/kg ketamine | |
| Kairaluoma et al., 200626 | Double-blinded RCT, 59 | Pre-incisional PVB | |
| Elkaradawy et al., 201228 | RCT, 50 | Ultrasound-guided TPVB with GA | |
| Ilfeld et al., 201827 | Double-blinded RCT, 60 | Ultrasound-guided continuous PVB catheter | |
| Gacio et al., 201821 | Non-randomized prospective observational study, 66 | GA and single-injection PVB | |
| Qian et al., 201922 | Double-blinded RCT, 184 | Ultrasound-guided single-injection multilevel TPVB | |
| Anesthesia | Cho et al., 201229 | Retrospective comparative study, 228 | Propofol vs. sevoflurane anesthesia |
| Lefebvre-Kuntz et al., 201532 | Prospective cohort study, 328 | Halogenated anesthetic vs. propofol | |
| Grigoras et al., 201330 | Double-blinded RCT, 36 | Perioperative IV lidocaine | |
| Terkawi et al., 201738 | Double-blinded RCT, 61 | Intraoperative IV lidocaine infusion | |
| Kim et al., 201737 | Double-blinded RCT, 116 | Intraoperative systemic lidocaine vs. magnesium | |
| Kendall et al., 201834 | Double-blinded RCT, 121 | Perioperative IV lidocaine | |
| Khan et al., 2019*35 | Double-blinded RCT, 100 | Perioperative pregabalin and lidocaine infusion | |
| Jain et al., 201236 | Double-blinded RCT, 69 | Intraoperative IV dexmedetomidine | |
| Kang et al., 202033 | Double-blinded RCT, 168 | Perioperative IV ketamine | |
| Sun et al., 201231 | Double-blinded RCT, 60 | Perioperative IV flurbiprofen axetil | |
| Oral medications | Fassoulaki et al., 200239 | Double-blinded RCT, 67 | Perioperative oral mexiletine vs. gabapentin capsules |
| Lee et al., 201340 | Single-blinded RCT, 51 | Multimodal analgesia (perioperative pregabalin and PVB catheter of local anesthetic) | |
| Vig et al., 201942 | Double-blinded RCT, 71 | Perioperative pregabalin | |
| Reyad et al., 201943 | Double-blinded RCT, 181 | Perioperative pregabalin | |
| Fassoulaki et al., 200144 | Double-blinded RCT, 96 | Perioperative mexiletine and regional ropivacaine block | |
| Amr et al., 200946 | Double-blinded RCT, 150 | Perioperative gabapentin and venlafaxine | |
| Khan et al., 2019*35 | Double-blinded RCT, 100 | Perioperative pregabalin and lidocaine infusion | |
| Hah et al., 199645 | Double-blinded RCT, 41 | Perioperative gabapentin | |
| Van Helmond et al., 201641 | Double-blinded RCT, 94 | Perioperative parecoxib injection and celecoxib | |
| Fassoulaki et al., 200547 | Double-blinded RCT, 44 | Multimodal analgesia (Gabapentin, EMLA cream near incision, irrigation of brachial plexus block) | |
| Na et al., 201848 | Double-blinded RCT, 83 | Intraoperative IV nefopam | |
| Surgical interventions | Salmon et al., 199855 | Double-blinded RCT, 128 | Preservation of ICBN |
| Torresan et al., 200751 | Double-blinded RCT, 85 | Preservation of ICBN | |
| Freeman et al., 200353 | Double-blinded RCT, 73 | Preservation of ICBN | |
| Taira et al., 201454 | Non-blinded RCT, 140 | Preservation of ICBN | |
| Tasmuth et al., 199950 | Retrospective observational study, 221 | High-volume surgical units vs. small-volume units | |
| Yang et al., 201252 | Double-blinded RCT, 99 | Spray of HA–CMC gel onto surface of pectoralis major and serratus anterior muscles | |
| Topical medications | Fassoulaki et al., 200056 | Double-blinded RCT, 45 | Intraoperative EMLA on sternal area |
*Included in two categories
RCT – Randomized controlled trial
GA – Generalized anesthesia
TPVB – Thoracic paravertebral block
ICBN – Intercostal-brachial nerve
HA–CMC – Hyaluronic acid–carboxymethyl cellulose
IV – Intravenous
Physical Therapy
Two double-blinded RCTs evaluated the effects of physical therapy (PT) on incidence and severity of PMPS in women undergoing breast cancer surgery with ALND, with little effect.11,12 The follow-up time in both studies was 12 months, and the total study population was 301 patients. Ammitzbøll et al. reported that a 1-year progressive resistance training program initiated at 3 weeks after breast cancer surgery with ALND conferred no benefit over usual care in reducing chronic pain incidence or intensity.11 Similarly, an 8-week myofascial therapy program had no added benefit compared with standard PT.12
Cognitive Therapy
Two studies on the efficacy of cognitive behavioral therapy in the prevention of PMPS met inclusion criteria, one of which was an RCT.13,14 The mean follow-up time for both studies was 26 months, and the total study population was 112 patients. Lacroix et al. reported that in 42 patients undergoing mastectomy with ALND, perioperative hypnosedation led to significantly lower incidence of PMPS at 4 years postoperatively compared with general anesthesia (GA) (P = 0.008).13 Pre‐surgical stress management training (SMT) did not significantly influence postoperative pain or anxiety after 3 months compared with control.14
Nerve Blocks
Fourteen studies on the efficacy of perioperative nerve blocks in the prevention of PMPS met inclusion criteria (Table 3). 15–20 Eight of these were double-blinded RCTs, while the rest comprised two retrospective cohort studies, two prospective randomized studies, and two prospective observational comparative studies. The mean follow-up time across all studies was 8 months, and the total study population involved 1511 patients.
Table 3.
Nerve Block Studies
| Reference | Procedure | Intervention | Follow-up (mo), Avg (Range) | Outcome measures | Findings |
|---|---|---|---|---|---|
| Besch et al., 201019 | Breast cancer surgery | Perioperative GA with pectoral nerve block | 6 | Presence of pain (Breast Cancer Pain Questionnaire), Pain intensity | Pectoral block had no effect on incidence nor severity of persistent post-surgical pain |
| De Cassai et al., 201918 | Mastectomy or lateral quadrantectomy | PECS II block | 12 | Presence of pain, intensity of pain (NRS) | PECS II decreased incidence of chronic pain only for 3 months compared to GA alone (P = 0.039). It did not decrease pain intensity. |
| Fujii et al., 201820 | Mastectomy | PECS II block | 6 | Presence of pain, pain intensity (NRS), health-related QOL (EQ-5D-3L questionnaire) | PECS II reduced incidence of moderate and severe chronic pain compared to serratus plane block (P = 0.02). |
| Strazisar et al., 201415 | Breast cancer surgery with ALND | Wound catheter with continuous infusion of local anesthetic | 6 | Presence of pain | Infusion of local anesthetic did not significantly decrease incidence of neuropathic pain compared to standard IV analgesia (20% vs. 40%, P = 0.09) |
| Mohamed et al., 201817 | Modified radical mastectomy with ALND | Wound irrigation with bupivacaine plus 5, 10 or 15 mg morphine | 3 | Location, intensity, nature and duration of pain, analgesic medication use, LANSS Pain Scale | The lowest mean LANSS score was recorded in the Morphine 15 group compared with Morphine 5 and Morphine 10 (P < 0.006). |
| Albi-Feldzer et al., 200016 | Breast cancer surgery | Wound infiltration with repeated injections of local anesthetic | 12 | Presence of pain, pain intensity at rest and movement, pain interference (BPI), neuropathic pain (DN4) | Ropivacaine wound infiltration did not decrease chronic pain intensity or incidence. |
| Gacio et al., 201821 | Major resection for breast cancer | Single TPVB injection | 6 | ||
| Pain intensity at rest and with movement in ipsilateral arm (VAS), neuropathic pain (DN3), QOL (EORTC QLQ-C30) | Single-injection PVB had no effect on intercostobrachial neuralgia (P = 0.3). | ||||
| Kairaluoma et al., 200626 | Conservative breast surgery for cancer with SENTINEL lymph node biopsy | Single TPVB injection | 12 | Presence of pain, pain intensity at movement and rest (NRS), pain characteristics | PVB lowered pain incidence (P = 0.003), intensity at rest (P = 0.011) and movement (P = 0.003). |
| Shimizu et al., 201523 | Breast cancer surgery | Ultrasound-guided single TPVB injection | 15 (13, 17) | Pain intensity (VRS) | TPVB significantly lowered incidence of chronic pain (P = 0.039). |
| Karmakar et al., 201724 | Modified radical mastectomy with ALND | Single injection or continuous infusion with catheter of TPVB | 6 | Incidence of pain, pain severity (VRS) at rest and during movement, health-related QOL, Chronic Pain Symptom and Sign Score | No difference in incidence of chronic pain between groups (P = 0.79). Patients receiving TPVB had lower chronic pain scores (P < 0.05) and had fewer symptoms of chronic pain (P ≤ 0.01). |
| Elkaradawy et al., 201228 | Conservative breast surgery for cancer | Ultrasound-guided TPVB with GA | 9 | Neuropathic pain (NPS), Pain intensity (NRS) | TPVB decreased pain intensity (P ≤ 0.05). |
| Ilfeld et al., 201827 | Uni- or bilateral mastectomy | Ultrasound- guided continuous PVB catheter | 12 | Presence of pain, pain intensity and interference (BPI) | PVB catheter significantly decreased incidence (P = 0.011) and intensity of pain (P = 0.007). |
| Qian et al., 201922 | Unilateral partial mastectomy | Ultrasound-guided single-injection multilevel TPVB | 6 | Presence of pain, pain intensity and interference (BPI) | PVB significantly reduced severity (P < 0.001). and incidence (P = 0.03) of chronic pain. |
| Kamal et al., 201925 | Modified radical mastectomy and ALND | Ultrasound-guided multilevel TPVB with 0.5 mg/kg or 1 mg/kg ketamine | 3 | DN4 questionnaire for chronic neuropathic pain | No difference in mean DN4 scores between control, 0.5 mg/kg and 1 mg/kg ketamine (P = 0.132) |
NRS – Numerical rating scale
QOL – Quality of life
LANSS- Leeds Assessment of Neuropathic Symptoms and Signs
BPI – Brief Pain Inventory
DN4 – Douleur neuropathique 4
TPVB – Thoracic paravertebral block
VAS – Visual analogue scale
DN3 – Douleur neuropathique 3
VRS – Verbal rating scale
Wound infiltration with local analgesia, whether through continuous infusion with a wound catheter or through repeat injections, was ineffective across two double-blinded RCTs. 15,16 In a third study by Mohamed et al., higher doses of morphine for wound irrigation were more effective in decreasing intensity of chronic pain (P < 0.006).17
Three studies with a total of 403 patients investigated the utility of pectoral nerve blocks (PECS) in preventing chronic pain after breast cancer-related surgery. 18, 19, 20 While PECS administered with GA was ineffective, 19 the results of PECS II were equivocal; one study found its benefit versus GA to be limited to 3 months, and another found that it reduced incidence of moderate/severe chronic pain when compared to serratus plane block (P = 0.02).18, 20
Eight studies with a total of 722 patients investigated the effects of thoracic paravertebral blocks (TPVBs) on reducing chronic pain after breast cancer.21, 22, 23, 24, 25, 26, 27, 28 Five of these studies used ultrasound guidance for TPVB administration. While one study found no effect on intercostobrachial neuralgia, 21 six studies found that TPVBs reduced incidence and intensity of chronic pain. Single injection versus continuous infusion with a catheter did not differ in efficacy. 24 Ketamine administration with TPVB had no effect.25
General Anesthesia
Ten studies on the efficacy of anesthesia in the prevention of PMPS met inclusion criteria (Table 4).29, 30, 31, 32, 33, 34, 35, 36, 37, 38 These included one retrospective study, one prospective study, and eight double-blinded RCTs. The mean follow-up time for all ten studies was 9 months, and the total study population was 1287 patients.
Table 4.
Anesthesia Studies
| Reference | Procedure | Intervention | Follow-up (mo), Avg (Range) | Outcome measures | Findings |
|---|---|---|---|---|---|
| Cho et al., 201229 | Breast cancer surgery | Propofol vs. sevoflurane anesthesia | 39 (30, 48) | Presence of pain, pain intensity (NRS), pain interference with daily life | Sevoflurane resulted in higher incidence of chronic pain compared to propofol (P = 0.007), but not pain intensity. |
| Lefebvre-Kuntz et al., 201532 | Breast cancer surgery | Propofol vs. halogenated anesthetic | 6 | Presence of pain, Neuropathic pain (DN4, Neuropathic Pain Symptom Inventory), pain intensity (VAS), | Type of general anesthetic had no effect on incidence nor intensity of pain. |
| Grigoras et al., 2013 | Mastectomy or wide local excision with ALND | IV lidocaine infusion | 3 | Presence of pain, intensity of pain (SF-MPQ, VAS) character of pain, interference with daily life | Lidocaine resulted in lower pain incidence (P = 0.031) and intensity (0.025) compared to control. |
| Terkawi et al., 201738 | Mastectomy | IV lidocaine infusion | 6 | Presence of pain, pain intensity (NRS), pain characteristics | Lidocaine was associated with a 20-fold decrease in incidence of CPSP compared to control (P = 0.013). |
| Kim et al., 201737 | Breast cancer surgery | Systemic lidocaine infusion vs. magnesium | 3 | Presence of pain, pain intensity and quality (Korean SF-MPQ) | Lidocaine significantly decreased pain intensity compared to control (P = 0.046), but had no effect on chronic pain incidence. Magnesium had no effect. |
| Kendall et al., 201834 | Breast cancer surgery | IV lidocaine infusion | 6 | Presence of pain, pain intensity (NRS, BPI, SF-MPQ), neuropathic pain (S-LANSS) | Lidocaine reduced pain incidence (P = 0.04) compared to control and had no effect on intensity. |
| Khan et al., 2019*35 | Unilateral or bilateral mastectomy or lumpectomy | Perioperative pregabalin and lidocaine infusion | 3 | Presence of pain, pain intensity (BPI, SF-MPQ2), neuropathic pain (DN4) | Lidocaine decreased incidence of persistent neuropathic pain (P = 0.049) compared to control, but not intensity. Pregabalin had no effect. |
| Sun et al., 201231 | Mastectomy with ALND | Perioperative IV flurbiprofen axetil | 12 | Presence of pain, pain intensity (NRS), nature of pain | Flurbiprofen axetil resulted in lower pain incidence for 6 months postoperatively, but not 12 months. It significantly lowered pain intensity (P < 0.05). |
| Kang et al., 202033 | Unilateral breast cancer surgery | IV ketamine | 6 | Presence of pain, pain intensity at rest and movement (NRS), Neuropathic pain (DN4) | Ketamine did not reduce pain intensity compared with control. Pain incidence was lower at 3 months, but not 6. |
| Jain et al., 201236 | Breast cancer surgery | IV dexmedetomidine | 3 | Presence of pain, pain intensity (BPI, SF-MPQ2) | Dexmedetomidine decreased pain intensity (P < 0.001) and incidence (P < 0.001) compared with control. |
NRS – Numerical rating scale
DN4 – Douleur neuropathique 4
VAS – Visual analogue scale
SF-MPQ – Short-form McGill Pain Questionnaire
BPI – Brief Pain Inventory
S-LANSS – Self-administered Leeds Assessment of Neuropathic Symptoms and Signs
QOL – Quality of life
IV – Intravenous
A retrospective study found that incidence of chronic pain was significantly higher in the sevoflurane anesthesia group compared with the propofol anesthesia group (P = 0.007).29 However, a multicenter prospective study by Lefebvre-Kuntz et al. with a greater sample size found no difference between propofol and other halogenated agent anesthetics.32 Neither study found an effect of anesthesia type on pain intensity.
Across five studies, intravenous (IV) lidocaine infusion significantly decreased chronic pain incidence, intensity, or both.30,34,35,37,38 Pregabalin and magnesium were both comparatively ineffective. 35,37
The remaining three studies in this cohort investigated several other anesthetic agents. Intraoperative low-dose ketamine reduced pain incidence at 3 months postoperatively compared with a control, but these results did not continue through 6 months.33 IV flurbiprofen axetil significantly decreased both incidence of pain and pain scores at 6 months postoperatively compared with a control.31 Perioperative infusion of dexmedetomidine significantly decreased the incidence and intensity of chronic pain.36
Oral Medications
Eleven RCTs regarding the efficacy of oral medications met inclusion criteria (Table 5).37,39, 40, 41, 42, 43, 44, 45, 46, 47, 48 Of these, ten were double-blinded RCTs. Across all studies, the mean follow-up time was 5 months, and there were 978 total patients.
Table 5.
Oral Medication Studies
| Reference | Procedure | Intervention | Follow-up (mo), Avg. (Range) | Outcome measures | Findings |
|---|---|---|---|---|---|
| Fassoulaki et al., 200144 | Modified radical mastectomy or lumpectomy with ALND | Mexiletine and regional ropivacaine block | 3 | Presence of pain, pain intensity | Oral mexiletine, regional block nor their combination decreased incidence or intensity of chronic pain compared with control. |
| Fassoulaki et al., 200239 | Breast surgery for cancer | Mexiletine vs. gabapentin | 3 | Presence of pain, pain intensity (NRS) | Neither mexiletine nor gabapentin affected pain incidence or intensity compared with control. |
| Hah et al., 199645 | Unilateral/ bilateral mastectomy or breast lumpectomy | Gabapentin | 12 | Presence of pain, pain intensity (BPI) | Gabapentin did not decrease incidence of pain compared with control. |
| Amr et al., 200946 | Partial or radical mastectomy with ALND | Gabapentin and venlafaxine | 6 | Presence of pain, pain intensity (VAS), pain characteristics | Venlafaxine decreased pain intensity (P < 0.0001) and incidences of burning and stabbing pain compared with gabapentin and control. |
| Fassoulaki et al., 200547 | Breast cancer surgery | Multimodal analgesia (oral gabapentin, EMLA cream near incision, irrigation of brachial plexus block) | 6 | Presence of pain, pain intensity | Multimodal analgesia intervention did not decrease incidence of chronic pain. |
| Vig et al., 201942 | Modified radical mastectomy | Pregabalin | 3 | Presence of pain, pain intensity (NRS) | Pregabalin did not decrease incidence nor intensity of chronic pain compared with control. |
| Reyad et al., 201943 | Modified radical mastectomy or conservative breast surgery with ALND | Pregabalin | 6 | Presence of pain, neuropathic pain (GSNP), pain intensity at rest and movement (VAS) | Pregabalin decreased incidence (P < 0.001) and intensity (P = 0.002) of neuropathic pain compared with control. |
| Khan et al., 2019*35 | Unilateral or bilateral mastectomy or lumpectomy | Pregabalin and lidocaine infusion | 3 | Presence of pain, pain intensity (BPI, SF-MPQ2), neuropathic pain (DN4) | Lidocaine decreased incidence of persistent neuropathic pain (P = 0.049) compared with control, but not severity. Pregabalin had no effect. |
| Lee et al., 201340 | Breast surgery with ALND | Multimodal analgesia (pregabalin and PVB catheter of local anesthetic) | 3 | Presence of pain, pain characteristics (SF-MPQ) | Multimodal analgesic regimen did not decrease pain incidence compared with control. |
| Van Helmond et al., 201641 | Breast cancer surgery | Parecoxib injection and oral celecoxib | 12 | Presence of pain, pain intensity at rest and during movement (VAS), electric pain and pressure pain tolerance thresholds | COX-2 inhibition had no effect on pain intensity compared with control. |
| Na et al., 201848 | Lumpectomy with ALND or SLNB | IV nefopam | 3 | Pain intensity (NRS) | Nefopam decreased |
| incidence of chronic pain (P = 0.04), but had no effect on pain intensity compared with control. |
NRS – Numerical rating scale
BPI – Brief Pain Inventory
VAS – Visual analogue scale
GSNP- Grading system for neuropathic pain
SF-MPQ – Short-form McGill Pain Questionnaire
DN4 – Douleur neuropathique 4
Four studies testing the efficacy of gabapentin showed negative results, whether it was administered alone, with another medication, or as part of a multimodal analgesia course.39,45, 46, 47 Four studies evaluated the efficacy of pregabalin.37,40,42,43 While it had no effect on incidence or intensity of chronic pain in three studies, Reyad et al. found that the medication decreased both incidence and intensity of chronic pain at 6 months postoperatively.43
Various other medications had mixed results in reducing PMPS incidence. Mexiletine and COX2 inhibitors were both ineffective, while venlafaxine, a selective serotonin and norepinephrine reuptake inhibitor (SSNRI), significantly reduced incidence and intensity of chronic pain at 6 months postoperatively.41,44,46,49 Preventative IV nefopam significantly decreased the incidence, but not intensity, of chronic pain.48
Surgical Techniques
Six articles focusing on the potential role of surgical techniques in prevention of chronic neuropathic pain after breast cancer-related surgery were included (Table 6).50, 51, 52, 53, 54, 55 These consisted of four double-blinded RCTs, one non-blinded RCT, and one retrospective observational study. Across all studies, the mean follow-up time was 16 months and a total of 746 patients were included.
Table 6.
Surgical Intervention Studies
| Reference | Procedure | Intervention | Follow-up (mo), Avg (Range) | Outcome measures | Findings |
|---|---|---|---|---|---|
| Salmon et al., 199855 | Mastectomy or conservative breast cancer surgery | Preservation of ICBN | 16 (10, 22) | Presence of pain or sensitivity in region of the ICBN | ICBN preservation had no effect on pain. |
| Torresan et al., 200751 | Axillary lymphadenectomy | Preservation of ICBN | 3 | Presence, intensity and type of sensitivity deficits and pain | ICBN preservation decreased incidence of anesthesia, hypoesthesia or hyperesthesia (P < 0.01). |
| Freeman et al., 200353 | Breast cancer surgery | Preservation of ICBN | 36 (32-38) | Sensation for light touch, presence of neuromas | Preservation of ICBN had no effect on pain. |
| Taira et al., 201454 | Breast cancer surgery | Preservation of ICBN | 24 | Presence of dysesthesia, paresthesia and pain sensation in upper arm, health related QOL (FACT-B) | Preservation of ICBN had no effect on incidence or severity of pain compared to dissection. |
| Yang et al., 201252 | Total mastectomy | Spray of HA-CMC gel onto surface of pectoralis major and serratus anterior muscles | 6 | Presence of motion-related pain and intensity of pain (NRS), DASH questionnaire | HA-CMC decreases pain intensity related to flexion (P < 0.001) and abduction (P = 0.034) compared to control. |
| Tasmuth et al., 199950 | Unilateral breast cancer surgery with axillary clearance | High-volume surgical units (HVU) vs. small-volume units (LVU) | 12 | Presence of pain, pain intensity (VAS, Finnish MPQ), interference with sleep | Patients in LVU had higher incidence of chronic pain (P < 0.05). |
ICBN - Intercostal-brachial nerve
QOL – Quality of life
FACT-B – Functional Assessment of Cancer Therapy – Breast
NRS – Numerical rating scale
DASH – Disabilities of the arm, shoulder, and hand
VAS – Visual analogue scale
MPQ- McGill Pain Questionnaire
Preservation of the intercostal-brachial nerve (ICBN) was discussed in four RCTs. 51,53, 54, 55 Three of these studies concluded that preserving the ICBN conferred no significant differences in incidence or intensity of chronic pain. 53–55 However, Torresan et al. found that ICBN preservation decreased overall incidence of anesthesia, hypoesthesia, or hyperesthesia. 51
A mixed solution of sodium hyaluronate and carboxymethylcellulose (HA–CMC) on the surface of the pectoralis major and serratus anterior muscles resulted in significantly lower pain intensity related to flexion and horizontal abduction at 6 months postoperatively. 52 According to an observational study, pain at 1 year was less common in patients receiving breast cancer surgery at high-volume surgical units than low-volume ones (P <0.05).50
Topical Medication Therapy
A double-blinded RCT of 45 patients evaluating topical medication for prevention of PMPS concluded that perioperative application of eutectic mixture of local anesthetics (EMLA) cream on the sternal, supraclavicular, and axillary area significantly decreased incidence (P = 0.002) and intensity (P = 0.003) of pain in the chest wall and axilla 3 months postoperatively.56
DISCUSSION
Summary of main results
This systematic review aimed to identify effective interventions for prevention of PMPS. Interventions were categorized into seven unique modalities that could, in turn, be categorized based on pathophysiologic targets that are either peripherally or centrally mediated. Peripheral nerve sensitization is caused by iatrogenic injury and compression by scar tissue, which reduce firing thresholds of nociceptors’ terminals in the axilla and chest wall and their associated primary afferent neurons.57 Other interventions target the central nervous system (CNS) by interfering with neurotransmission. This prevents maladaptive central sensitization caused by repeated stimulation of nociceptive pathways.58
Interventions for peripheral sensitization (topical medication, systemic and local anesthesia, peripheral nerve surgery)
Of all forms of anesthesia, perioperative IV lidocaine showed the most positive results in our review, with multiple RCTs demonstrating its efficacy in reducing incidence and severity of PMPS. Systemic administration of local anesthetics has analgesic, anti-inflammatory, and anti-hyperalgesic effects, simultaneously targeting peripheral nociceptors and central sensitization. 30,59 However, Kendall et al. hypothesize that relying on a binary yes/no criterion to detect persistent pain rather than a validated pain instrument, as all but one of these studies do, may lead to overestimation of lidocaine's efficacy.34
Administration of local anesthetic suppresses afferent nociceptive signals and inflammatory reaction after nerve injury, but appears limited in its capacity to prevent chronic pain. This indicates that while there is ample evidence for severe acute postoperative pain being a risk factor for development of PMPS,1 modulation of acute pain alone is not sufficient to prevent chronic pain. Meanwhile, topical EMLA seems to significantly reduce the incidence and severity of PMPS, whether through anesthetic effect on cutaneous areas where injured nerves project, or due to systemic uptake.56
Intercostobrachial neuralgia due to sectioning of the ICBN is the most common cause of post-mastectomy chronic pain, and a common consequence of ALND.60,61 Across multiple studies, ALND increases the incidence of chronic pain compared to sentinel lymph node biopsy (SLND) and no axillary procedures.62, 63, 64, 65, 66 While the efficacy of ICBN preservation is widely debated in the literature, it has no effect on PMPS based on the findings of this review. Importantly, evidence has shown that breast reconstruction, whether implant or autologous, does not increase the incidence of PMPS despite additional tissue dissection and potential donor site morbidity.67 Long-term prospective cohort studies with standardized pain management among patients to compare different approaches to breast reconstruction are necessary.
Interventions for central sensitization (psychiatric treatment, oral medications)
Cortical reorganization plays an essential role in neuropathic pain after mastectomy. Anxiety, depression, and pain catastrophizing increase intensity of pain perception in the acute postsurgical period and are associated with development of PMPS.68, 69, 70 Behavioral cognitive therapy may help alter perception of noxious stimuli. Accordingly, perioperative hypnosis and pre-surgical stress management performed appear to be effective in preventing PMPS.
Medications targeting central sensitization showed variable benefits in this review, with wide ranges of drug doses and administration duration across studies. Nefopam and venlafaxine were both effective. Despite its efficacy in treatment of PMPS, gabapentin was ineffective in pain prevention, while results of pregabalin studies were equivocal. Gabapentinoids, such as gabapentin and pregabalin, interfere with afferent pain signals by inhibiting glutamate release in the dorsal horn of the spinal cord. Nefopam has properties of a monoamine reuptake inhibitor and an NMDA receptor antagonist, preventing pain signal transmission to secondary afferent neurons. Tricyclic antidepressants (TCAs), SSNRIs, such as venlafaxine, and SSNRIs inhibit pain transmission by: 1) binding to α2-adrenergic receptors in the dorsal horn of the spinal cord, preventing release of excitatory neurotransmitters that lead to pain perception and 2) increasing noradrenaline levels at the locus coeruleus of the brainstem, improving the function of an impaired descending noradrenergic inhibitory system.71 While our review found COX-2 inhibitors to be ineffective, IV flurbiprofen axetil, a nonselective COX inhibitor with a high affinity for inflammatory tissues due to its composition as emulsified lipid microspheres, effectively reduced pain incidence, indicating that blocking inflammatory responses plays a role in preventing PMPS.31 This supports the idea that therapy limited to pre- and intraoperative periods are likely to be insufficient for pain prevention due to the inflammatory reaction of damaged tissue providing a source of sensory signals that could induce central sensitization, even if it was prevented during the operation.
Given the close link between acute postoperative pain and chronic pain, medications that reduce perioperative anxiety may play a role in PMPS prevention. Ketamine has both mood stabilization and analgesic effects achieved through NMDA receptor blockade and decreased reuptake of serotonin and norepinephrine. 33 However, it was shown to be ineffective for PMPS prevention in this review, both alone and when administered with TPVB, indicating that targeting central sensitization alone is insufficient.
Interventions for central sensitization (nerve blockade)
Nerve blocks prevent integration of nociceptive impulses to the CNS, preventing the development of hyperalgesia and chronic pain.72 We conclude that single injection and continuous TPVB are effective in reducing severity and incidence of chronic pain.
Ultrasound-guided thoracic interfascial plane blocks are alternatives to PVBs. The PECS II block entails two interfascial injections of local anesthetic between the pectoralis major and minor muscles, while the serratus plane block is a single injection between the serratus anterior and latissimus dorsi muscles. As found in our review, PECS II was effective compared with serratus plane block; however, its efficacy compared with that of GA was not significant after 3 months. Considering the apparent positive results of nerve blocks for chronic pain, RCTs are necessary to compare the efficacy of PVB with PECS II block, and future work is needed to elucidate the exact medications and dosages necessary for optimal results.
Interventions for musculoskeletal dysfunction
Breast surgery often results in a decrease in motility of myofascial tissues relative to each other, creating trigger points (TrPs) and contributing to chronic postoperative upper limb pain.73 The aim of PT is to manually release these TrPs and tissue adhesions. However, our review found no benefit of myofascial therapy. Progressive resistance training was similarly ineffective. These results indicate a need to identify subgroups of patients for whom PT would be effective, specifically those whose pain is contributed to by myofascial dysfunctions. While pectoralis tightness and pain from adhesion can be prevented by stretching exercises, this intervention poses a risk for seroma formation.74 The effectiveness of HA–CMC as an alternative seen in our review shows promise in attenuating early postoperative adhesions that may evolve into chronic pain.
Limitations of this study
Lack of access to potentially negative studies that remain unpublished may be a source of publication bias that our review strategy was unable to overcome. Another potential limitation of this study is reviewer bias. To minimize this bias, two independent reviewers screened articles in the method supported by the PRISMA-P systematic review protocol.9 Due to the heterogeneity of patient populations, follow-up times and treatments across studies, subgroup analysis was not feasible. Future studies are necessary to guide treatment recommendations tailored to specific patient profiles.
CONCLUSION
This comprehensive review of strategies for prevention of chronic pain after breast cancer-related surgery highlights several effective intervention modalities. As PMPS is a phenomenon mediated through multiple mechanisms, our review supports the use of a multimodal intervention involving preoperative nerve block, intraoperative lidocaine infusion and postoperative medications to prevent the onset of pain hypersensitivity. Future research should evaluate the potential of personalized and multimodal prevention strategies that utilize combination therapy to more effectively prevent chronic post-mastectomy pain. Further, that many medications are effective for treatment of acute postoperative pain, but not prevention of chronic neuropathic pain after breast cancer surgery highlights the need for further investigation into the pathogenesis of post-mastectomy pain and novel pharmacological targets of prevention.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram illustrating the flow of information through phases of the systematic review.
Declaration of Competing Interest
Dr. Ellis, Ms. Yuksel, Dr. Chappell, Dr. Jackson and Ms. Wescott have nothing to disclose.
Acknowledgments
Funding statement
No funding was received for this article.
Ethical Approval statement
N/A
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpra.2021.10.009.
Appendix. Supplementary materials
References
- 1.Tait RC, Zoberi K, Ferguson M, et al. Persistent Post-Mastectomy Pain: Risk Factors and Current Approaches to Treatment. J Pain. 2018;19(12):1367–1383. doi: 10.1016/j.jpain.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couceiro TC de M, Valença MM, Raposo MCF, Orange FA, Amorim MMR. Prevalence of post-mastectomy pain syndrome and associated risk factors: a cross-sectional cohort study. Pain Manag Nurs. 2014;15(4):731–737. doi: 10.1016/j.pmn.2013.07.011. de. [DOI] [PubMed] [Google Scholar]
- 3.Harvey AM. Classification of chronic pain—descriptions of chronic pain syndromes and definitions of pain terms. Clin J Pain. 1995;11(2):163. doi: 10.1097/00002508-199506000-00024. [DOI] [Google Scholar]
- 4.Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;(7) doi: 10.1002/14651858.CD008307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 6.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7(9):626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meretoja TJ, Leidenius MHK, Tasmuth T, Sipilä R, Kalso E. Pain at 12 months after surgery for breast cancer. JAMA. 2014;311(1):90–92. doi: 10.1001/jama.2013.278795. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammitzbøll G, Andersen KG, Bidstrup PE, et al. Effect of progressive resistance training on persistent pain after axillary dissection in breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2020;179(1):173–183. doi: 10.1007/s10549-019-05461-z. [DOI] [PubMed] [Google Scholar]
- 12.De Groef A, Van Kampen M, Vervloesem N, et al. Myofascial techniques have no additional beneficial effects to a standard physical therapy programme for upper limb pain after breast cancer surgery: a randomized controlled trial. Clin Rehabil. 2017;31(12):1625–1635. doi: 10.1177/0269215517708605. [DOI] [PubMed] [Google Scholar]
- 13.Lacroix C, Duhoux FP, Bettendorff J, et al. Impact of perioperative hypnosedation on postmastectomy chronic pain: preliminary results. Integr Cancer Ther. 2019;18 doi: 10.1177/1534735419869494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garssen B, Boomsma MF, Meezenbroek E de J, et al. Stress management training for breast cancer surgery patients. Psychooncology. 2013;22(3):572–580. doi: 10.1002/pon.3034. [DOI] [PubMed] [Google Scholar]
- 15.Strazisar B, Besic N. Comparison of continuous local anaesthetic and systemic pain treatment after axillary lymphadenectomy in breast carcinoma patients - a prospective randomized study. Radiol Oncol. 2013;47(2):145–153. doi: 10.2478/raon-2013-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albi-Feldzer A, Mouret-Fourme E E, Hamouda S, et al. A double-blind randomized trial of wound and intercostal space infiltration with ropivacaine during breast cancer surgery: effects on chronic postoperative pain. Anesthesiology. 2013;118(2):318–326. doi: 10.1097/ALN.0b013e31827d88d8. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed SA-B, Abdel-Ghaffar HS, Kamal SM, Fares KM, Hamza HM. Effect of topical morphine on acute and chronic postmastectomy pain: what is the optimum dose? Reg Anesth Pain Med. 2016;41(6):704–710. doi: 10.1097/AAP.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 18.De Cassai A, Bonanno C, Sandei L, Finozzi F, Carron M, Marchet A. PECS II block is associated with lower incidence of chronic pain after breast surgery. Korean J Pain. 2019;32(4):286–291. doi: 10.3344/kjp.2019.32.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besch G, Lagrave-Safranez C, Ecarnot F, et al. Pectoral nerve block and persistent pain following breast cancer surgery: an observational cohort study. Minerva Anestesiol. 2018;84(6):769–771. doi: 10.23736/S0375-9393.18.12544-2. [DOI] [PubMed] [Google Scholar]
- 20.Fujii T, Shibata Y, Akane A, et al. A randomised controlled trial of pectoral nerve-2 (PECS 2) block vs. serratus plane block for chronic pain after mastectomy. Anaesthesia. 2019;74(12):1558–1562. doi: 10.1111/anae.14856. [DOI] [PubMed] [Google Scholar]
- 21.Gacio MF, Lousame AMA, Pereira S, Castro C, Santos J. Paravertebral block for management of acute postoperative pain and intercostobrachial neuralgia in major breast surgery. Braz J Anesthesiol. 2016;66(5):475–484. doi: 10.1016/j.bjane.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Qian B, Fu S, Yao Y, Lin D, Huang L. Preoperative ultrasound-guided multilevel paravertebral blocks reduce the incidence of postmastectomy chronic pain: a double-blind, placebo-controlled randomized trial. J Pain Res. 2019;12:597–603. doi: 10.2147/JPR.S190201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu H, Kamiya Y, Nishimaki H, Denda S, Baba H. Thoracic paravertebral block reduced the incidence of chronic postoperative pain for more than 1 year after breast cancer surgery. JA Clin Rep. 2015;1(1):19. doi: 10.1186/s40981-015-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmakar MK, Samy W, Li JW, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med. 2014;39(4):289–298. doi: 10.1097/AAP.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 25.Kamal SM, Ahmed BM, Refaat A. Effect of ketamine–bupivacaine combination in multilevel ultrasound- assisted thoracic paravertebral block on acute and chronic post-mastectomy pain. Egyptian Journal of Anaesthesia. 2019;35(1):33–41. doi: 10.1080/11101849.2019.1589715. [DOI] [Google Scholar]
- 26.Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006;103(3):703–708. doi: 10.1213/01.ane.0000230603.92574.4e. [DOI] [PubMed] [Google Scholar]
- 27.Ilfeld BM, Madison SJ, Suresh PJ, et al. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017–2025. doi: 10.1245/s10434-014-4248-7. [DOI] [PubMed] [Google Scholar]
- 28.Elkaradawy S, Nasr M, Elkerm Y, Deeb ME, Yassine O. The effect of multimodal balanced anaesthesia and long term gabapentin on neuropathic pain, nitric oxide and interleukin-1β following breast surgery. Egyptian Journal of Anaesthesia. 2012;28(1):67–78. doi: 10.1016/j.egja.2011.10.005. [DOI] [Google Scholar]
- 29.Cho A-R, Kwon J-Y, Kim K-H, et al. The effects of anesthetics on chronic pain after breast cancer surgery. Anesth Analg. 2013;116(3):685–693. doi: 10.1213/ANE.0b013e31827ee372. [DOI] [PubMed] [Google Scholar]
- 30.Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain. 2012;28(7):567–572. doi: 10.1097/AJP.0b013e31823b9cc8. [DOI] [PubMed] [Google Scholar]
- 31.Sun M, Liao Q, Wen L, Yan X, Zhang F, Ouyang W. Effect of perioperative intravenous flurbiprofen axetil on chronic postmastectomy pain. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38(7):653–660. doi: 10.3969/j.issn.1672-7347.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Lefebvre-Kuntz D, Dualé C, Albi-Feldzer A, et al. General anaesthetic agents do not influence persistent pain after breast cancer surgery: A prospective nationwide cohort study. Eur J Anaesthesiol. 2015;32(10):697–704. doi: 10.1097/EJA.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 33.Kang C, Cho A-R, Kim K-H, et al. Effects of Intraoperative Low-Dose Ketamine on Persistent Postsurgical Pain after Breast Cancer Surgery: A Prospective, Randomized, Controlled, Double-Blind Study. Pain Physician. 2020;23(1):37–47. [PubMed] [Google Scholar]
- 34.Kendall MC, McCarthy RJ, Panaro S, et al. The Effect of Intraoperative Systemic Lidocaine on Postoperative Persistent Pain Using Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials Criteria Assessment Following Breast Cancer Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Pain Pract. 2018;18(3):350–359. doi: 10.1111/papr.12611. [DOI] [PubMed] [Google Scholar]
- 35.Kim MH, Lee KY, Park S, Kim SI, Park HS, Yoo YC. Effects of systemic lidocaine versus magnesium administration on postoperative functional recovery and chronic pain in patients undergoing breast cancer surgery: A prospective, randomized, double-blind, comparative clinical trial. PLoS ONE. 2017;12(3) doi: 10.1371/journal.pone.0173026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain G, Bansal P, Ahmad B, Singh DK, Yadav G. Effect of the perioperative infusion of dexmedetomidine on chronic pain after breast surgery. Indian J Palliat Care. 2012;18(1):45–51. doi: 10.4103/0973-1075.97354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan JS, Hodgson N, Choi S, et al. Perioperative pregabalin and intraoperative lidocaine infusion to reduce persistent neuropathic pain after breast cancer surgery: A multicenter, factorial, randomized, controlled pilot trial. J Pain. 2019;20(8):980–993. doi: 10.1016/j.jpain.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M. Perioperative lidocaine infusion reduces the incidence of post-mastectomy chronic pain: a double-blind, placebo-controlled randomized trial. Pain Physician. 2015;18(2):E139–E146. [PubMed] [Google Scholar]
- 39.Fassoulaki A, Patris K, Sarantopoulos C, Hogan Q. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesth Analg. 2002;95(4):985–991. doi: 10.1097/00000539-200210000-00036. table of contents. [DOI] [PubMed] [Google Scholar]
- 40.Lee, McAuliffe N, Dunlop, Palanisamy, Shorten. A comparison of the effects of two analgesic regimens on the development of persistent post-surgical pain (PPSP) after breast surgery.
- 41.van Helmond N, Steegers MA, Filippini-de Moor GP, Vissers KC, Wilder-Smith OH. Hyperalgesia and Persistent Pain after Breast Cancer Surgery: A Prospective Randomized Controlled Trial with Perioperative COX-2 Inhibition. PLoS ONE. 2016;11(12) doi: 10.1371/journal.pone.0166601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vig S, Kumar V, Deo S, Bhan S, Mishra S, Bhatnagar S. Effect of Perioperative Pregabalin on Incidence of Chronic Postmastectomy Pain Syndrome: A Prospective Randomized Placebo-Controlled Pilot Study. Indian J Palliat Care. 2019;25(4):508–513. doi: 10.4103/IJPC.IJPC_85_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyad RM, Omran AF, Abbas DN, et al. The Possible Preventive Role of Pregabalin in Postmastectomy Pain Syndrome: A Double-Blinded Randomized Controlled Trial. J Pain Symptom Manage. 2019;57(1):1–9. doi: 10.1016/j.jpainsymman.2018.10.496. [DOI] [PubMed] [Google Scholar]
- 44.Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. Regional block and mexiletine: the effect on pain after cancer breast surgery. Reg Anesth Pain Med. 2001;26(3):223–228. doi: 10.1053/rapm.2001.23205. [DOI] [PubMed] [Google Scholar]
- 45.Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: A randomized clinical trial. JAMA Surg. 2018;153(4):303–311. doi: 10.1001/jamasurg.2017.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amr YM, Yousef AAA-M. Evaluation of efficacy of the perioperative administration of Venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26(5):381–385. doi: 10.1097/AJP.0b013e3181cb406e. [DOI] [PubMed] [Google Scholar]
- 47.Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005;101(5):1427–1432. doi: 10.1213/01.ANE.0000180200.11626.8E. [DOI] [PubMed] [Google Scholar]
- 48.Na H-S, Oh A-Y, Koo B-W, Lim D-J, Ryu J-H, Han J-W. Preventive Analgesic Efficacy of Nefopam in Acute and Chronic Pain After Breast Cancer Surgery: A Prospective, Double-Blind, and Randomized Trial. Medicine (Baltimore) 2016;95(20):e3705. doi: 10.1097/MD.0000000000003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo KK, Aycock JK. A blinded randomized controlled trial to evaluate the use of botulinum toxin for pain control in breast reconstruction with tissue expanders. Ann Plast Surg. 2015;74(3):281–283. doi: 10.1097/SAP.0b013e31829be8d8. [DOI] [PubMed] [Google Scholar]
- 50.Tasmuth T, Blomqvist C, Kalso E. Chronic post-treatment symptoms in patients with breast cancer operated in different surgical units. Eur J Surg Oncol. 1999;25(1):38–43. doi: 10.1053/ejso.1998.0597. [DOI] [PubMed] [Google Scholar]
- 51.Torresan RZ, Cabello C, Conde DM, Brenelli HB. Impact of the preservation of the intercostobrachial nerve in axillary lymphadenectomy due to breast cancer. Breast J. 2003;9(5):389–392. doi: 10.1046/j.1524-4741.2003.09505.x. [DOI] [PubMed] [Google Scholar]
- 52.Yang EJ, Kang E, Jang JY, et al. Effect of a mixed solution of sodium hyaluronate and carboxymethyl cellulose on upper limb dysfunction after total mastectomy: a double-blind, randomized clinical trial. Breast Cancer Res Treat. 2012;136(1):187–194. doi: 10.1007/s10549-012-2272-5. [DOI] [PubMed] [Google Scholar]
- 53.Freeman SRM, Washington SJ, Pritchard T, Barr L, Baildam AD, Bundred NJ. Long term results of a randomised prospective study of preservation of the intercostobrachial nerve. Eur J Surg Oncol. 2003;29(3):213–215. doi: 10.1053/ejso.2002.1409. [DOI] [PubMed] [Google Scholar]
- 54.Taira N, Shimozuma K, Ohsumi S, et al. Impact of preservation of the intercostobrachial nerve during axillary dissection on sensory change and health-related quality of life 2 years after breast cancer surgery. Breast Cancer. 2014;21(2):183–190. doi: 10.1007/s12282-012-0374-x. [DOI] [PubMed] [Google Scholar]
- 55.Salmon RJ, Ansquer Y, Asselain B. Preservation versus section of intercostal-brachial nerve (IBN) in axillary dissection for breast cancer–a prospective randomized trial. Eur J Surg Oncol. 1998;24(3):158–161. doi: 10.1016/s0748-7983(98)92793-7. [DOI] [PubMed] [Google Scholar]
- 56.Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. EMLA reduces acute and chronic pain after breast surgery for cancer. Reg Anesth Pain Med. 2000;25(4):350–355. doi: 10.1097/00115550-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Meijuan Y, Zhiyou P, Yuwen T, Ying F, Xinzhong C. A retrospective study of postmastectomy pain syndrome: incidence, characteristics, risk factors, and influence on quality of life. ScientificWorldJournal. 2013;2013 doi: 10.1155/2013/159732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 59.Koppert W, Ostermeier N, Sittl R, Weidner C, Schmelz M. Low-dose lidocaine reduces secondary hyperalgesia by a central mode of action. Pain. 2000;85(1-2):217–224. doi: 10.1016/s0304-3959(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 60.Kaur N, Jain A. Post mastectomy Chronic Pain in Breast Cancer Survivors: An Update on Definition, Pathogenesis, Risk Factors,Treatment and Prevention. Clin Oncol. 2017;2:1293. [Google Scholar]
- 61.Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1–13. doi: 10.1016/s0304-3959(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 62.Steegers MA, Wolters B, Evers AW, Strobbe L, Wilder-Smith OH. Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain. 2008;9(9):813–822. doi: 10.1016/j.jpain.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Miguel R, Kuhn AM, Shons AR, et al. The effect of sentinel node selective axillary lymphadenectomy on the incidence of postmastectomy pain syndrome. Cancer Control. 2001;8(5):427–430. doi: 10.1177/107327480100800506. [DOI] [PubMed] [Google Scholar]
- 64.Spivey TL, Gutowski ED, Zinboonyahgoon N, et al. Chronic pain after breast surgery: A prospective, observational study. Ann Surg Oncol. 2018;25(10):2917–2924. doi: 10.1245/s10434-018-6644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baron RH, Fey JV, Borgen PI, Stempel MM, Hardick KR, Van Zee KJ. Eighteen sensations after breast cancer surgery: a 5-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Ann Surg Oncol. 2007;14(5):1653–1661. doi: 10.1245/s10434-006-9334-z. [DOI] [PubMed] [Google Scholar]
- 66.Fu Y, Chung D, Cao M-A, Apple S, Chang H. Is axillary lymph node dissection necessary after sentinel lymph node biopsy in patients with mastectomy and pathological N1 breast cancer? Ann Surg Oncol. 2014;21(13):4109–4123. doi: 10.1245/s10434-014-3814-3. [DOI] [PubMed] [Google Scholar]
- 67.Henderson JR, Tao A, Kirwan CC, Barr L. Immediate breast reconstruction does not increase postmastectomy pain. Ann Surg Oncol. 2014;21(1):113–117. doi: 10.1245/s10434-013-3293-y. [DOI] [PubMed] [Google Scholar]
- 68.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Weinrib AZ, Azam MA, Birnie KA, Burns LC, Clarke H, Katz J. The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention and management. Br J Pain. 2017;11(4):169–177. doi: 10.1177/2049463717720636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montgomery GH, Bovbjerg DH, Schnur JB, et al. A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. J Natl Cancer Inst. 2007;99(17):1304–1312. doi: 10.1093/jnci/djm106. [DOI] [PubMed] [Google Scholar]
- 71.Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci. 2017;18(11) doi: 10.3390/ijms18112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sandkühler J, Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol. 2012;12(1):18–27. doi: 10.1016/j.coph.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fourie WJ. Considering wider myofascial involvement as a possible contributor to upper extremity dysfunction following treatment for primary breast cancer. J Bodyw Mov Ther. 2008;12(4):349–355. doi: 10.1016/j.jbmt.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 74.Cheville AL, Tchou J. Barriers to rehabilitation following surgery for primary breast cancer. J Surg Oncol. 2007;95(5):409–418. doi: 10.1002/jso.20782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.