Highlights
-
•
Patients with brain metastases at diagnosis have limited life expectancy.
-
•
A patient’s insurance is associated with different overall survivals.
-
•
Those with private insurance were most likely to receive all treatments modalities.
-
•
Black patients are disproportionally represented in Medicaid or uninsured groups.
Keywords: National cancer database, Brain neoplasms, Synchronous brain metastases, Payer status, Insurance status
Abstract
Background
Synchronous brain metastases (SBMs) are a presentation of stage IV cancers with limited treatment options. This study examines the association between health insurance status and overall survival (OS) of patients with SBMs using the National Cancer Database (NCBD).
Methods
We queried the NCDB for patients with SBMs from 2010 to 2015. Included cases were from seven primary cancers. Patients were grouped based on their insurance status. We assessed the association of insurance with OS using a Cox proportional hazards model adjusted for age at diagnosis, sex, race, education level, income level, residential area, treatment facility type, Charlson-Deyo comorbidity status, year of diagnosis, primary tumor type, and receipt of chemotherapy, radiation therapy (RT), immunotherapy, and primary site surgery.
Results
Of 97,659 patients included, those who had Medicaid, Medicare, or without health insurance were less likely to receive brain RT, chemotherapy, and/or surgery of the primary cancer site compared to privately insured patients. In multivariable COX analysis, patients with Medicare (HR = 1.11, 95% CI: 1.09–1.14, P < 0.001), Medicaid (HR = 1.11, 95% CI: 1.09–1.13, P < 0.001), or no insurance (HR = 1.18, 95% CI: 1.14–1.22, P < 0.001) were associated with decreased OS compared to private insurance.
Conclusion
After retrospective analysis, Medicaid, Medicare, and no insurance were all associated with worse OS compared to private insurance. Future studies can focus on determining the factors associated with insurance status and factors contributing to improved OS stratified by insurance status.
Introduction
Brain metastases (BMs) are the most common type of Central Nervous System (CNS) tumor in the United States, with estimates of incidence ranging between 8 and 14 per 100,000 population [1] and occurring in 5 – 10% of cancer patients [2], [3]. Approximately half of BMs originate from lung cancer, and the remaining half are derived from breast, melanoma, colorectal, and renal tumors [4], [5]. Patients with BMs generally have poor morbidity and mortality related to mass effect or from treatment toxicities, with estimated overall survival (OS) ranging from three to fifteen months [6], [7], [8], [9], [10]. A subset of patients present with BMs at the time of primary cancer diagnosis, defined in this study as synchronous brain metastasis (SBM). A study by Kormer et al. estimates that SBMs occur in about 1.7% of primary cancers and found differences in frequency of SBMs based on primary tumor type such as lung cancer (10.8%), esophageal cancer (1.5%), renal cancer (1.4%), and melanoma (1.2%) [11]. Outcomes in this population are poor with an estimated median survival between 2 and 20 months depending on primary tumor site [12].
Currently, primary treatment modalities of SBMs are similar to BMs and include surgery, stereotactic radiosurgery (SRS) and whole brain radiation therapy (WBRT) or some combination of the three [13], [14]. Treatment of SBMs is guided by the primary tumor histology, KPS status, in addition to the number, location, and size of both intracranial and extracranial metastases [15]. For patient with a single intercranial metastatic tumor, surgical resection has been shown to have a survival benefit in some trials [16], [17]. In patients with a limited number of intracranial metastases and size less than three centimeter, SRS has become a popular treatment option with good local control rate and improved quality of life with similar OS to WBRT[6], [18], [19], [20]. Regardless of type of primary tumor or the burden of brain metastases, these treatments require access to a multidisciplinary team of medical, surgical, and radiation oncologists which may be less available to patients with Medicare, Medicaid, or without insurance compared to patients with private insurance.
In the United States, health insurance is complex with great variability in terms of covered services between different insurers. The majority of the working population receives coverage by employer-supplemented private insurance. Private insurers typically collect monthly premiums and have a deductible that must be met by the patient before the insurer pays for services. Government programs, namely Medicare and Medicaid, provide coverage to citizens outside of the working pool, such as the retired and unemployed. People qualify for Medicare at age 65, at which point they may receive Medicare benefits. Medicare is operated by the US government at the federal level; in contrast to Medicaid, which is administered at the state level. Generally, Medicaid provides coverage for those that make below 138% of federal poverty level, which in the year 2021 is approximately 17,774 $USD for a single member household [21]. People may be uninsured because of their immigration status, by choice, or their salary disqualifies them from Medicaid, but they still cannot afford the premiums and deductibles associated with private insurance [22].
As of 2018, approximately 27.5 million (8.5%) and 57.8 million (17.9%) people in the United States are uninsured or enrolled in Medicaid, respectively [22]. Disparities in cancer outcomes, based on insurance status, have been documented throughout the literature [23], [24]. While the effects of socioeconomic status (SES) and insurance status have been documented in relation to SBMs[25], many studies choose to focus on one primary cancer site [26], or specific treatment modalities [27], [28]. To fully understand how health insurance status impacts patients enduring SBMs, identifying disparities among groups of patients with different types of insurance is necessary to improve outcomes for patients of all backgrounds. Health insurance can further dictate which treatments are offered to patients, perpetuating disparities in healthcare outcomes in terms of morbidity and mortality [29].
It is unknown how health insurance status affects overall survival outcomes in SBM patients, regardless of the primary cancer type or undergone treatments. We therefore design this study, using the National Cancer Database (NCDB), to examine if health insurance status is associated with overall survival of cancer patients with SBMs at diagnosis.
Materials & methods
Data source
The National Cancer Database (NCDB), a nationwide joint program sponsored by both the American College of Surgeons and the American Cancer Society, serves to provide national surveillance and quality improvement benchmarks for cancer outcomes across the United States. The NCDB collects data from over 1500 Commission on Cancer facilities and captures approximately 70% of newly diagnosed cancer in the US, with 34 million historical records. All patient data are de-identified and therefore exempt from institutional review board approval. The data are freely available by entering an agreement through the NCDB at https://www.facs.org/quality-programs/cancer/ncdb/puf.
Study population
We identified 97,659 patients in the NCDB from 2010 to 2015 with a primary tumor from seven origins and SBMs for study inclusion. 2010 was the initial year that the NCDB began recording data regarding BMs. Patients were grouped based on their insurance status: private insurance, Medicaid, Medicare, and uninsured. Patients were excluded for missing information related to treatments or insurance status. The seven primary tumors included in the study are breast cancer, Non-Small Cell Lung Cancer (NSCLC), Small Cell Lung Cancer (SCLC), other types of lung cancer, melanoma, colorectal cancer, and renal cancer.
Endpoints
The primary outcome was overall survival (OS), which was calculated from the date of diagnosis to the date of death. Those alive or lost to follow-up were censored. We also reported the odds ratio (OR) for the probability of receiving brain RT, surgery of the primary site, chemotherapy, radiation therapy, or some combination in patients with Medicare, Medicaid, and no insurance using private insurance as a reference.
Explanatory variables
The main predictor was type of insurance, which included private insurance, Medicare, Medicaid and uninsured. Other covariates included age at diagnosis, sex, race, education level, income level, residential area, treatment facility type, Charlson-Deyo comorbidity score, year of diagnosis, primary tumor type, and treatments including chemotherapy, surgery, RT, and immunotherapy.
Statistical analyses
Descriptive statistics for categorical and continuous variables are reported. A chi-square test was used to determine the association of insurance type with certain demographics and treatment related factors. We used logistic regression analysis to report the association of health insurance type and the probability of receiving a specific treatment.
ORs are reported as the measure of association with likelihood of receiving chemotherapy, surgery, radiation therapy or a combination of therapies. Survival time was measured in months from the date of diagnosis to the date of death. We used the Kaplan-Meier (KM) method to generate survival curves and analyzed the differences between groups using the log-rank test.
Cox proportional hazards regression analysis was conducted to estimate the hazard ratio (HR) and its associated 95% confidence interval (CI). The multivariable Cox regression model included variables significant at P < 0.20 in univariable models. P values of 0.05 were used to define statistical significance, and we used SAS 9.4 (SAS Institute Inc.) for the analysis.
Results
Patient characteristics
A total of 97,659 cases of SBM were queried from the NCDB between 2010 and 2015. Among them, 32,131 (32.90%) had private insurance, 10,679 (10.93%) had Medicaid, 49,168 (50.35%) had Medicare, and 5,681 (5.82%) were uninsured. We included seven primary cancer sites: 4.52% of the study population from breast cancer, 65.27% from NSCLC, 15.49% from SCLC, 6.68% from other types of lung cancer, 3.72% from melanoma, 1.30% from colorectal cancer, and 3.02% from kidney cancer.
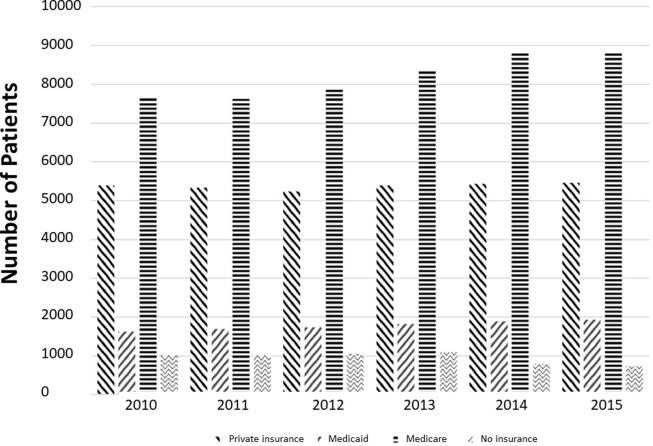
Demographic characteristics of the cohort are reported in Table 1, and a time trend graph of patients by insurance type seen in Fig. 1. The median age at diagnosis across all groups was 64.8 (standard deviation = 11.04), with private insurance, Medicaid, Medicare, and uninsured groups having mean ages of 58.74 (8.94), 55.81 (8.48), 71.70 (8.29), 56.37 (8.33), respectively. The majority of patients, 97.7%, analyzed in this study were from urban areas. Patients, who are white, have higher education, and have higher income were more likely to have private insurance or Medicare. Across all insurance groups, most patients received their treatment at community hospitals, 65.90% overall. Whites composed 84.81% of the cohort while blacks made up 11.56%, and all other races composed the remaining 3.63%. The distribution of black patients in the four insurance groups did not mirror white patients. In the reported white population, 33.5% had private insurance, 9.4% had Medicaid, 51.8% had Medicare, 5.3% were uninsured. However, within the black population 26.8% had private insurance, 20.1% had Medicaid, 44.0 %, and 9.1% were uninsured.
Table 1.
Baseline characteristics of the study population.
| Variable | Private N (%) 32,131 (32.9) |
Medicaid N (%) 10,679 (10.9) |
Medicare N (%) 49,168 (50.4) |
Uninsured N (%) 5,681 (5.8) |
P | |
|---|---|---|---|---|---|---|
| Age at diagnosis, Median (range) | 58.74 (8.9) | 55.81 (8.5) | 71.70 (8.3) | 56.37 (8.3) | 0.001 | |
| Sex | Male | 15,621 (48.6) | 5,279 (49.43) | 25,362 (51.6) | 2,994 (52.7) | |
| Female | 16,510 (51.4) | 5,400 (50.6) | 23,806 (48.4) | 2,687 (47.3) | 0.001 | |
| Race | White | 27,556 (86.5) | 7,736 (73.0) | 42,564 (87.1) | 4,370 (77.5) | |
| Black | 2,999 (9.4) | 2,256 (21.3) | 4,934 (10.1) | 1,014 (18.0) | 0.001 | |
| Other | 1,321 (4.1) | 603 (5.7) | 1,346 (2.8) | 252 (4.5) | ||
| Unknown | 255 | 84 | 324 | 45 | ||
| Education | >=13% NHSD* | 13,575 (42.4) | 6,562 (61.6) | 22,735 (46.3) | 3,510 (62.0) | 0.001 |
| <13% NHSD* | 18,475 (57.6) | 4,084 (38.4) | 26,343 (53.7) | 2,152 (38.0) | ||
| Unknown | 81 | 33 | 90 | 19 | ||
| Income | >=$35,000 | 19,579 (61.1) | 4,372 (41.1) | 26,708 (54.5) | 2,341 (41.4) | |
| <35,000 | 12,451 (38.9) | 6,268 (58.9) | 22,341 (45.6) | 3,314 (58.6) | 0.001 | |
| Unknown | 101 | 39 | 119 | 26 | ||
| Place of Living | Urban | 30,670 (98.0) | 10,203 (97.5) | 46,754 (97.4) | 5,425 (97.6) | |
| Rural | 615 (2.0) | 259 (2.5) | 1,235 (2.6) | 134 (2.4) | 0.001 | |
| Unknown | 846 | 217 | 1,179 | 122 | ||
| Hospital Type | Community | 20,089 (63.7) | 5.914 (57.2) | 34,168 (69.6) | 3,417 (61.9) | 0.001 |
| Academic | 11,440 (36.3) | 4,419 (42.8) | 14,939 (30.4) | 2,103 (38.1) | ||
| Unknown | 602 | 346 | 61 | 161 | ||
| Charlson/Deyo Score | 0 | 22,786 (70.9) | 6,861 (64.3) | 27,834 (56.6) | 3,873 (68.2) | |
| 1 | 2,619 (8.2) | 1,153 (10.8) | 7,642 (15.5) | 528 (9.3) | ||
| >=2 | 6,726 (20.9) | 2,665 (25.0) | 13,692 (27.9) | 1,280 (22.5) | 0.001 | |
| Primary site surgery | Yes | 1,477 (4.6) | 329 (3.1) | 1,235 (2.5) | 153 (2.7) | |
| No | 30,508 (95.4) | 10,309 (96.9) | 47,783 (97.5) | 5,499 (97.3) | 0.001 | |
| Chemotherapy | Yes | 20,982 (67.2) | 5,903 (57.2) | 21,714 (45.5) | 2,813 (51.3) | |
| No | 10,254 (32.8) | 4,413 (42.8) | 25,962 (54.5) | 2,675 (48.7) | 0.001 | |
| Radiation Therapy | Yes | 24,984 (78.1) | 7,920 (74.4) | 33,138 (67.7) | 3,941 (69.7) | |
| No | 7,014 (21.9) | 2,721 (25.6) | 15,811 (32.3) | 1,712 (30.3) | 0.001 | |
| Immunotherapy | Yes | 1,515 (4.7) | 315 (3.0) | 1,245 (2.5) | 152 (2.7) | |
| No | 30,538 (95.3) | 10,337 (97.0) | 47,824 (97.5) | 5,520 (97.3) | 0.001 | |
| Year of Diagnosis | 2010–2013 | 21,244 (66.1) | 6,859 (64.2) | 31,550 (64.2) | 4,163 (73.3) | 0.001 |
| 2014–2015 | 10,887 (33.9) | 3,820 (35.8) | 17,618 (35.8) | 1,518 (26.7) | ||
| Primary Cancer Type | Breast | 1,785 (5.6) | 715 (6.7) | 1,554 (3.2) | 359 (6.3) | 0.001 |
| NSCLC | 21,452 (66.8) | 7,000 (65.6) | 31,643 (64.4) | 3,650 (64.3) | ||
| SCLC | 4,523 (14.1) | 1,641 (15.4) | 8,154 (16.6) | 814 (14.3) | ||
| Other types of lung cancer | 1,323 (4.1) | 555 (5.2) | 4,265 (8.7) | 380 (6.7) | ||
| Melanoma | 1,435 (4.5) | 336 (3.2) | 1,649 (3.4) | 209 (3.7) | ||
| Colorectal | 426 (1.3) | 124 (1.2) | 649 (1.3) | 72 (1.3) | ||
| kidney | 1,187 (3.7) | 308 (2.9) | 1,254 (2.6) | 197 (3.5) | ||
NHSD = no high school degree.
Fig. 1.
Distribution of patients by insurance type from left to right: Private Insurance, Medicaid, Medicare, and no insurance.
In every treatment category, the private insurance group had the highest usage proportion. Primary site surgery was performed in 4.62% of privately insured patients, 3.09% of Medicaid patients, 2.52% of Medicare patients, and 2.71% of uninsured patients. Chemotherapy was performed in 67.17%, 57.22%, 45.54%, and 51.26% of privately insured, Medicaid, Medicare, and uninsured patients respectively. Radiotherapy was performed in 78.08%, 74.43%, 67.70%, and 69.72% of privately insured, Medicaid, Medicare, and uninsured patients respectively. Immunotherapy was used in 4.73%, 2.96%, 2.54%, 2.68% of privately insured, Medicaid, Medicare, and uninsured patients respectively. Among the patients who received radiotherapy, whole brain radiotherapy and stereotactic radiotherapy were performed in 47% and 47.9% of Medicare patients, 35% and 40% of privately insured patients, 11.8% and 9.1% of Medicaid patients, and 6.2% and 3% of uninsured patients. (Supplemental Table 1).
Treatment by insurance type
We performed univariate and multivariable logistic regression analysis between insurance status and the types of treatment, including brain or other site radiotherapy, chemotherapy, and surgery (Table 2). For Medicaid, Medicare, and uninsured groups, any combination (surgery, radiation, chemotherapy) of therapy had statistically significant decreased ORs in both univariate and multivariable analysis compared to the private insurance group. A common pattern emerges with ORs decreasing in the following sequence: private insurance, Medicare, Medicaid and lastly uninsured. This suggests a lower association of receiving any type of treatment combination for those without private insurance. For example, Medicare patients that received chemotherapy and brain radiotherapy had an OR of 0.79 (95% CI: 0.746–0.84), Medicaid had an OR of 0.52 (95% CI: 0.48–0.56), and uninsured had an OR of 0.36 (95% CI: 0.33–0.39) in comparison to private insurance. There were three exceptions to this pattern with no significant difference between private insurance and Medicare for ORs of brain radiotherapy, other site radiotherapy, and surgery of primary cancer site.
Table 2.
Univariate and multivariable logistic regression analysis of receiving a specific treatment by insurance type.
| Combinations | N (%) | Univariate | P | Multivariable | P |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||||
| Only brain RT | |||||
| Private | 7,929 (22.9) | Reference | Reference | ||
| Medicaid | 3,471 (10.0) | 0.85 (0.78–0.92) | 0.001 | 0.81 (0.74–0.88) | 0.001 |
| Medicare | 21,176 (61.1) | 0.74 (0.70–0.78) | 0.001 | 0.97 (0.91–1.03) | 0.26 |
| Uninsured | 2,067 (6.0) | 0.69(0.62–0.76) | 0.001 | 0.66 (0.59–0.73) | 0.001 |
| Only Other RT | |||||
| Private | 4,837 (21.6) | Reference | Reference | ||
| Medicaid | 2,300 (10.3) | 0.97 (0.869–1.08) | 0.56 | 0.89 (0.79–0.99) | 0.04 |
| Medicare | 13,809 (61.5) | 0.69 (0.65–0.75) | 0.001 | 0.93 (0.85–1.01) | 0.08 |
| Uninsured | 1,492 (6.7) | 0.90 (0.795–1.03) | 0.12 | 0.84 (0.73–0.96) | 0.009 |
| Only chemotherapy | |||||
| Private | 6,027 (25.3) | Reference | Reference | ||
| Medicaid | 2,377 (10.0) | 0.59 (0.54–0.66) | 0.001 | 0.54 (0.48–0.59) | 0.001 |
| Medicare | 13,991 (58.6) | 0.41 (0.38–0.44) | 0.001 | 0.79 (0.73–0.85) | 0.001 |
| Uninsured | 1,465 (6.1) | 0.47 (0.41–0.53) | 0.001 | 0.42 (0.36–0.47) | 0.001 |
| Only surgery of the primary cancer site | |||||
| Private | 34,989 (20.5) | Reference | Reference | ||
| Medicaid | 1,648 (9.7) | 0.51 (0.35–0.75) | 0.007 | 0.45 (0.30–0.69) | 0.002 |
| Medicare | 10,800 (63.4) | 0.54 (0.43–0.67) | 0.001 | 0.86 (0.66–1.12) | 0.25 |
| Uninsured | 1,088 (6.4) | 0.45 (0.28–0.73) | 0.001 | 0.35 (0.20–0.59) | 0.001 |
| Chemotherapy plus brain RT | |||||
| Private | 16,319 (33.7) | Reference | Reference | ||
| Medicaid | 5,288 (10.9) | 0.59 (0.55–0.63) | 0.001 | 0.52 (0.48–0.56) | 0.001 |
| Medicare | 24,035 (49.7) | 0.33 (0.32–0.35) | 0.001 | 0.79 (0.746–0.84) | 0.001 |
| Uninsured | 2,766 (5.7) | 0.41 (0.38–0.45) | 0.001 | 0.36 (0.33–0.39) | 0.001 |
| Chemotherapy plus other RT | |||||
| Private | 7,483 (28.3) | Reference | Reference | ||
| Medicaid | 2,786 (10.5) | 0.592 (0.542–0.647) | 0.001 | 0.505 (0.459–0.556) | 0.001 |
| Medicare | 14.519 (54.91) | 0.305 (0.287–0.323) | 0.001 | 0.712 (0.662–0.766) | 0.001 |
| Uninsured | 1.654 (6.3) | 0.447 (0.400–0.499) | 0.001 | 0.375 (0.333–0.423) | 0.001 |
| Surgery plus brain RT | |||||
| Private | 3,654 (21.0) | Reference | Reference | ||
| Medicaid | 1,688 (9.7) | 0.52 (0.40–0.68) | 0.001 | 0.46 (0.35–0.62) | 0.001 |
| Medicare | 10,933 (62.9) | 0.39 (0.34–0.46) | 0.001 | 0.72 (0.59–0.88) | 0.001 |
| Uninsured | 1,101 (6.3) | 0.35 (0.24–0.50) | 0.001 | 0.32 (0.22–0.47) | 0.001 |
| Surgery plus other RT | |||||
| Private | 3,412 (20.4) | Reference | Reference | ||
| Medicaid | 1,623 (9.7) | 0.35 (0.16–0.74) | 0.006 | 0.29 (0.13–0.67) | 0.003 |
| Medicare | 10,606 (63.5) | 0.21 (0.14–0.33) | 0.001 | 0.41 (0.24–0.71) | 0.001 |
| Uninsured | 1,075 (6.4) | 0.39 (0.17–0.92) | 0.03 | 0.25 (0.09–0.70) | 0.008 |
| Surgery plus chemotherapy | |||||
| Private | 3,518 (20.8) | Reference | Reference | ||
| Medicaid | 1,657 (9.8) | 0.57 (0.40–0.80) | 0.001 | 0.48 (0.33–0.69) | 0.001 |
| Medicare | 10,692 (63.1) | 0.24 (0.19–0.31) | 0.001 | 0.70 (0.52–0.95) | 0.01 |
| Uninsured | 1,087 (6.4) | 0.37 (0.23–0.60) | 0.001 | 0.34 (0.21–0.57) | 0.001 |
| Surgery plus chemotherapy plus brain RT | |||||
| Private | 4,024 (22.6) | Reference | Reference | ||
| Medicaid | 1,741 (9.8) | 0.39 (0.33–0.49) | 0.001 | 0.36 (0.29–0.45) | 0.001 |
| Medicare | 10,949 (61.4) | 0.18 (0.16–0.201) | 0.001 | 0.53 (0.45–0.63) | 0.001 |
| Uninsured | 1,123 (6.3) | 0.26 (0.19–0.34) | 0.001 | 0.23 (0.17–0.31) | 0.001 |
| Surgery plus chemotherapy plus other RT | |||||
| Private | 4,837 (21.6) | Reference | Reference | ||
| Medicaid | 2,300 (10.3) | 0.54 (0.37–0.78) | 0.001 | 0.39 (0.25–0.59) | 0.001 |
| Medicare | 13,809 (61.5) | 0.14 (0.10–0.19) | 0.001 | 0.48 (0.33–0.69) | 0.001 |
| Uninsured | 1,492 (6.7) | 0.32 (0.19–0.56) | 0.001 | 0.27 (0.15–0.47) | 0.001 |
Overall survival
Kaplan-Meier curves were plotted for all insurance groups and median survivals were compared, Fig. 2. Median survival for private insurance, Medicare, Medicaid, and Uninsured patients were 7.69 months (95% CI: 7.59–7.82, log-rank p < 0.0001), 6.05 months (95% CI: 5.85–6.24, log-rank p < 0.0001), 3.81 months (95% CI: 3.78–3.88, log-rank p < 0.0001), and 4.63 months (95% CI: 4.44–4.90, log-rank p < 0.0001), respectively.
Fig. 2.
Kaplan Meier curves of Overall Survival for (dash-dotted line) private insurance, (dashed line) Medicare, (solid line) Medicaid, and (dotted line) no insurance.
In univariable analysis, patients with Medicare, Medicaid, or no insurance were associated with higher hazard of death compared to those with private insurance. Other notable factors associated with a higher risk of death included income less than $35,000, treatment at community programs, and Charlson-Deyo scores of one or greater than one.
In multivariable analysis, after adjustment for the above-mentioned positive factors, Medicaid, Medicare, and no insurance were all associated with worse OS compared to private insurance, seen in Table 3. Comparatively, patients with Medicare (HR = 1.11, 95% CI: 1.09–1.14, P < 0.001) or Medicaid (HR = 1.11, 95% CI: 1.09–1.13, P < 0.001) were associated with statistically significant worse OS compared to private insurance. Patients without any insurance were associated with the worst OS of the insurance groups (HR = 1.18, 95% CI: 1.14–1.22, P < 0.001) compared to private insurance. The results stayed the same with Medicaid (HR: 1.07, 95% CI: 1.04–1.11, P < 0.0001), Medicare (HR: 1.10, 95% CI: 1.07–1.14, P < 0.0001), and no insurance (HR: 1.16, 95% CI: 1.11–1.21, P < 0.0001) all associated with worsened OS compared to private insurance when the analysis was restricted to patients who received all of first course treatments at the reporting facility and to patients who had only one lifetime tumor or the tumor was the first of multiple tumors. In the analyses stratified by tumor types, Medicaid, Medicare, and no insurance were associated with worse OS compared to private insurance for breast cancer, NSCLC, other lung cancer, and melanoma, while there was no difference in the OS of patients who had Medicaid compared to private insurance in the tumor histology of SCLC, colorectal cancer, and kidney cancer. The results are provided in Table 4.
Table 3.
Univariable and multivariable Cox proportional regression analysis of factors associated with OS in BMs patients.
| Variable | Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | ||
| Age at diagnosis (continuous) | 1.02 (1.02–1.02) | 0.001 | 1.01 (1.01–1.01) | 0.001 | |
| Insurance type | Private | Reference | Reference | ||
| Medicaid | 1.17 (1.14–1.20) | 0.001 | 1.11 (1.09–1.14) | 0.001 | |
| Medicare | 1.55 (1.53–1.57) | 0.001 | 1.11 (1.09–1.13) | 0.001 | |
| No insurance | 1.30 (1.26–1.34) | 0.001 | 1.18 (1.14–1.22) | 0.001 | |
| Sex | Male | Reference | Reference | ||
| Female | 0.82 (0.81–0.83) | 0.001 | 0.86 (0.85–0.87) | 0.001 | |
| Race | White | Reference | Reference | ||
| Black | 0.95 (0.93–0.97) | 0.001 | 0.94 (0.91–0.96) | 0.001 | |
| non-white non-black | 0.68 (0.65–0.70) | 0.001 | 0.73 (0.70–0.76) | 0.001 | |
| Education | >=13% NHSD* | 1.06 (1.05–1.07) | 0.001 | 0.98 (0.97–0.99) | 0.03 |
| <13% NHSD* | Reference | Reference | |||
| Income | >=$35,000 | Reference | Reference | ||
| <$35,000 | 1.11 (1.10–1.13) | 0.001 | 1.05 (1.04–1.07) | 0.001 | |
| Place of Living | Urban | Reference | |||
| Rural | 1.08 (1.04–1.13) | 0.003 | |||
| Hospital Type | Academic | Reference | Reference | ||
| Community | 1.24 (1.22–1.26) | 0.001 | 1.18 (1.16–1.19) | 0.001 | |
| Charlson/Deyo Score | 0 | Ref | Reference | ||
| 1 | 1.26 (1.24–1.28) | 0.001 | 1.14 (1.12–1.16) | 0.001 | |
| >=2 | 1.51 (1.48–1.54) | 0.001 | 1.22 (1.19–1.24) | 0.001 | |
| Primary Site Surgery | Yes | Reference | 0.001 | Reference | |
| No | 2.21 (2.12–2.30) | 2.14 (2.05–2.24) | 0.001 | ||
| Chemotherapy | Yes | Reference | Reference | ||
| No | 2.33 (2.30–2.36) | 0.001 | 2.17 (2.132–2.20) | 0.001 | |
| Radiation Therapy | Yes | Reference | Reference | ||
| No | 1.57 (1.55–1.60) | 0.001 | 1.24 (1.22–1.26) | 0.001 | |
| Immunotherapy | Yes | Reference | Reference | ||
| No | 1.89 (1.82–1.97) | 0.001 | 1.44 (1.38–1.51) | 0.001 | |
| Year of Diagnosis | 2010–2013 | 1.09 (1.07–1.11) | 0.001 | 1.07 (1.05–1.08) | 0.001 |
| 2014–2015 | Reference | Reference | |||
| Primary Cancer Type | Breast cancer | 0.73 (0.69–0.77) | 0.001 | 0.75 (0.71–0.79) | 0.001 |
| NSCLC | 1.11 (1.07–1.16) | 0.001 | 1.06 (1.02–1.11) | 0.006 | |
| SCLC | 1.23 (1.18–1.29) | 0.001 | 1.243(1.19–1.30) | 0.001 | |
| Other types of lung cancer | 2.26 (2.16–2.37) | 0.001 | 1.38 (1.32–1.45) | 0.001 | |
| Melanoma | 0.98 (0.93–1.04) | 0.55 | 0.77 (0.73–0.82) | 0.001 | |
| Colorectal cancer | 1.19 (1.12–1.28) | 0.001 | 1.27 (1.18–1.37) | 0.001 | |
| Kidney cancer | Reference | Reference | |||
*NHSD = no high school degree.
Table 4.
Multivariable Cox regression analysis for stratified by primary tumor types
| Tumor type | Variable | HR (95% CI) | P |
|---|---|---|---|
| Breast cancer | Private insurance | Reference | |
| Medicaid | 1.224 (1.097–1.366) | 0.001 | |
| Medicare | 1.161 (1.048–1.287) | 0.001 | |
| No insurance | 1.326 (1.154–1.523) | 0.001 | |
| Non-small cell lung cancer | Private insurance | Reference | |
| Medicaid | 1.089 (1.056–1.124) | 0.001 | |
| Medicare | 1.102 (1.077–1.129) | 0.001 | |
| No insurance | 1.158 (1.113–1.205) | 0.001 | |
| Small-cell lung cancer | Private insurance | Reference | |
| Medicaid | 1.049 (0.986–1.117) | 0.13 | |
| Medicare | 1.102 (1.052–1.154) | 0.001 | |
| No insurance | 1.212 (1.118–1.315) | 0.001 | |
| Other types of lung cancer | Private insurance | Reference | |
| Medicaid | 1.159 (1.038–1.295) | 0.01 | |
| Medicare | 1.123 (1.039–1.213) | 0.003 | |
| No insurance | 1.186 (1.040–1.352) | 0.01 | |
| Melanoma | Private insurance | Reference | |
| Medicaid | 1.466 (1.264–1.700) | 0.001 | |
| Medicare | 1.119 (1.005–1.246) | 0.04 | |
| No insurance | 1.490 (1.251–1.775) | 0.001 | |
| Colorectal cancer | Private insurance | Reference | |
| Medicaid | 1.205 (0.948–1.532) | 0.13 | |
| Medicare | 1.338 (1.124–1.593) | 0.001 | |
| No insurance | 1.350 (1.005–1.814) | 0.04 | |
| Kidney cancer | Private insurance | Reference | |
| Medicaid | 1.037 (0.894–1.203) | 0.63 | |
| Medicare | 1.161 (1.033–1.305) | 0.01 | |
| No insurance | 0.902 (0.752–1.084) | 0.27 |
Discussion
To our knowledge, our study is the first to show that insurance status is associated with overall survival in patients with SBMs, regardless of primary cancer type. We additionally found that those with private insurance are most likely to receive all types of treatment modalities; followed by Medicare, then Medicaid, and lastly those without insurance. Lastly, black patients were disproportionally represented in the Medicaid and uninsured groups compared to white patients.
Conceptually, in the American health care system, insurance status can act as a proxy measurement for the ability to access healthcare as the uninsured have limited avenues compared to those with insurance. The health insurance landscape in the United States is complex with the majority of the non-elderly population covered by employer-provided private insurance. Government programs, such as Medicare and Medicaid, supplement citizens outside of the working pool, such as the unemployed and retired. Even with these programs in place, as of 2018, 27.5 million (8.5%) Americans are uninsured with limited access to healthcare[22]. Our hypothesis was that those without private insurance would be associated with the lower rates of receiving surgical, medical, radiation and immunotherapy and by extension would be associated with poorer OS.
We found that those with private insurance are most likely to receive all types of treatment modalities compared to Medicare, Medicaid, and those without insurance. Modh et al. investigated insurance status and usage rates of SRS between insured groups. In agreement with this study, they found that SRS usage was significantly higher in the private insurance group in comparison to the uninsured group [27]. Furthermore, we found that those with private insurance were associated with higher OS compared to Medicare, Medicaid, and the uninsured, which is consistent with studies investigating other cancers. Niu et al. found that in seven primary cancers, patients with Medicaid or no insurance were associated with poorer outcomes compared to private insurance [24]. Poorer outcomes associated with insurance status have been reported across breast, cervical, colorectal, lung, prostate, bladder, and non-hodgkin's lymphoma [24], [30], [31]. Unlike other cancers, synchronous brain metastases present a unique challenge because of innate heterogeneity in tumor behavior base on primary histology and subsequent treatment options. We performed a combined and stratified analysis by primary tumor type in effort to account for these differences, and the over-arching trend remains that people without private insurance are associated with lower HRs. Our results are consistent with others and may be unsurprising, but we believe this to be the first analysis investigating an association between insurance status and survival outcome from SBMs.
In addition to our primary findings, our combined analysis found that not receiving primary site surgery, chemotherapy, radiotherapy, or immunotherapy were associated with increased HRs compared to those who received those treatments as seen in Table 3. When we combine this with our other results that those without private insurance are associated with a decreased ORs of receiving treatments compared to private insurance, we may see these affects compound. Alternatively, privately insured patients had the highest usage rates of these treatments, and other studies have found privately insurance patients receive better access to cancer screenings, prompt appointments, and prescription medication. All of which could lead to earlier diagnosis of cancer and therefore more treatable disease [32], [33], [34], [35], [36], [37], [38], [39]. Uninsured and Medicaid patients have been shown to face many barriers regarding healthcare access [32], [40]. It’s harder to find a provider willing to take decreased or no reimbursement, and patients face longer wait times [41]. They may have more comorbid conditions or doctors may be influenced by perceptions that they may not comply with, or refuse treatment [32]. Aizer et al. found that increasing age, non-white race, and unmarried patients were more likely to refuse cancer treatment [42]. Other studies have found that uninsured patients are more likely to present with advanced diseased and are less likely to receive various treatments [11], [43], which agrees with our results.
We should consider the role that race plays in this study, particularly looking at which populations compose the uninsured and Medicaid groups. In this study, black patients were nearly twice as likely to have Medicaid or no insurance in comparison to white patients. Within black population 20.1% had Medicaid, and 9.1% were uninsured. This is in comparison to within the white population 9.4% having Medicaid, and 5.3% being uninsured. Unfortunately, this distribution is consistent with systemic racial biases and disadvantages have been recorded throughout medicine and in oncology [44].
Limitations
Our study has several limitations. Our data was retrospective and therefore a risk of coding misclassification is present. The NCDB does not record the size, number or location of SBMs or if the patients died from their primary cancer, consequences of their SBMs, or an unrelated cause. We also did not have information about surgery to the brain as this information is not available in the NCDB. NCDB only provides the patient’s insurance status at the time of diagnosis. Patients may have started in one insurance group and change during the course of their treatment, especially if they became Medicare eligible. Additionally, private insurance is not well defined, and the cohort was skewed toward private insurance and Medicare patients. Within the United States, many types of insurance plans exist with high variability as to what services are covered by the insurance, what providers are considered “in-network,” the payment structure of deductibles and premiums and these differences are not in the database. However, even with high variability in the term “private insurance”, this group had the highest survival and highest treatment rates, suggesting better access to care.
Conclusions
In this comprehensive retrospective analysis of the NCDB, we conclude that patients with SBMs and limited insurance, in the form of Medicaid, Medicare or being uninsured, suffer from poorer overall survival compared to those with private insurance. Notably, those with private insurance are the most likely to receive all types of treatment modalities; followed by Medicare, then Medicaid, and lastly those without insurance. Future prospective studies should record insurance status and asses its impact and determine the factors associated with insurance status and factors contributing to improved OS stratified by insurance status.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2021.11.004.
Contributor Information
Alex Kolomaya, Email: alexander.kolomaya@unmc.edu.
Saber Amin, Email: samin@unmc.edu.
Chi Lin, Email: clin@unmc.edu.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Nayak L., Lee E.Q., Wen P.Y. Epidemiology of Brain Metastases. Curr Oncol Rep. 2012;14(1):48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan J.S., Sloan A.E., Davis F.G., Vigneau F.D., Lai P., Sawaya R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 3.Davis F.G., Dolecek T.A., McCarthy B.J., Villano J.L., et al. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14(9):1171–1177. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller K.D., Nogueira L., Mariotto A.B., et al. Cancer treatment and survivorship statistics, 2019. CA: Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 5.Ostrom Q.T., Wright C.H., Barnholtz-Sloan J.S. Brain metastases: epidemiology. Handb Clin Neurol. 2018;149:27–42. doi: 10.1016/B978-0-12-811161-1.00002-5. [DOI] [PubMed] [Google Scholar]
- 6.Brown P.D., Jaeckle K., Ballman K.V., Farace E., Cerhan J.H., Anderson S.K., et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316(4):401. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoyama H., Tago M., Shirato H. Stereotactic Radiosurgery With or Without Whole-Brain Radiotherapy for Brain Metastases: Secondary Analysis of the JROSG 99–1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457. doi: 10.1001/jamaoncol.2015.1145. [DOI] [PubMed] [Google Scholar]
- 8.Sperduto P.W., Kased N., Roberge D., Xu Z., Shanley R., Luo X., et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocher M., Soffietti R., Abacioglu U., Villà S., Fauchon F., Baumert B.G., et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieder C., Haukland E., Mannsåker B., Pawinski A.R., Yobuta R., Dalhaug A. Presence of Brain Metastases at Initial Diagnosis of Cancer: Patient Characteristics and Outcome. Cureus. 2019;11(2):e4113. doi: 10.7759/cureus.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kromer C., Xu J., Ostrom Q.T., Gittleman H., Kruchko C., Sawaya R., et al. Estimating the annual frequency of synchronous brain metastasis in the United States 2010–2013: a population-based study. J Neurooncol. 2017;134(1):55–64. doi: 10.1007/s11060-017-2516-7. [DOI] [PubMed] [Google Scholar]
- 12.Singh R., Stoltzfus K.C., Chen H., et al. Epidemiology of synchronous brain metastases. Neurooncol Adv. 2020;2(1):vdaa041. doi: 10.1093/noajnl/vdaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graber J.J., Cobbs C.S., Olson J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Use of Stereotactic Radiosurgery in the Treatment of Adults With Metastatic Brain Tumors. Neurosurgery. 2019;84(3):E168–E170. doi: 10.1093/neuros/nyy543. [DOI] [PubMed] [Google Scholar]
- 14.Nahed B.V., et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Surgery in the Management of Adults With Metastatic Brain Tumors. Neurosurgery. 2019;84(3):E152–E155. doi: 10.1093/neuros/nyy542. [DOI] [PubMed] [Google Scholar]
- 15.Sperduto P.W., Chao S.T., Sneed P.K., Luo X., Suh J., Roberge D., et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Patchell R.A., Tibbs P.A., Walsh J.W., Dempsey R.J., Maruyama Y., Kryscio R.J., et al. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N Engl J Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 17.Noordijk E.M., Vecht C.J., Haaxma-Reiche H., Padberg G.W., Voormolen J.H.C., Hoekstra F.H., et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29(4):711–717. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 18.Aoyama H., Shirato H., Tago M., Nakagawa K., Toyoda T., Hatano K., et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 19.Chang E.L., Wefel J.S., Hess K.R., Allen P.K., Lang F.F., Kornguth D.G., et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 20.Robin T.P., Rusthoven C.G. Strategies to Preserve Cognition in Patients With Brain Metastases: A Review. Frontiers. Oncology. 2018;8 doi: 10.3389/fonc.2018.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochran N. In D.o.H.a.H. Services, editor. Annual Update of the HHS Poverty Guidelines; 2021.
- 22.Berchick ER, Barnett JC, Upton RD. In U.S.C. Bureau, editor. Health Insurance Coverage in the United States: 2018; 2019.
- 23.Ellis L., Canchola A.J., Spiegel D., Ladabaum U., Haile R., Gomez S.L. Trends in Cancer Survival by Health Insurance Status in California From 1997 to 2014. JAMA Oncology. 2018;4(3):317. doi: 10.1001/jamaoncol.2017.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu X., Roche L.M., Pawlish K.S., Henry K.A. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403–411. doi: 10.1002/cam4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuño M., Mukherjee D., Elramsisy A., Nosova K., Lad S.P., Boakye M., et al. Racial and gender disparities and the role of primary tumor type on inpatient outcomes following craniotomy for brain metastases. Ann Surg Oncol. 2012;19(8):2657–2663. doi: 10.1245/s10434-012-2353-z. [DOI] [PubMed] [Google Scholar]
- 26.Weiner A.B., Matulewicz R.S., Tosoian J., Feinglass J., Schaeffer E. The effect of socioeconomic status, race, and insurance type on newly diagnosed metastatic prostate cancer in the United States (2004–2013) Urol Oncol. 2018;36(3) doi: 10.1016/j.urolonc.2017.10.023. p. 91 e1–91 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modh A., Doshi A., Burmeister C., Elshaikh M.A., Lee I., Shah M. Disparities in the Use of Single-fraction Stereotactic Radiosurgery for the Treatment of Brain Metastases From Non-small Cell Lung Cancer. Cureus. 2019;11(2):e4031. doi: 10.7759/cureus.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alphonse-Sullivan N., Taksler G.B., Lycan T., Weaver K.E., McTyre E.R., Shenker R.F., et al. Sociodemographic predictors of patients with brain metastases treated with stereotactic radiosurgery. Oncotarget. 2017;8(60):101005–101011. doi: 10.18632/oncotarget.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klapheke A., Yap S.A., Pan K., Cress R.D. Sociodemographic disparities in chemotherapy treatment and impact on survival among patients with metastatic bladder cancer. Urol Oncol. 2018;36(6) doi: 10.1016/j.urolonc.2018.03.008. p. 308 e19–308 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayanian J.Z., Kohler B.A., Abe T., Epstein A.M. The Relation between Health Insurance Coverage and Clinical Outcomes among Women with Breast Cancer. N Engl J Med. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 31.Han X., Jemal A., Flowers C.R., Sineshaw H., Nastoupil L.J., Ward E. Insurance status is related to diffuse large B-cell lymphoma survival. Cancer. 2014;120(8):1220–1227. doi: 10.1002/cncr.28549. [DOI] [PubMed] [Google Scholar]
- 32.Ward E., Halpern M., Schrag N., Cokkinides V., DeSantis C., Bandi P., et al. Association of Insurance with Cancer Care Utilization and Outcomes. CA Cancer J Clin. 2008;58(1):9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 33.Koroukian S.M., Xu F., Dor A., Cooper G.S. Colorectal Cancer Screening in the Elderly Population. Disparities by Dual Medicare?Medicaid Enrollment Status. 2006;41(6):2136–2154. doi: 10.1111/j.1475-6773.2006.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coughlin S.S., Uhler R.J., Bobo J.K., Caplan L. Breast cancer screening practices among women in the United States, 2000. Cancer Causes Control. 2004;15(2):159–170. doi: 10.1023/B:CACO.0000019496.30145.62. [DOI] [PubMed] [Google Scholar]
- 35.Sambamoorthi U., McAlpine D.D. Racial, ethnic, socioeconomic, and access disparities in the use of preventive services among women. Prev Med. 2003;37(5):475–484. doi: 10.1016/s0091-7435(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 36.Ioannou G.N., Chapko M.K., Dominitz J.A. Predictors of colorectal cancer screening participation in the United States. The American Journal of Gastroenterology. 2003;98(9):2082–2091. doi: 10.1111/j.1572-0241.2003.07574.x. [DOI] [PubMed] [Google Scholar]
- 37.Parker J., Gebretsadik T., Sabogal F., Newman J., Lawson H.W. Mammography Screening Among California Medicare Beneficiaries: 1993–1994. Am J Prevent Med (Baltimore) 1998;(15):198–205. doi: 10.1016/s0749-3797(98)00045-2. [DOI] [PubMed] [Google Scholar]
- 38.Chen J., Diamant A., Pourat N., Kagawa-Singer M. Racial/Ethnic Disparities in the Use of Preventive Services Among the. Elderly. 2005;29(5):388–395. doi: 10.1016/j.amepre.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Davis M.M., Renfro S., Pham R., Hassmiller Lich K., Shannon J., Coronado G.D., et al. Geographic and population-level disparities in colorectal cancer testing: A multilevel analysis of Medicaid and commercial claims data. Prev Med. 2017;101:44–52. doi: 10.1016/j.ypmed.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Himmelstein D.U., Warren E., Thorne D., Woolhandler S. Illness And Injury As Contributors To Bankruptcy. Health Aff. 2005;24(Suppl1) doi: 10.1377/hlthaff.w5.63. p. W5-63–W5-73. [DOI] [PubMed] [Google Scholar]
- 41.Hing E., Burt, Catherine W. Characteristics of Office-Based Physicians and Their Medical Practices: United States, 2005–2006. Nat Center Health Stat Vital Health Stat. 2008;13(166) [PubMed] [Google Scholar]
- 42.Aizer A.A., Chen M.-H., Parekh A., Choueiri T.K., Hoffman K.E., Kim S.P., et al. Refusal of curative radiation therapy and surgery among patients with cancer. Int J Radiat Oncol Biol Phys. 2014;89(4):756–764. doi: 10.1016/j.ijrobp.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Walker G.V., Grant S.R., Guadagnolo B.A., Hoffman K.E., Smith B.D., Koshy M., et al. Disparities in Stage at Diagnosis, Treatment, and Survival in Nonelderly Adult Patients With Cancer According to Insurance Status. J Clin Oncol. 2014;32(28):3118–3125. doi: 10.1200/JCO.2014.55.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woods L.M., Rachet B., Coleman M.P. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.