Abstract
According to the 2020 data released by the International Agency for Research on Cancer, breast cancer has surpassed lung cancer as the world's most newly diagnosed first-time cancer. Compared with patients with other types of cancer, those with breast cancer experience greater mental stress and more severe psychological impacts because of the life-threatening diagnosis, physical changes, treatment side effects, and family and social life dysfunctions. These usually manifest as anxiety, depression, nervousness, and insomnia, all of which elicit stress responses. Particularly under chronic stress, the continuous release of neurotransmitters from the neuroendocrine system can have a highly profound impact on the occurrence and prognosis of breast cancer. However, because of the complex mechanisms underlying chronic stress and the variability in individual tolerance, evidence of the role of chronic stress in the occurrence and evolution of breast cancer remains unclear. This article reviewed previous research on the correlation between chronic stress and the occurrence and development of breast cancer, particularly the molecular mechanism through which chronic stress promotes breast cancer via neurotransmitters secreted by the nervous system. We also review the progress in the development of potential drugs or blockers for the treatment of breast cancer by targeting the neuroendocrine system.
Keywords: Chronic stress, Neuroendocrine system, Neurotransmitters, Breast cancer, Drugs, Antagonists
Abbreviations: ACH, acetylcholine; ΑDR-α1, α1 adrenergic receptors; ΑDR-α2, α2 adrenergic receptors; AC, adenylate cyclase; α7-nAChR, α7 nicotinic acetylcholine receptors; BCSCs, breast cancer stem cells; BDNF, brain-derived neurotrophic factor; β-AR/ΑDR-β, β-adrenergic receptor; β-END, β-endorphin; CCK, cholecystokinin; CNS, central nervous system; CCL2, CC chemokine ligand; CCR2, CC chemokine receptor; CUS, chronic unpredictable stress; CTCs, circulating tumor cells; CRS, chronic restraint stress; CSC, cancer stem cells; CBSM, cognitive-behavioral stress management; CTNNB1, catenin β1; cAMP, cyclic adenosine monophosphate; DA, dopamine; DR, dopamine receptors; DAG, diacylglycerol; EPAC, exchange protein directly activated by cAMP; EGR-1, early growth response gene-1; EGFR, epidermal growth factor receptor; ERK1/2, extracellular signal-regulated kinase 1/2; EMT, epithelial–mesenchymal transformation; E, epinephrine; FAK, focal adhesion kinase; GPCR, G-protein-coupled receptor; GB1e, GABAB1e; GAD, glutamic acid decarboxylase; GABA, γ-aminobutyric acid; GC, glucocorticoids; HPA, hypothalamic–pituitary–adrenal axis; HSP, heat shock proteins; HA, histamine; IRS, insulin receptor substrate; IGF-1R, insulin-like growth factor-1 receptor; IP3, inositol triphosphate; JNK, c-Jun NH2-terminal kinase; LDHA, lactate dehydrogenase A; mAChRs, muscarinic acetylcholine receptors; MAPK, mitogen-activated protein kinase; MPI, medical and psychological intervention; mTOR, mammalian target of rapamycin; mGluR, glutamic acid; NTS, neurotensin; NTSR, neurotensin receptor; nAChR, nicotinic acetylcholine receptor; NE, norepinephrine; NPY, neuropeptide Y; PRL, lactogen; PKC, protein kinase C; PRL, prolactin; PKA, protein kinase A; PLC, phospholipase C; PROP, propranolol; POU5F1, POU domain class 5 transcription factor 1; PMN, premetastatic niche; SSADH, succinate semialdehyde dehydrogenase; SNS, sympathetic nervous system; TrkB, tyrosine kinase receptor B; TPH1, tryptophan hydroxylase 1; VP, vasopressin; VEGF, vascular endothelial growth factor; 5-HT, 5-hydroxytryptamine)
Graphical abstract
Introduction
According to the 2020 data released by the International Agency for Research on Cancer of the World Health Organization, the incidence of new diagnoses of breast cancer has surpassed that of lung cancer for the first time. The prevention and treatment of breast cancer remains a critical research topic across the world. Presently, breast cancer is mostly regarded as a physical disease by modern medicine and its neuropsychological factors are ignored. Since the biopsychosocial medicine model was proposed by the American scholar Engel in 1977, emotions have been considered as another vital sign along with body temperature, pulse, breathing, and blood pressure. Compared with patients with other types of cancers, those with breast cancer have a significantly higher incidence of depression due to the side effects of radiotherapy and chemotherapy, mastectomy, menopause, and other factors. In such emotionally loaded microenvironment, these factors may even induce breast cancer recurrence and metastasis, thus directly affecting the prognosis and survival rate of patients with breast cancer. An increasingly larger number of problems arise with therapies that target breast cancer cells; therefore, future research may shift from a single-purpose biomedical model to a more socially integrated model of medicine.
With the change in medical strategy, people have gradually realized that the development of breast cancer is not only related to physiological factors but also closely related to the stress caused by personal neuropsychological factors and social environment. Stress responses are a series of nonspecific adaptive responses that are produced when the body senses that the homeostatic balance is threatened or altered. Based on the duration and intensity of the stress, it can be classified as acute and chronic stress. Acute stress has a positive effect on the body. Conversely, chronic stress indicates that the body has been in a state of “overdrive” for a long period of time, which can cause serious complications and even promote tumor occurrence and metastasis. Common stressors are categorized as physical (various physical, chemical, and biological stimuli), psychological (conflict, frustration, hatred, fear, etc.), and social (professional competition, work burden, etc.) [10,71].
Research on the relationship between psychosocial factors and breast cancer has been increasing. In this review, we discuss the biological pathways and mechanisms of chronic psychological stress regulation in breast cancer progression. We explore the effect of the continuous secretion of certain neurotransmitters on the occurrence and development of breast cancer, and we summarize the potential drugs or blockers that influence the neuroendocrine system for the treatment of breast cancer, further clarify the mechanism of neurotransmitter action, and suggest novel strategies to develop drugs against breast cancer.
Relationship between chronic stress, the neuroendocrine system, and breast cancer
In today's fast-paced society, an individual experiences a high degree of personal stress, which usually manifests as anxiety, depression, tension, and insomnia. In addition to these, patients with breast cancer face changes in their body image, side effects of treatment, and family and social factors that lead to mental stress and psychological distress, all of which lead to chronic psychological stress [88]. Currently, the role of chronic psychological stress in tumor progression is one of the prominent topics in oncology research [76]. Chronic stress refers to the continuous, nonspecific, adaptive response of the body caused by the activation of the classical hypothalamic–pituitary–adrenal (HPA) axis [160]. Chronic psychological stress has been confirmed to be involved in various physiological and pathological processes of the human body and has been shown to promote disease progression by weakening the mental or physical health of the patient. In particular, the evolution of malignant tumors and the remodeling of the tumor microenvironment have severe adverse effects [10].
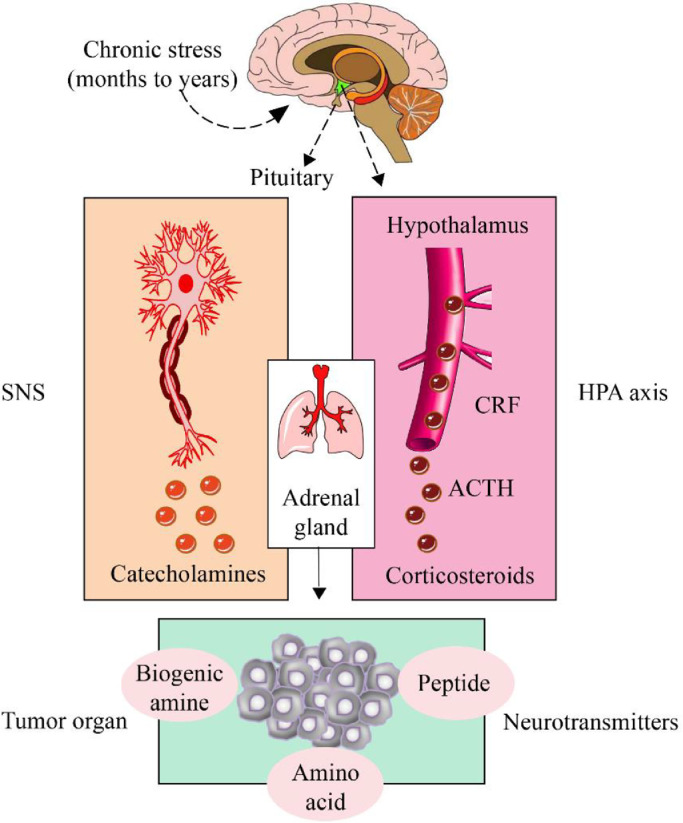
Increasing evidence has shown that chronic psychological stress can induce the occurrence and prognosis of breast cancer through the neuroendocrine system [126]. The ancient Greek physician Galen pointed out that psychology and physiology are connected and that organic diseases of the body can be cured by psychotherapy [141]. Nerves mainly transmit information from the central nervous system (CNS) to organs and tissues. Sensory nerves, motor nerves, and autonomic nerves (sympathetic and parasympathetic) form a neural network directly or indirectly connected to the neuroendocrine system, thus enabling functional communication between various parts of the body [56]. Other physical stressors, such as surgical stress and cold stress, have also been shown to promote the growth of breast cancer cells [16,64]. Regardless of the stress source, the nonspecific response of the body is mainly produced by the HPA axis and sympathetic nervous system (SNS). The HPA axis primarily mediates the secretion of glucocorticoids (GCs), whereas SNS mainly mediates the secretion of catecholamines such as epinephrine (E) and norepinephrine (NE) [22]. SNS rapidly improves the body's alertness and resilience during stress, which enables the body to cope with stress [34]; however, the continuous activation of the SNS under chronic stress significantly elevates the expression levels of GC, E, NE, and other neurotransmitters in the tumor microenvironment, thereby promoting the cell growth and metastasis of breast cancer [75] (Fig. 1).
Fig. 1.
Relationship between chronic stress, the neuroendocrine system, and breast cancer
Chronic stress significantly affects the occurrence of breast cancer, including promoting tumor cell proliferation and invasion, tumor angiogenesis, and evasive immune system monitoring. Stress responses are mainly mediated by the hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system (SNS). SNS rapidly improves the body's alertness and strain capacity during rapid stress, which is beneficial to the body; however, the continuous activation of neurotransmitters during chronic stress is detrimental to the body. Neurotransmitters can be classified as biogenic amines, amino acids, or peptides and are known to play complex and varied roles in breast cancer.
Neurotransmitters are chemical substances that transmit messages among neurons or between neurons and effectors. The role of neurotransmitters in breast cancer is complex and varied. Based on their chemical structures, neurotransmitters can be categorized as biogenic amines, amino acids, peptides, etc. Biogenic amine neurotransmitters include dopamine (DA), NE, E, and serotonin (5-HT, also known as 5-hydroxytryptamine). Amino acid neurotransmitters include γ-aminobutyric acid (GABA), glycine, glutamic acid (mGluR), histamine (HA), and acetylcholine (ACH). Peptide neurotransmitters include endogenous opioid peptide, neurovasopressin, cholecystokinin, and neuropeptide Y (NPY). This paper summarized the mechanisms regulating the development of breast cancer using the abovementioned classification framework. Obtaining mechanistic insights into the differential biological responses of different neurotransmitters under chronic stress allows us to understand into how drugs can be used to block the neuroendocrine system in breast cancer treatment.
Biogenic amine neurotransmitters regulate the pathological mechanism of breast cancer
α-epinephrine and breast cancer
Surgery, environment, and psychosocial stress factors activate a series of signal transduction pathways in the human body, triggering the stress response in the autonomic nervous system. Adrenergic receptors are the principal SNS mediators that regulate organ function, maintain systemic homeostasis in a resting state, and trigger the body's “fight or flight” response under stress. Following the activation and excitation of adrenergic receptors, the sympathetic nerves release E and NE that initiate various intracellular signaling cascades [2,29]. There is increasing evidence demonstrating that α-adrenergic receptors play an important role in breast cancer cell proliferation, migration, and invasion [145,153] (Fig. 2A). Despite evidence that the application of adrenergic receptor antagonists can slow down cancer progression and reduce patient mortality, there are still some controversies about whether they are absolutely beneficial for patients with cancer. For example, people pay more attention to psychological stress and use the restraint stress model or the chronic unpredictable stress model to study its mechanism. There are few studies on other physical stressors, such as how changes in environmental temperature affect tumor progression in ways other than the through the immune system. As another example, the studies mentioned below cannot rule out the influence of other hormones involved in the complex neuroendocrine response under stress on tumor growth. However, it is certain that adrenaline signals are the key effector mediators that play a role in stress and tumors driving.
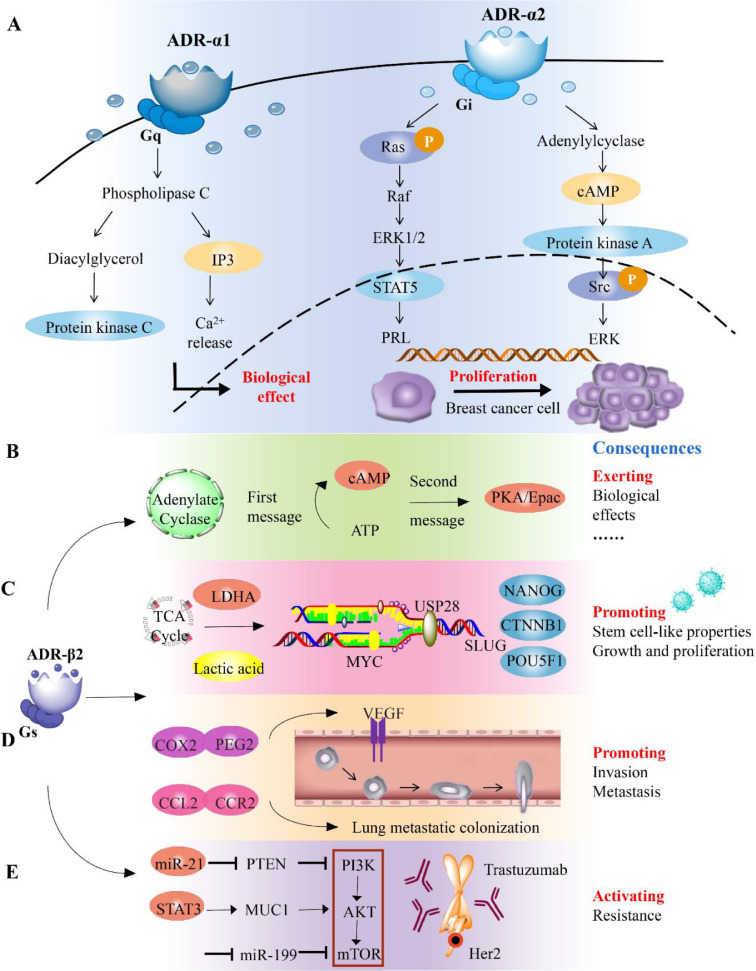
Fig. 2.
Biogenic amine neurotransmitters regulate the pathological mechanism of breast cancer
A. ΑDR-α1 is coupled to Gq protein and exerts physiological effects via the PLC/IP3/Ca2+ and DAG/PKC pathways. ΑDR-α2 coupled with Gi protein inhibits AC expression and decreases the intracellular cAMP level. ΑDR-α2 stimulates ERK phosphorylation via the Ras/Rαf/MAPK signaling pathway and Src-independent pathway to promote breast cancer cell proliferation. B. Upon stimulation by adrenaline, ΑDR-β and GS protein conjugate to activate AC, resulting in the production of the second messenger cAMP, which then activates its two downstream target molecules PKA or EPAC, leading to biological effects. C. Under chronic stress, ΑDR-β2 promotes glucose homeostasis in breast cancer cells to shift toward glycolysis and promotes LDHA expression. The resultant weakly acidic microenvironment enhances the deubiquitination of MYC by USP28, improves the stability of MYC, and activates the promoter of Slug, ultimately promoting the stem cell-like properties of breast cancer. D. The increased expression of CCL2 and CCR2 in chronically stressed mice results in the accumulation of macrophages in the lungs, which ultimately leads to a significant increase in the metastasis and colonization of circulating breast cancer cells in the lungs. E. Trastuzumab resistance-dependent PI3K/Akt/mTOR pathway is controlled by catecholamine-induced ΑDR-β2 activation.
Classification and biological function of receptors
ΑDR-α1 is coupled to Gq protein through the phospholipase C/inositol triphosphate/Ca2+ pathway, and the diacylglycerol/protein kinase C (PKC) pathway plays a role. ΑDR-α2 coupled with Gi protein inhibits adenylate cyclase (AC) and decreases the intracellular levels of cyclic adenosine monophosphate (cAMP) [51,101]. ΑDR-α is found in arteries and capillaries and helps regulate blood supply to many organs, including the breasts.
Promotion of proliferation
ΑDR-α2 is a key regulator of human breast cancer cell proliferation. Some researchers have studied its mechanism, mainly involving the mitogen-activated protein kinase (MAPK) signaling pathway. In the breast cancer cell lines MCF-7 and T47D, activation of ADR-α2 increases prolactin (PRL) levels and activates STΑT5, extracellular signal-regulated kinase (ERK1/2), and Akt [152]. ERK1/2 is one of the key signaling proteins of the Ras/Rαf/MΑPK signaling pathway, and it participates in various functions, including the regulation of carcinogenesis and the progression of tumor cells. In addition, PRL can activate ERK1/2 and Akt through Src, and ΑDR-α2 can stimulate ERK phosphorylation through Src-independent pathways [1].
Chemical resistance
Research has shown that ADR-α can affect chemotherapy drugs used to treat breast cancer. Su et al. [139] demonstrated that the activation of α-adrenergic signaling leads to the upregulation of MDR1. Increased MDR1 expression increases the resistance of MCF-7 cells to paclitaxel. When MCF-7 cells were implanted subcutaneously in severe combined immunodeficiency (SCID) mice, the stress condition led to the upregulation of MDR1 expression.
β-epinephrine and breast cancer
Chronic stress can activate β-adrenergic receptor (ΑDR-β), which plays an important role in growth, invasion, metastasis, angiogenesis, immunosuppression, etc. [39]. It can also reduce the therapeutic effect of anticancer drugs, which negatively affects the prognosis of patients with breast cancer. Evidence has shown that the application of ΑDR-β antagonists can slow down the progression of breast cancer, reduce the mortality rates of patients, and improve their prognosis. Such antagonists are expected to become biomarkers for diagnosis, monitoring the treatment effect, and determining the prognosis of breast cancer [107]. Ultimately, there exists a link between stress, ΑDR-β, and breast cancer. The following section focuses on the key mediators involved in ΑDR-β signaling implicated in the stress response and the progression of breast cancer.
Classification and biological function of receptors
ΑDR-β is distributed in the liver, kidney, lung, brain, breast, lymphatic tissue, and vasculature [62]. β1, β2, and β3 receptors bind to GS proteins and, when stimulated by adrenaline, activate AC to produce cAMP, a second messenger. cAMP production reactivates its two main downstream target molecules: protein kinase A (PKA) or exchange protein directly activated by cAMP (EPAC) [72]. The production and activation of cAMP and its downstream effectors lead to an array of signaling pathways that affect the biological response as indicated in Fig. 2B. ΑDR-β signaling can also regulate the biological activities of fibroblasts, epithelial cells, and vascular endothelial cells, which are related to cancer cells, especially lymphatic and immune cells [75,84].
Induced stem cell-like properties
Compared with common breast cancer cells, breast cancer stem cells show enhanced tumorigenesis, invasion, and migration abilities, which is considered essential for the proliferation, invasion, and other malignant biological characteristics of breast cancer cells. Cui et al. used NOD/SCID mice to establish a stress model and found that ΑDR-β2 promotes glucose metabolism balance in breast cancer cells, shifting it toward glycolysis, and also promotes the expression of lactate dehydrogenase A under chronic stress. The product of this is lactic acid that changes the pH of the local environment which then promotes the formation of a weakly acidic microenvironment, thus consequently enhancing the deubiquitination of MYC by the deubiquitination enzyme USP28 and improving the stability of MYC [38]. MYC activates the transcription factor Slug, promotes the expression of the “self-renewal” related genes catenin β1, POU domain class 5 transcription factor 1, and NANOG and improves the efficiency of breast cancer cell microsphere formation in vitro and in vivo; in other words, the transcription factor Slug ultimately promotes the stem cell-like properties of breast cancer and induces tumor growth and proliferation (Fig. 2C). Kang et al. used 4T1 cells to construct a mouse chronic stress model of breast cancer and found that after its activation, ΑDR-β2 regulated hexokinase-2 expression in cancer cells and promoted glucose metabolism in tumors [73]. In addition, ΑDR-β2 stimulation in osteoblasts promoted the adhesion of breast cancer cells to bone marrow endothelial cells in an IL-1β- and selectin-dependent manner [35].
Promotion of migration and invasion
Changes in neuroendocrine responses in the body during chronic stress can result in a “premetastatic microenvironment” in the distal organs during the premetastatic tumor stage, making it more conducive to the survival of tumor cells. In other words, the premetastatic niche (PMN) is another important factor in the promotion of tumor metastasis. ΑDR-β overexpression in human breast cancer cells induces an invasive phenotype, whereas significantly reduced ΑDR-β expression reduces the effect of stress on metastasis in vivo [119]. The final site that tumor cells colonize and whether the tumor can survive in distant organs, far away from the primary focus, is affected by the microenvironment of the distant organs. The formation of PMN and survival of metastatic cells are closely related to the recruitment of macrophages in PMN. Chen et al. [28] used the ΑDR-β agonist isoproterenol in a mouse model of chronic unpredictable stress response before metastasis and showed that the number of monocytes in the peripheral blood, spleen, and bone marrow was significantly increased after isoproterenol administration. Furthermore, the expressions of CC chemokine ligand 2 and monocyte/macrophage chemokine receptor 2 in lung tissues were upregulated, leading to the accumulation of macrophages in the lung. The result was a significant increase in the metastatic colonization of circulating tumor cells in the lungs of the breast cancer mouse model. In addition, Le et al. [78] found that chronic stress can regulate the COX2-PEG2 inflammatory pathway related to tumor-associated macrophages through ΑDR-β signal transduction of inflammatory cells, increase the expression of vascular endothelial growth factor (VEGF) and the density of lymphatic vessels in the primary tumor of breast cancer, induce stable expansion of lymphatic vessels draining the primary tumor, and increase lymph flow, thereby enhancing the ability of tumor cells to migrate and spread through the lymphatic vessels (Fig. 2D).
Chemical resistance
In addition to promoting the proliferation, migration, and invasion of breast cancer cells, ΑDR-β2 reverses chemical resistance to the breast cancer drug Herceptin (trastuzumab). The therapeutic antibody Herceptin is currently a first-line drug for the clinical treatment of breast cancer; it can specifically target breast cancer cells overexpressing HER2. However, drug resistance has become a critical problem, restricting the clinical efficacy of the drug. ΑDR-β2 and HER2 constitute a positive feedback loop in human breast cancer cells, affecting the biological behavior of breast cancer cells, suggesting that ΑDR-β2 activation is involved in trastuzumab resistance [83]. Further studies have found that in patients with breast cancer with HER2 overexpression, ΑDR-β2 expression is negatively correlated with the response to trastuzumab. ΑDR-β2 upregulates the expression of miR-21 and muc1 by activating STAT3 and the PI3K/Akt pathway. It also inhibits miR-199a/b-3p expression and induces the activation of mammalian target of rapamycin (mTOR). Therefore, the trastuzumab resistance-dependent PI3K/Akt/mTOR pathway is controlled by catecholamine-induced β2-AR activation (Fig. 2E). Furthermore, the ΑDR-β antagonist propranolol (PROP) not only enhances the antitumor activities of trastuzumab but also resensitizes resistant cells to trastuzumab. The next research direction needs to accurately assess the expression of ADR-β subtypes in tumor cells of patients with breast cancer and selectively use β-receptor blockers. Only prospective, multicenter clinical studies could provide reliable evidence for β-blockers to become a new adjuvant treatment for patients with breast cancer.
Dopamine and breast cancer
DA is a catecholamine neurotransmitter present in the mammalian neuroendocrine system [114]. Chronic stress causes the overexpression of DRs. The DA imbalance theory, which explains the mechanism of stress, is widely accepted [89]. Under chronic stress, rats continue to be in a state of depression; DA levels in the prefrontal lobe, striatum, hippocampus, and other brain regions are decreased; and the synaptic transmission function of dopaminergic neurons is significantly downregulated [93]. Imaging studies in patients with severe depression showed abnormal DRD1 binding [23]. Long-term stress stimulation has been reported to significantly increase DRD1 levels in the rat limbic system by 29%, which decreases after antidepressant treatment [102]. Selective DRD1 agonists can reverse the decrease in DA levels caused by chronic unpredictable stress and can ameliorate behavioral despair in mice [42,[111]. Abnormal function of the DA system can cause diseases of the central nervous system, such as schizophrenia and Parkinson's disease. Numerous recent studies have found that the DR signaling pathway is closely related to the occurrence, development, and prognosis of breast cancer. DRs may become new targets for the targeted therapy of tumors. For example, the DR antagonist methithioprazine has been explored in basic research and clinical practice of pituitary tumors [9]. DA agonists such as bromocriptine and carergoline are first-line agents for the treatment of prolactinoma, and some researchers are currently researching the application of DA agonists for the treatment of breast cancer [37].
Overexpression of DRs can induce breast cancer. In 2003, Comings et al. concluded that the D2 receptor gene is a risk factor for breast cancer (P = 0.018) in a study on the genetic risk factors of breast cancer. Studies have found that individuals with chronic stress are susceptible to several cancers, including breast cancer. Interestingly, the expression levels of DRD2–DRD4 in patients with breast cancer are elevated [108]. The expression levels of DRD2–DRD4 in patients with breast cancer without psychological intervention were significantly higher than those in patients who received the intervention [3]. Bakhtou et al. [12] found a difference in gene expression between patients with breast cancer and healthy people and found that DRD2 and DRD4 were overexpressed in peripheral blood mononuclear cells of patients at stage II and III breast cancer. At the same time, cellular experiments revealed that DRD2 agonists could induce apoptosis of breast cancer cells. Sachlos et al. [117] found that pluripotent stem cells in healthy subjects did not express DRs, whereas CSCs expressed all five DRs, suggesting that DRs are a potential biomarker for screening CSCs and a potential target for triple-negative breast cancer therapy. However, many questions about DRs and tumors need to be further studied, especially the specific molecular mechanism of the antitumor effect of compounds through the DR signaling pathway. The important role of DR agonists and antagonists in tumors has been receiving increased attention from researchers. However, the role of DRs in food intake and glucose metabolism is still unclear. Future research can focus on tumor metabolism and discover the role of dopamine in other tumors. Based on current research results, it is expected to play a clinical guiding role.
Noradrenaline and breast cancer
Evidence suggests that the tumor-driven effect of β-adrenergic signaling is mainly dependent on local sympathetic ending-derived NE [36]. When SNS is activated by chronic stress, adrenaline and NE are first secreted by the adrenal medulla and distributed through the bloodstream to the tumor site. Sympathetic endings entering tumor tissue can release NE, which interacts directly with tumor cells [143]. A study on breast cancer demonstrated that mice with restraint stress exhibit rapid tumor growth and increased tumor innervation. Antagonism of the nicotinic ACh receptor (NACHR) in the peripheral nervous system with hexamethylammonium bromide completely eliminated cell growth. However, removal of the adrenal gland had no significant effect on tumor growth and nerve count increase induced by restraint stress. This result confirms the dominant role of NE derived from local nerve fibers [7]. The author also proposed a feedforward circuit innervated by tumors: under restraint stress, the brain source is upregulated by activating the β3-AR/cAMP/Epac/c-Jun NH2-terminal kinase (JNK) pathway. The expression of brain-derived neurotrophic factor increases tumor tissue innervation through the action of tyrosine kinase receptor B. This new growth of nerve endings can release NE to promote tumor growth, thereby forming a feedforward circuit. In addition, the depressive state increases the level of NE, increases the phosphorylation of p38MAPK, and increases the malignancy of breast cancer cells [119]. NE activates the Akt signaling pathway under anxiety and promotes the development of breast cancer [147].
Promotion of migration and invasion
NE released under chronic stress can promote the invasion and metastasis of breast cancer cells. Sloan et al. [128] constructed a stress model of mice using binding methods and found that chronic stress enhanced the metastasis of breast cancer cells to distant tissues (including lung and lymph nodes) but had little effect on the growth of the primary tumor. This stress-induced metastasis is mediated by promoting macrophage infiltration, and stress simultaneously induces the expression of premetastatic genes such as TGFb, Arg1, and CSF1 in the primary tumor microenvironment. Stress also promotes the adhesion of tumor cells and vascular endothelial cells. Isoproterenol can induce integrin-mediated cell adhesion through the β−2AR/cAMP/Epac/Rap1 pathway [110]. Studies have shown that NE can induce the release of the chemokine GROa from vascular endothelium and promote β1-integrin-mediated adhesion of breast cancer cells to vascular endothelium [138]. Under chronic stress, NE can activate focal adhesion kinase through the ADRB2/Src signal axis, thereby inhibiting the anoikis of breast cancer cells [133]. As a result, a small portion of the surviving circulating tumor cells adheres to the vascular endothelial cells of the distant organs, escapes the circulatory system, and are transferred to specific remote organs. Yamazaki et al. [155] treated MDA-MB-231 breast cancer cells with NE and found that NE can induce the production of reactive oxygen species and promote the expression of heme oxygenase-1, MMP-2, and MMP-9 genes, thereby increasing the invasiveness of breast cancer cells.
Promotion of angiogenesis and lymphangiogenesis
NE can promote the formation of tumor blood vessels and lymph vessels. Chen et al. found that VEGF expression in breast cancer cells can be upregulated by NE and that the culture supernatant of breast cancer cells treated with NE promoted the formation of a capillary-like network of endothelial cells. In a study on cocultured endothelial and breast cancer cells, NE was found to activate the Notch pathway of endothelial cells and significantly upregulate the expression of Notch ligand Jagged-1 on breast cancer cells. The Notch pathway is closely related to the interaction of tumor cells and mesenchymal cells and angiogenesis. The upregulation of Jagged-1 caused by NE can be reversed by β2-AR, PKA, and mTOR inhibitors, indicating that β2-AR/PKA/mTOR pathway is involved in this process.
5-hydroxytryptamine and breast cancer
One of the causes of chronic stress is the decreased release of 5-HT in the CNS, which reduces the content of the synaptic cleft. 5-hydroxytryptamine (5-HT) was first isolated from serum by author Rapport, and is more commonly known as serotonin [130]. 5-HT is present in a large amount in the digestive system and a small amount in the CNS. It participates in the regulation of mood and cognition, as well as the regulation of the functions of the gastrointestinal tract, such as movement, secretion, and immunity [60,[94]. Studies have confirmed that 5-HT levels in the CNS of patients with depression are reduced. The 5-HT level was also significantly reduced in a chronic restraint stress mouse model [46,118]. 5-HT6 receptor antagonists are closely related to the CNS and are also involved in the regulation of neurotransmitters, such as ACH, DA, and GABA, playing an important role in alleviating anxiety and depression [61]. Furthermore, The HT3 receptor is an important target for controlling digestive disorders such as anorexia and bulimia. Activation of the 5-HT3 receptor can increase intracellular Ca2+ levels, promote the release of neurotransmitters and neuropeptides, and stimulate central and peripheral neurons, leading to vomiting and inflammation. 5-HT3 receptor antagonists can effectively counter eating disorders and vomiting caused by early chemotherapy and radiotherapy [164]. Therefore, 5-HT3 receptor antagonists can be clinically developed to inhibit dopaminergic neurons and promote the increase of adrenocorticotropic hormone levels to relieve stress response.
In the mammary glands, 5-HT produced by the prolactin-induced expression of the 5-HT biosynthesis gene tryptophan hydroxylase 1 induces lactation-to-involution switching via 5-HT7 receptor-dependent tight junction disruption [66]. Although 5-HT has an inhibitory effect on the growth of normal breast epithelial cells, it stimulates the proliferation of breast cancer cells at submicromolar concentrations. Although the mechanism by which 5-HT affects the malignant phenotype of breast cancer remains unclear, the angiogenic effect of 5-HT suggests that it stimulates the proliferation and invasion of cancer cells, which is necessary for cancer progression [55,57]. Additionally, in the triple-negative breast cancer cell line MDA-MB-231, 5-HT was shown to promote cell invasion and proliferation through the 5-HTR7 receptor and had a stronger proliferation effect in the first stage of metastasis than that after local infiltration [50]. Depression often promotes the dysregulation of the immune system of patients with cancer and accelerates tumor recurrence and metastasis. Compared with the nondepressive group, patients with breast cancer with depression had higher 5-HT levels, higher 5-HTR1 expression, and lower overall survival rates [85].
Amino acid neurotransmitters regulate the pathological mechanism of breast cancer
γ-aminobutyric acid and breast cancer
Inhibitory neurotransmitter GABA is involved in important life activities such as human growth and development and nitrogen balance. Recent studies have reported that GABA can regulate tumor cell proliferation, migration, differentiation, and apoptosis [113]. The decrease in GABA levels in the cerebrospinal fluid and plasma of patients with depressive disorder results in weakening of the inhibitory effect on central neuron activity, leading to symptoms such as anxiety and insomnia, and further acceleration of the occurrence of chronic stress [14,43].
GABA receptors can be categorized into ion channels and metabotropic receptors according to their sensitivity to agonists and antagonists. Among them, GABAA and GABAC receptors are ion channels, whereas GABAB receptors are metabotropic, belonging to the G-protein-coupled receptor (GPCR) family [20]. GABAB comprises two subunits, GABAB1 and GABAB2. The prominent feature of GABAB1 is its molecular diversity; to date, 14 splicing isomers ranging from GABAB1a to GABAB1n have been identified. GABAB1e (GB1e) was shown to promote the proliferation and migration of MCF-7 cells and protect cells from apoptosis caused by Tm and Tg that are stressed by the endoplasmic reticulum [68]. GB1e can also significantly promote the cloning and microsphere formation of MCF-7 cells and the expression of the breast cancer stem cell marker molecule CD44. Molecular experiments showed that GB1e may promote the enhancement of cancer stem cell characteristics by activating the MAPK and ERK1/2 signaling pathways [20]. This is the first exploration of the function of GABAB1 spliceosome in tumors and the first to establish a connection between GABAB receptors and the tumor microenvironment and tumor stem cells.
ABAT, a key enzyme in GABA metabolism, catalyzes the degradation of GABA into succinate hemialdehyde and l-glutamic acid, thereby contributing to the reduction of GABA levels [67]. Based on an analysis of a breast cancer metabolomics database, we found that tumor tissues with low ABAT expression had high GABA content and that the promoter of ABAT contained four conservative Snail-binding site E-box sequences (CAGGTG). Accordingly, we hypothesized that due to the change in the copy number of the ABAT genome and Snail inhibition, the ABAT expression level in basal-like breast cancer was decreased, leading to an increase in the levels of its metabolic substrate GABA and activation of related signaling pathways through GABA, thereby promoting the occurrence and development of breast cancer. In addition, GABA-mediated activation of the Ca2+-NFAT1 axis downregulated ABAT expression in basal-cell-like breast cancer, thereby promoting the occurrence and metastasis of breast cancer [31] (Fig. 3A). Gina et al. silenced GABRP in two BLBC cell lines, HCC1187 and HCC70, and found that BLBC-related cytokeratin expression decreased, such as the expressions of KRT5, KRT6B, KRT14, and KRT17. Simultaneously, the silencing of GABRP expression reduced ERK1/2 phosphorylation in the two cell lines and ultimately inhibited tumorigenic potential and migration in vitro [127]. GABRA3 activated the Akt pathway to promote breast cancer cell migration, invasion, and metastasis [53].
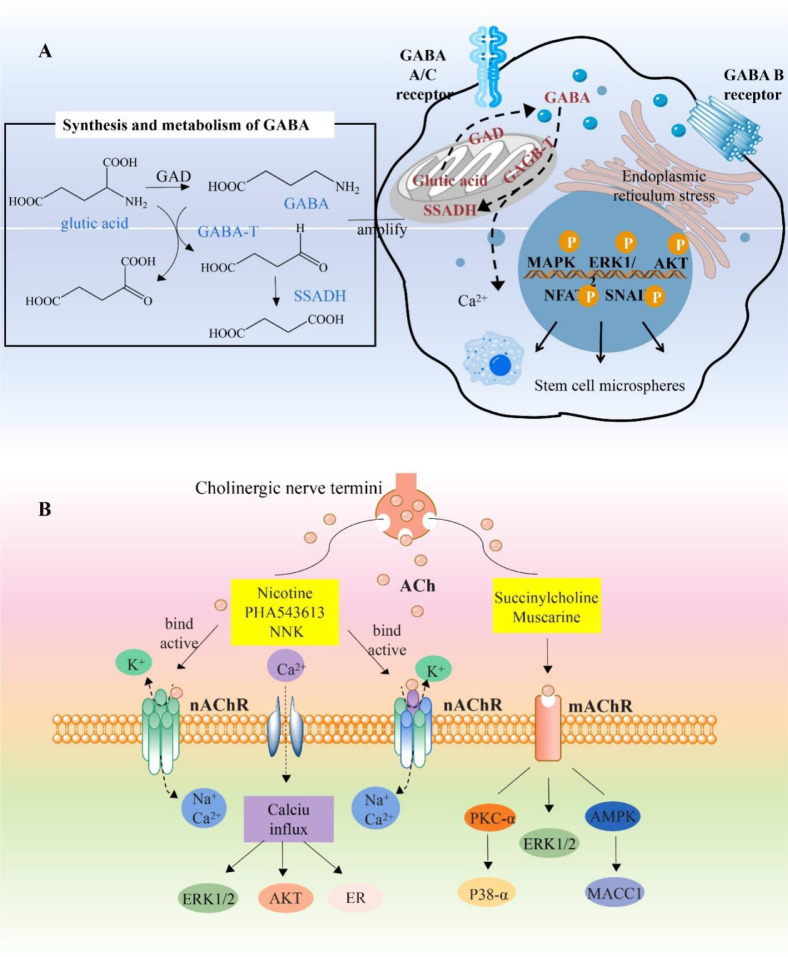
Fig. 3.
Amino acid neurotransmitters regulate the pathological mechanism of breast cancer
A. GABAA and GABAC receptors are ionotropic channels, whereas GABAB receptors are metabotropic. ABAT is a key enzyme in GABA metabolism that catalyzes the degradation of GABA to produce succinic semialdehyde and l-glutamic acid, thereby contributing to reducing GABA levels. The downregulation of ABAT expression in basal cell-like breast cancer may be due to GABA-mediated activation of the Ca2+-NFAT1 axis. In addition, GABAB1e (GB1e) may significantly enhance the cloning and microsphere formation ability of MCF-7 cells through endoplasmic reticulum stress and further activates the MAPK/AKT/ERK signaling pathway to promote breast cancer cell proliferation, migration, and invasion. B. Neuronal nAChRs are categorized as heteropentamers and homopentamers. Their structures and pharmacological functions are different. When activated by an agonist, the influx of Na+ and Ca2+ and the efflux of K+ occurs, regulating the expressions of MAPK, PKC, VEGF, etc. These proteins are closely associated with the occurrence and development of breast cancer.
Acetylcholine and breast cancer
Classification and biological function of receptors
Cholinergic receptors are divided into muscarinic ACh receptors and nAChRs, which mediate the production of the neurotransmitter ACH. In mammals, nAChRs are divided into neuronal and muscle nAChRs [30]. Neuronal nAChRs can be divided into α and β subunits. α subunits (α2, α3, α4, and α6) and β subunits (β2, β3, and β4) can form heteropentamers, and α7 and α9 subunits can form homopentamers [140]. Obviously, the different structures of α7 and α9 lead to completely different pharmacological functions. But their structures are so similar that they are difficult to distinguish (Table 1). As a specific antagonist of neuronal nAChRs, α-conotoxin has a strong ability to distinguish between different α and β subunit interfaces of neuronal nAChR subtypes. Therefore, α-conotoxin is a valuable molecular probe for studying the fine structure and physiological and pharmacological functions of various nAChR subtypes, and it is also a source of new drugs for the treatment of nAChR-related diseases [79]. The nAChRs are closely associated with the occurrence and development of diseases, and the different nAChR subtypes determine the different pathological and physiological process (Fig. 3B).
Table 1.
Differences between nAChRs and mAChRs.
| Subtypes | FamilyMetabotropic | Structure | SubunitsM1-M5 | Agonists | Antagonists |
|---|---|---|---|---|---|
| nAChRs | Ionotrophic (ligand-gated ion channel receptors) | Pentameric transmembrane proteins | Neuronal: α2-α10 and β2-β4 Muscle-type: α1, β1, 8, γ (developmental muscle), and ε (mature muscle) |
Nicotine PHA543613 Tobacco, nitrosamines Acetycholine NNK |
α-Bungarotoxin |
| mAChRs | G-protein-coupled receptors | Seven-transmembrane proteins | M1-M5 | Succinylcholine Muscarine |
Atropine |
Overexpression of α7 and α9-nAChR
Mounting evidence has shown that the overexpression of ACHRs mediates the proliferation, apoptosis, angiogenesis, and epithelial–mesenchymal transformation of breast cancer cells [103]. Members of the nAChR family, α7-nAChR and α9-nAChR, have been found to be expressed in both MCF-7 and MDA-MB-468 cells [30]. Aldehyde dehydrogenase (ALDH), which is responsible for deoxidation in cells, has been used as a specific marker of stem cells in recent years. It has been mainly used to identify the enrichment of CSC subsets and the determination of poor prognosis in patients with breast cancer, which is mainly associated with increased metastasis and chemotherapy resistance [121]. Nicotine, an N-choline receptor agonist, can induce human MCF-7 breast cancer cells to develop resistance to the anticancer drug Adriamycin. Additionally, nicotine promotes the migration of breast cancer by activating PKC, and the number of ALDH-positive CSCs in MCF-7 cells is dependent on the concentration of nicotine [74]. The proliferation of ALDH-positive cells in MCF-7 cells treated with the α7-nAChR specific agonist PHA 543,613 was increased, and this effect was blocked by the N-choline antagonist α-BTX [59]. In vivo studies have shown that nicotine may promote the growth of solid tumors by inducing cancer cell proliferation, adhesion, and angiogenesis.
Higher expression of α9-nAChR mRNA was detected in ER (+) tumor tissues than in ER (-) tumor tissues. Mouse transplant tumor models and the NNK human mammary epithelial cell line MCF-10A can undergo cancerization on exposure to nicotine [81]. Further studies showed that NNK could not only upregulate the phosphorylation level of ERK1/2 and stimulate the expression of genes encoding tumor-related factors but also downregulate the expression of the tumor suppressor gene CDKN2A, thereby inducing cancer [52]. Small RNA interference experiments confirmed that the proliferation of breast cancer cells induced by nicotine or NNK can be inhibited by silencing the expression of α9-nAChR [49]. In contrast, α9-nAChR overexpression can stimulate the proliferation of breast cancer cells [151]. It was demonstrated that nicotine and NNK regulate the pathological processes of cancer by inducing the expression of genes related to cell process or metabolism, and this is closely related to the activation or high expression of nAChR.
Glutamate and breast cancer
mGluR is a major excitatory neurotransmitter in the mammalian neuroendocrine system. Strong or chronic repeated stress can induce excessive and persistently activated excitation, resulting in increased free radical generation, causing pathological damage through oxidative stress [47]. Increasing evidence proves that mGluR is involved in the development of breast cancer [161]. Xiao et al. constructed an mGluR4 eukaryotic expression plasmid capable of high expression in MCF-7 cells. Immunofluorescence results showed that mGluR4 was localized in the cell membrane and cytoplasm. CCK-8 experiments showed that high mGluR4 expression significantly promoted the proliferation of breast cancer cells [26]. Speyer et al. [135] discussed the potential role of mGluR1 in mediating endothelial cell (EC) proliferation and tumor-induced angiogenesis. Using mGluR1-specific inhibitors or shRNA silencing, the growth of EC and the formation of stromal tubes was shown to be dependent on the mGluR1 signaling pathway. Additionally, in mouse models of breast cancer, the loss of mGluR1 activity led to decreased angiogenesis. We think mGluR1 acts as a pro-angiogenic factor and a mediator of tumor progression in breast cancer. Therefore, researchers suggest that mGluR1 is a potential new molecular target for anti-angiogenesis therapy in breast cancer. Although the mechanism of mGluR in the nervous system has been studied in detail, its role in the occurrence and development of breast cancer remains to be elucidated.
Histamine and breast cancer
HA is involved in the pathological process of physiological allergies and various diseases as a central neurotransmitter [90]. Martinel et al. applied restraint-cold stress to stimulate rats to determine the HA level in the heart, lungs, liver, kidneys, and stomach. The results showed that the HA levels in the heart in both the stress and the control groups were not significantly different [90]. The HA level showed a significant increase in the stress group but remained unchanged in the control group. Under various stress conditions, the level of released HA increases, and, through its receptors, it participates in the regulation of the autonomic nervous system; cardiovascular activities; behavior; and secretion of neuroendocrine hormones such as corticotropin releasing hormone, ACTH, vasopressin, PR, β-endorphin, and NE [91]. HA expression and biosynthesis have been detected in breast cancer cells. Breast cancer cells treated with the H4R agonists clobenpropit, VUF8430, and JNJ28610244 showed enhanced cell migration and invasion. The increased levels of ERK1/2 phosphorylation and TGF-β1 were detected using western blotting, the increased expression of Smad2/3-positive nuclei was detected using indirect immunofluorescence, and the formation of tumors was detected using a mammosphere assay [48]. Increased histidine decarboxylase gene expression is associated with improved relapse-free and overall survival among patients with breast cancer. HA treatment (5 mg kg−1) in 4T1 tumor-bearing mice reduced tumor growth and increased apoptosis. Although no immunomodulatory effects were observed in wild-type mice, significant correlations between tumor weight and cytotoxic lymphocyte infiltration were detected in H4R knockout mice. H4R is differentially modulated by agonists and antagonists impacting tumor growth and immunity in 4T1 tumor-bearing mice [100]. For this reason, HD could be a potential biomarker for breast cancer.
Peptide neurotransmitters regulate the pathological mechanism of breast cancer
NPY and breast cancer
NPY is one of the most abundant neuropeptides and plays an important role in regulating emotions and stress-related behaviors [159]. Studies have shown that NPY is involved in stress response to varying degrees and can freely cross the blood–brain barrier [58]. The NPY system is significantly altered during chronic stress. Zambello et al. [158] found that the expressions of NPY mRNA in the hippocampus, Y1 receptor mRNA in the ventral and medial hypothalamus, and Y2 receptor mRNA in the ventral and medial hypothalamus were significantly lower in mice under chronic stress than in those in the control group. The anxiety and depression symptoms shown by the mice in the stress group were significantly increased compared with those of the mice in the control group. This suggests a functional connection between the hippocampus and hypothalamus NPY system and that chronic stress may damage the NPY system, indirectly leading to the occurrence of emotional problems [17].
Receptor autoradiography showed that 85% of breast cancer cases had NPY receptors. The NPY family includes three peptides: NPY, polypeptide YY, and pancreatic polypeptide; of these, NPY is more important in tumors [125]. NPY acts through four GPCRs: Y1, Y2, Y4, and Y5. Y1, Y2, and Y5 are associated with various aspects of tumorigenesis and angiogenesis [6]. NPY receptors are expressed in the colon, kidneys, adrenal gland, reproductive organs, testicles, and breasts [19]. Y1 is the dominant receptor in tumors, whereas Y2 is expressed preferentially in nontumor breast tissue. Reubi et al. proposed that oncogenic transformation may be conferred from Y2 to Y1 subtype [112]. Recent in vitro studies have implicated Y5 receptors in breast cancer growth and angiogenesis. Additionally, NPY inhibited the inflammatory response and enhanced the activity of MCF-7 cells in a hypoxic environment [40]. This is probably because NPY affects the inflammatory response, which in turn affects HIF-1 expression in tumor cells.
Neurotensin and breast cancer
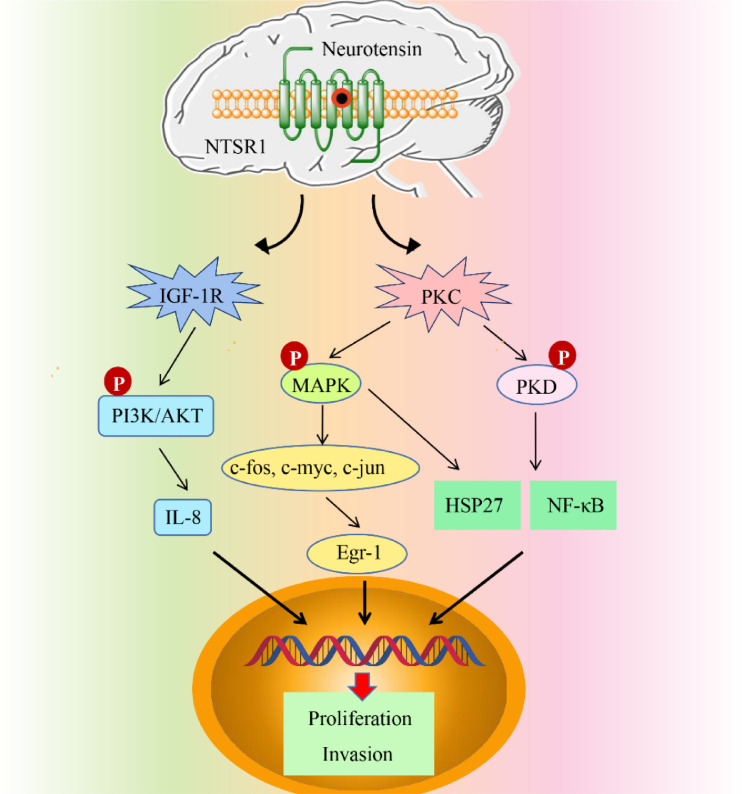
Neurotensin (NTS) shows various central and peripheral effects by binding to NTS receptor (NTSR) [45]. There are three known subtypes of NTSR: NTSR1, NTSR2, and NTSR3. NTSR1, the high-affinity NTSR, has been well studied and is associated with the occurrence, development, proliferation, invasion, and prognosis of various malignant tumors [87,97,98]. However, currently, there are only few studies on the role of NTSR1 in breast cancer. In this study, the relevant literature on the occurrence and development of breast cancer mediated by the NTS/NTSR1 signaling pathway in recent years has been reviewed. NTS and NTSR1 are explored as potential diagnostic markers and drug action targets for breast cancer treatment.
Somaï et al. [131] found that NTSR1 and NST activation can promote the transcriptional activation of Bcl-2 in breast cancer cells and promote the growth of tumor cells. Bakirtzi et al. [13,162] showed that binding of NTS and NTSR1 promoted the expression of interleukin-8, which was dependent on NF-κB, and NF-κB activated the insulin-like growth factor-1 receptor (IGF-1R)-mediated PI3K/Akt pathway. This activation promoted the expression of NF-κB-dependent related genes so that epithelial cells could evade apoptosis, thereby leading to cell proliferation. Studies on insulin receptor substrates (IRSs) confirmed that the combination of IRSs and IGF-1R does play an important role in the proliferation and metastasis of breast cancer. Souazé et al. [134] found that NTS and NTSR1 were expressed in MDA-MB-231 cells. Exogenous NT can enhance the movement and invasion of cancer cells after activating NTR1. Watters et al. found that NTSR is the target gene of estradiol and that estradiol can increase the transcription of NTSR in the hypothalamic arcuate nucleus neuroendocrine nucleus and preoptic region. MAPK signals activated by NTS can also significantly increase the HSP27 phosphorylation at site 82. The overexpression of heat shock proteins in patients with breast cancer promoted the proliferation and invasion of cancer cells, which is a marker of poor prognosis for malignant breast cancer and is related to the degree of tumor invasion [8]. High HSP27 expression in breast cancer can affect the sensitivity of tumor cells to hormone therapy [45,[54,150]. In addition, MAPK signals activated by NTS can also significantly increase the phosphorylation of HSP27 at site 82 [54] (Fig. 4). NTS can also express early growth response gene-1 (EGR-1), and the promoter region of epidermal growth factor receptor (EGFR) has an EGR-1 binding site [163]. Thus, EGFR expression is promoted, which is closely related to breast cancer occurrence. EGFR is a benzimidazole compound. At present, there are clinically specific antibodies and small-molecule kinase inhibitors against EGFR receptors used in breast cancer treatment. Some studies have pointed out that there are EGFR mutants in breast cancer, and these mutants are produced due to the deletion, mutation, and rearrangement of EGFR. The deletion of certain domains of EGFR leads to the destruction of receptor downregulation mechanisms, activation of abnormal signal transduction pathways, and inhibition of cell apoptosis. The EGFR ligand also has a great influence on intracellular signal transduction. Coexpression of the two often indicates a poor tumor prognosis. In addition, KRAS protein is downstream of the EGFR signaling pathway. Therefore, KRAS gene testing can screen those patients with breast cancer for whom EGFR-targeted therapeutic drugs are effective and realize the individualized treatment of patients with breast cancer.
Fig. 5.
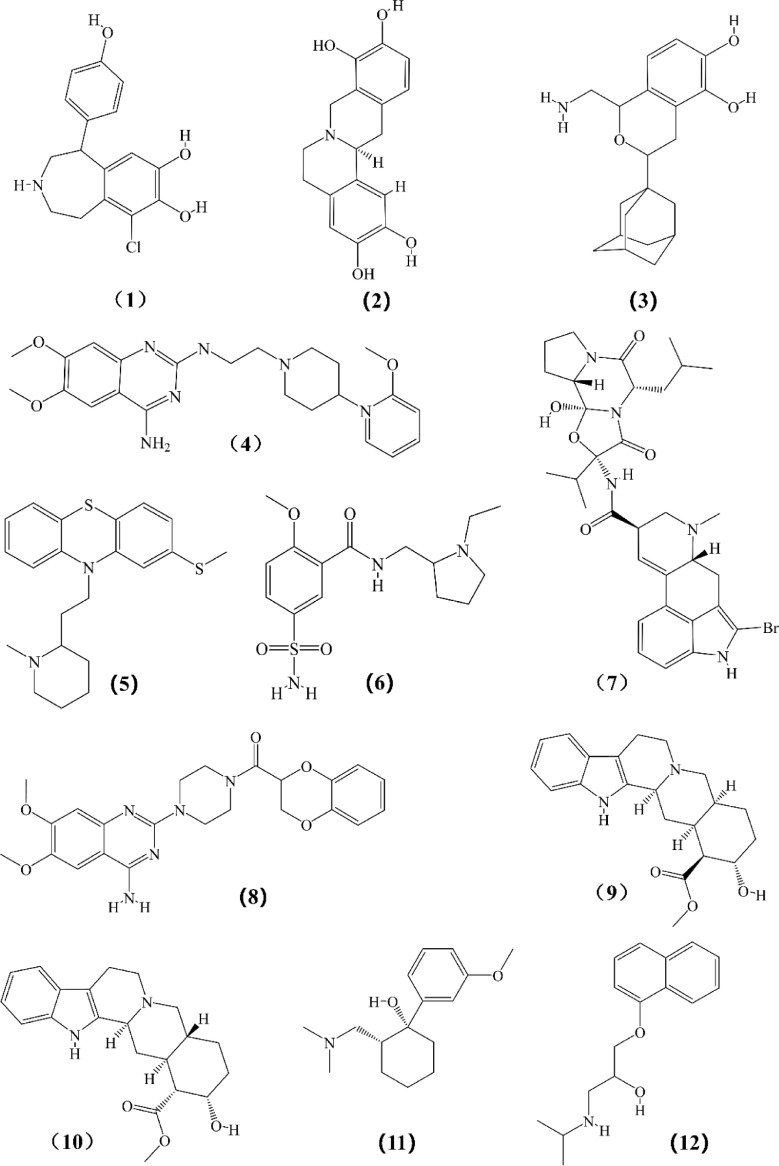
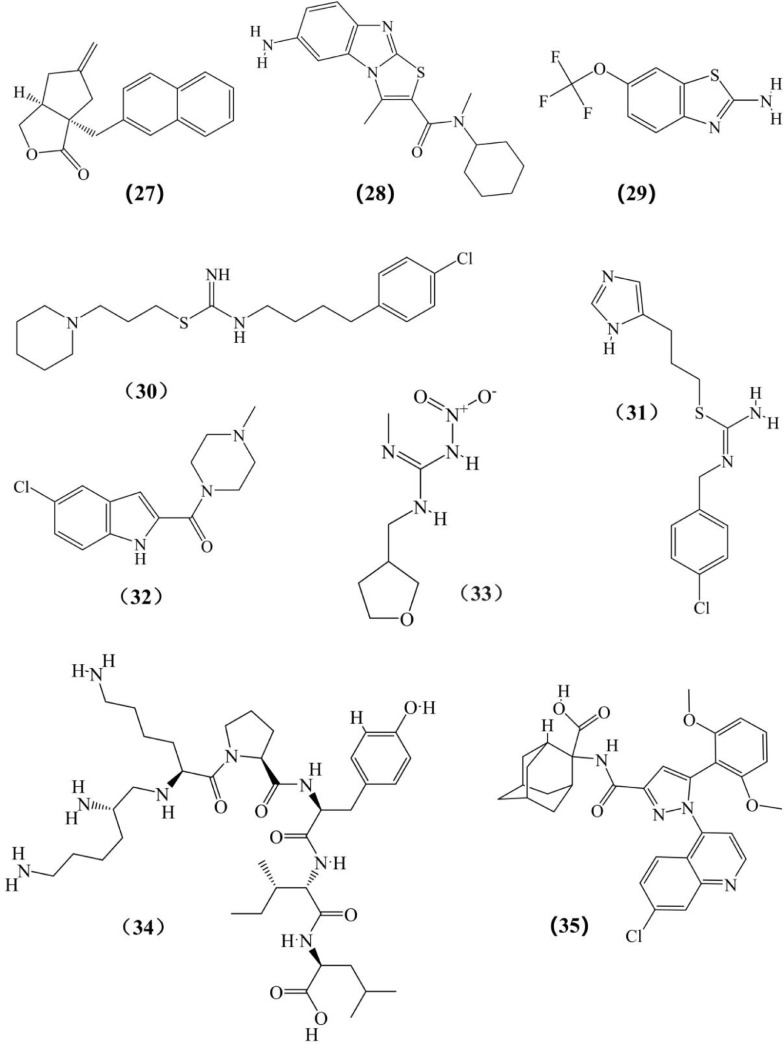
Structures of other chemicals expected to reverse or interfere in the occurrence and development of breast cancer which listed in Tables III to VII. (1) Fenoldopam; (2) l-Stepholidine; (3) A77636; (4) C17; (5) Thioridazine; (6) Sulpiride; (7) Bromocriptine; (8) Doxazosin; (9) Rauwolscine; (10) Yohimbine (11) Tramadol; (12) Propranolol; (13) Quercetin 3-O-glucuronide; (14) ICI 118,551; (15) Atenolol; (16) Isoproterenol; (17) SB269970; (18) 4-DAMP; (19) BJ-1113; (20) Cevimeline; (21) Garcinol; (22) Darifenacin; (23) Luteolin; (24) EGCG; (25) Quercetin; (26) YM 298,198; (27) BAY36–7620; (28) YM 298,198; (29) Riluzole; (30) OUP-186; (31) Clobenpropit; (32) JNJ7777120; (33) Scorpion venom; (34) JMV449; (35) SR-48,692.
Fig. 4.
The NTS/NTSR1 signaling pathway mediates the occurrence and development of breast cancer.
Thus, NTSR1 can serve as an important target for breast cancer diagnosis and treatment, but many of its mechanisms have not yet been fully determined. For example, it is not understood if other molecules are involved in the invasion and metastasis of NTS–NTSR1 mediated by ECM degrading enzymes. In various NTSR1-positive malignant tumors, such as pancreatic and breast cancers, NTS usually regulates the growth and proliferation of tumor cells through an autocrine mechanism. Therefore, it has been proposed that the stability of NTS mediated by MMPs may be a new regulation mode of tumor cells [24]. Then, the question arises whether the elevated MMP level in the process of NTS-NTSR1 promoting breast cancer invasion and metastasis causes the degradation of NTS and terminate this signal. Studies have found that the regulation of NTS stability by MMP in tumor cells may be more complicated and that the regulation of NTS by MMP may be related to the degree of dependence of tumor cells on sex hormones [44,124]. Then, is the role of MMPs positive or negative and what is the relationship between MMPs and the regulation of estrogen signaling? These questions need to be further investigated. Table 2 summarizes the roles and molecular mechanisms of the above neurotransmitters in promoting breast cancer during chronic stress.
Table 2.
Effects of neurotransmitters on breast cancer in chronic stress.
| Category | Neurotransmitter | Changes in content | Effects on breast cancer cells | Mechanism |
|---|---|---|---|---|
| Biogenic amines | α-Epinephrine | ΑDR-α1↑ ΑDR-α2↑ |
Proliferation↑, resistance to paclitaxel | Ras/Raf/MAPK; PKA/Src/Erk |
| β-Epinephrine | ADR-β1↑ ADR-β2↑ |
Stem cell-like properties↑, migration↑, invasion↑, resistance to trastuzumab | β2-AR/PKA/Epac; β2-AR/STAT3/muc1; PI3K/Akt/miR-199a/b-3p |
|
| DA | DRD2↑ DRD4↑ |
Apoptosis↑ | — | |
| NE | ↑ | Adhesion↑, proliferation↑, migration↑, invasion↑, angiogenesis↑, lymphangiogenesis↑ | β3-AR/cAMP/Epac/c-Jun; β−2AR/cAMP/Epac/Rap; ADRB2/Src/FAK; β2-AR/PKA/mTOR |
|
| 5-HT | 5-HT3↓ 5-HTR7↓ |
Proliferation↑, Migration↑, Invasion↑ | — | |
| Amino acids | GABA | GABAB1e↑ | Stem cell-like properties↑, migration↑, invasion↑ | Ras/MAPK; PI3K/Akt |
| ACh | α7-nAChR↑ α9-nAChR↑ |
Proliferation↑, migration↑, invasion↑, Angiogenesis↑ | Upregulated phosphorylation level of ERK1/2 | |
| mGluR | ↑ | Proliferation↑, angiogenesis↑ | mGluR1 signaling pathway | |
| HA | ↑ | Migration↑, invasion↑ | TGF-β/Smad | |
| Peptides | NPY | Y5↑ | Proliferation↑, angiogenesis↑ | — |
| NTS | NTSR1↑ | Proliferation↑, migration↑, invasion↑ | NTS/NTSR1; NF-κB/IGF-1R/PI3K/Akt; PKC/MAPK |
Research progress of the treatment of breast cancer by drugs affecting the neuroendocrine system
DR agonists/antagonists
Currently, most antipsychotic drugs are DR antagonists. Antipsychotic drugs block D2 receptors to cause hyperprolactinemia, which plays an important role in the occurrence and development of breast cancer. This preliminarily explains the relationship between antipsychotic drugs and breast cancer ([95,116]). For example, the antipsychotic drug methionidazine significantly promoted apoptosis of MDA-MB-231 and MCF-7 cells and inhibited tumor growth in a mouse model of breast cancer [157]. Wang et al. [148] found that in the sunitinib (receptor tyrosine kinase inhibitor)-resistant breast cancer cell line MCF-7, DA can cooperate with sunitinib to inhibit the proliferation of drug-resistant breast cancer cells. The inhibitory effect was also observed in a xenograft mouse model. In addition, thioridazine, a DR antagonist, in combination with doxorubicin significantly inhibited the growth of breast cancer cells compared with doxorubicin alone [70]. Li et al. [82] found that although the oral DRD2 antagonist sulpiride alone had no inhibitory effect on tumor growth in MCF-7 cell xenograft models; the inhibitory effect was obvious when combined with dexamethasone and the combined treatment could significantly reduce the expression of MMP2 in xenograft tumor while increasing the expression of E-cadherin, thereby lowering the incidence of pulmonary metastasis in animal models. The addition of specific DRD2 agonists in combination therapy can reverse this inhibitory effect, indicating that sulpiride can significantly improve the efficacy of dexamethasone in the treatment of drug-resistant and metastatic breast cancer through the antagonism of DRD2. In summary, the DR signaling pathway and its expression levels are closely related to the occurrence and development of breast cancer. The following section summarizes the anti-breast cancer drugs and their mechanism of action through the DR signaling pathway (Table 3).
Table 3.
DR agonist/antagonists.
| Subtype | Drug | Material basis | Model | Effect | Mechanism | References |
|---|---|---|---|---|---|---|
| DR agonists | Fenoldopam | 1 | MDA-MB-231 cells BALB/c mice | Suppressed cell viability, inhibited invasion, and induced apoptosis in multiple breast cancer cell lines | Inhibited the cGMP/protein kinase G (PKG) pathway | [18] |
| l-stepholidine (l-SPD) | 2 | Pharmacodynamics, PD model | The reduction in the number of lung metastases was approximately proportional to the square of the drug dose | Inhibited the emission rate of tumor cells in situ to inhibit metastasis | [156] | |
| A77636 | 3 | MDA-MB-231 cells 4T1 cells BALB/c mice |
Inhibited the proliferation of breast cancer cells and the bone metastasis of breast cancer mice | The expression of activated T-nuclear factor 1 was downregulated to inhibit the development of osteoclasts, reduce osteolytic lesions, and improve the bone density of the femur and tibia | [92] | |
| C17 | 4 | 4T1 cells BALB/c mice |
Inhibited lung metastasis in 4TL in situ mouse model of breast cancer | Upregulated E-cadherin, downregulated Snail and N-cadherin | ||
| Bromocriptine | 7 | MCF-7 cells | Inhibited cell proliferation | Activated DRD2 | [109] | |
| DR antagonist | Thioridazine | 5 | MDA-MB-231 cells MCF-7 cells BALB/c mice | Promoted cell apoptosis and inhibited tumor growth in mouse models of breast cancer | Upregulated E-cadherin and downregulated β-catenin | [157] |
| Sulpiride | 6 | MCF-7 cells | There was no inhibitory effect of single use, but the inhibitory effect was obvious when combined with dexamethasone | Downregulated MMP2 and upregulated E-cadherin | [82] |
DR-α antagonists
ΑDR-α promote the proliferation and invasion of breast cancer cells. Therefore, ΑDR-α antagonists may serve as drugs for the treatment of breast cancer. Vázquez et al. [145] evaluated the effects of E, NE, yohimbine, and the synthetic ΑDR-α agonist clonidine on the growth of three breast cancer cell lines, ER-negative breast epithelial cell line (MCF10Α), ER-positive breast cancer cell line (MCF-7), and TNBC cell line (MDA-MB-231). Clonidine stimulated the proliferation of MCF10-A, MCF-7, and MDA-MB-231 cells, whereas yohimbine and rauphin, ΑDR-α2 specific antagonists, completely reversed the cell proliferation induced by clonidine. Xia et al. [153] reported that tramadol could inhibit the proliferation, migration, and invasion of breast cancer cell lines through ΑDR-α2. The ΑDR-α1 antagonist doxazosin could reduce the expression of phosphorylated EGFR, the level of pERK1/2, and the transcriptional activities mediated by NF-κB, AP-1, SRE, E2F, and CRE. EGF or TNF-α treatment alone could not block breast cancer cell apoptosis mediated by doxazosin, but combined treatment of EGF and TNF-α could completely block breast cancer cell apoptosis mediated by doxazosin. This shows that doxazosin can induce breast cancer cell apoptosis by blocking EGFR and the NF-κB signal transduction pathway. Therefore, researchers believe that doxazosin can be a potential drug for breast cancer treatment. The following section summarizes the research progress of ΑDR-α2 antagonists in the treatment of breast cancer (Table 4).
Table 4.
ΑDR-α antagonists.
| Subtype | Drug | Material basis | Model | Effect | Mechanism | References |
|---|---|---|---|---|---|---|
| ΑDR-α1 antagonists | Doxazosin | 8 | MDA-MB-231 MCF-7 MCF12A |
Induced apoptosis | Blocked EGFR and NF-κB signaling | [63] |
| ΑDR-α2 antagonists | Rauwolscine | 9 | MC4-L5 Mouse tumor C4-HD |
Inhibited the proliferation | [145] | |
| Yohimbine | 10 | MCF-7 SCID mice |
Reversed cell proliferation induced by clonidine | Enhanced the pump function of P-glycoprotein and confers resistance of MCF-7 cells to paclitaxel | [139] | |
| Tramadol | 11 | MDA-MB-231 BALB/c mice |
Inhibited proliferation, migration, and invasion | Reduced ERK expression | [153] |
ΑDR-β2 antagonists
The high expression of ΑDR-β in some breast cancer cell types affects the recurrence and metastasis of breast cancer, suggesting that blocking the ΑDR-β pathway is an effective strategy to slow down the progression of breast cancer [105]. A meta-analysis by Wang et al., a retrospective analysis by Zhao et al., a systematic analysis of cohort studies of breast cancer by Childers et al., and a clinical study analysis by Choy evaluated the relationship between ΑDR-β antagonists and breast cancer prognosis [32,33,149,165]. The results showed that BBS significantly reduced the risk of recurrence and distant metastasis, but most of these studies involved small samples and the follow-up time was relatively short; thus, larger studies are needed to further explore the relationship between BBS and breast cancer prognosis.
At present, PROP, labetalol, metoprolol, bisoprolol, and atenolol are commonly used clinical ΑDR-β antagonists in the treatment of heart diseases such as hypertension and arrhythmia. Recent studies have suggested a correlation between ΑDR-β antagonists and the prognosis of breast cancer. PROP is well tolerated and has high safety. It has been clinically used to treat infantile hemangioma, and a phase II clinical trial of its use for the treatment of breast cancer is underway. PROP is used in combination with chemotherapy drugs to prevent cardiotoxicity caused by anthracyclines and inhibit breast cancer metastasis induced by SNS activation caused by postoperative stress [80]. PORP can inhibit the infiltration of macrophages in the tumor parenchyma to a large extent, indicating that ΑDR-β signaling plays an important role in stress-mediated tumor metastasis. PROP can also inhibit glucose metabolism and uptake in 4T1 breast cancer cells. By inhibiting the ΑDR-β signal, PROP can reduce the Ki-67 proliferation index of breast cancer cells, reduce the expression of various cyclins, and enhance the expression of the tumor suppressor gene p53 protein in the cells. It has also been shown to induce apoptosis of breast cancer cells and significantly inhibit tumor growth in patients with advanced breast cancer [96]. Rivero et al. demonstrated that β-blockers can effectively reduce the number of lung metastases and tumor size of triple-negative breast cancer cells by simulating an MDA-MB-231 cell metastasis model in NSG-immunodeficient mice. The following section summarizes the research progress of ΑDR-β2 antagonists in the treatment of breast cancer (Table 5).
Table 5.
ΑDR-β2 antagonists.
| Subtype | Drug | Material basis | Breast cancer cell line | Effect | Mechanism | References |
|---|---|---|---|---|---|---|
| Natural products that inhibit ADR-β2 | Quercetin-3-O-glucuronide (Q3G) | 13 | MDA-MB-231 | Suppressed cell invasion and MMP-9 induction and inhibited the binding of [3H]-NA to ADR-β2 | Suppressed ROS generation, cAMP and RAS activation, phosphorylation of ERK1/2, and HMOX1, MMP2, and MMP9 gene expression | [155] |
| ADR-β2 antagonists | Propranolol | 12 | MDA-MB-435 MDA-MB-231 |
Inhibited the viability of cells, arrested cell cycle progression at G0/G1 and S phase, and induced cell apoptosis | ERK1/2 and COX-2 phosphorylation levels were significantly decreased after β-blocker treatment. | [154] |
| ICI 118,551 | 14 | MDA-MB-48 | ||||
| Atenolo | 15 | MDA-MB-453 | Inhibited cell proliferation | [21] | ||
| Isoproterenol | 16 | MDA-MB-231 ZR-75 |
Inhibited cell proliferation | Inhibited DNA synthesis | [129] |
Other receptor antagonists
The following section summarizes the research progress of other neurotransmitter receptors antagonists in the treatment of breast cancer (Table 6).
Table 6.
Other receptor antagonists.
| Subtype | Drug | Material basis | Model | Effect | Mechanism | References |
|---|---|---|---|---|---|---|
| 5-HT antagonists | SB269970 | 17 | MCF-7 T47D MDA-MB-231 |
Antiproliferation and anti-invasion | Gα-activated cAMP and Gβγ-activated kinase signaling during invasion and Gβγ-activated PI3K/Akt signaling during proliferation | [50] |
| BJ-1113 | 19 | MCF-7 MDA-MB-231 |
The inhibitory effect of BJ-1113 against MDA-MB-231 tumor growth was greater than that of SB269970, a 5-HT7 receptor antagonist | |||
| mAChR antagonists | Cevimeline | 20 | MCF-7 MDA-MB-231 |
Inhibited cell proliferation | PLC activation | [30] |
| 4-DAMP | 18 | MCF-7 MDA-MB-231 |
Inhibited cell proliferation | PLC activation | [69,120,123] | |
| Darifenacin | 22 | MCF-7 MDA-MB-231 |
Inhibited cell proliferation | PLC activation | [86,99,106] | |
| nAChR antagonists | Epigallocatechin-3-gallate (EGCG) |
24 | MCF-7 | Inhibited cell proliferation | Blocking α9-nAChR signaling pathway | [144] |
| Garcinol | 21 | MCF-10A MDA-MB-231 NOD.CB17-PRKDC (SCID) |
Inhibited cell proliferation | Downregulate the expression of α9-nAChR and cyclin D3 protein | [27] | |
| mGluR1-specific antagonists | Riluzole | 29 | Inhibited cell proliferation in a dose-response manner in all EC types | Inhibition of angiogenesis | [41,136] | |
| BAY36–7620 | 27 | [25,137] | ||||
| YM 298,198 | 28 | MCF-7 | Inhibited cell proliferation in all the cell types, except HUVEC | Inhibition of angiogenesis | [135] | |
| H3R antagonists | OUP-186 | 30 | MDA-MB-231 MCF-7 |
Induced breast cancer cell death | Activated caspase-3/7 | [142] |
| Clobenpropit | 31 | MDA-MB-231 MCF-7 |
Cell death was only slightly induced | Activated caspase-3/7 | ||
| JNJ7777120 | 32 | MDA-MB-231 MCF-7 |
Inhibited cell migration and invasion | Reduced E-cadherin, cytoplasmic and nuclear β-catenin, and nuclear Slug and an increase in vimentin and α-smooth muscle actin expression | [48] | |
| Peptide neurotransmitter antagonists | Scorpion venom | 33 | MDA-MB-231 | Reduced motility and invasion in breast cancer cells | Inhibited MMP activity; upregulation of p53 and downregulation of Bcl-xL and BID protein expression by modulating signaling proteins Erk1/2 and STAT3 and DNA damage in breast and colorectal cancer cell lines | [4,5] |
| NTS agonist | JMV449 | 34 | MCF-7 | Promoted apoptosis | MAPK activation caused the expression of B-cell lymphoma-2 (BCL-2) | [131] |
| NTS antagonist | SR48692 | 35 | [77] |
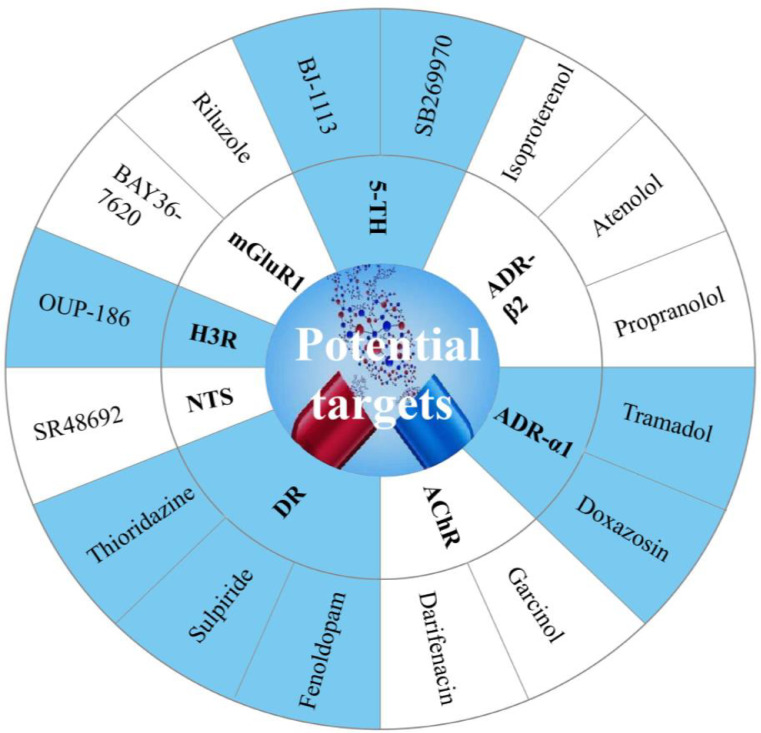
In summary, we highlight in the Fig. 5 which are the potential targets for drug development.
Nonpharmacological interventions to reverse chronic stress
Tumor treatment has already entered the era of comprehensive treatment, and exploratory research on stress management strategies is extremely challenging. Studies have suggested that adding medical and psychological intervention and cognitive-behavioral stress management to the treatment of patients with cancer can reduce the patients’ stress level and improve their quality of life, thereby improving clinical efficacy [15,146]. Benign stress often brings beneficial effects, and it is no exception for malignant tumors. The elevated levels of adrenaline and IL-6 induced by exercise can lead to tumor growth through the mobilization and redistribution of NK cells [65,104]. Results from mice models also verified that the benign stress of pancreatic cancer, lung cancer, and melanoma lung metastasis can stabilize the expression levels of CCR5 and NKG2D in NK cells through the activation of sympathetic nerves, thereby enhancing their antitumor effects [132]. Clinical studies have shown that behaviors such as mindfulness, meditation, and yoga are effective in reducing chronic stress and improving the quality of life of patients with cancer [11,115,122]. In short, a healthy lifestyle, a positive life attitude, and professional stress management guidance are essential for the prevention and treatment of tumors.
The COVID-19 pandemic continues to affect our lives and has led to several inconveniences for patients with breast cancer; this has made them prone to negative emotions, such as anxiety and depression, which affects the effectiveness of treatment. Strengthening the psychological care of patients undergoing chemotherapy after breast cancer surgery can relieve their distress and help them successfully complete the course of chemotherapy. One of the consequences of the COVID-19 pandemic is the need to prevent infections and delay in breast cancer screenings. The Canadian Association of Breast Imaging/Canadian Association of Radiologists has developed safety guidelines for breast imaging and concluded that delaying breast cancer screening should be avoided if possible even during the COVID-19 pandemic.
Summary and prospects
In the personalized treatment of patients with breast cancer, the emergence of chronic stress is always accompanied by neurological disorders and disorders that affect the prognosis of patients to varying degrees. Independently existing psychological problems are unrelated to the technical problems involved in the treatment process. Existing treatments for breast cancer may not reduce the continuous stress faced by patients due to the psychological effects. Considering that the mechanism of chronic stress promoting the occurrence and evolution of breast cancer has so far not been systematically and comprehensively revealed, this article reviews the research on the correlation between chronic stress and the occurrence and development of breast cancer. The molecular mechanism by which chronic stress promotes breast cancer development through related neurotransmitters progress in elucidating potential drugs or blockers in the treatment of breast cancer have been emphasized. Novel ideas for the prevention and treatment of breast cancer in clinical practice, and the theoretical basis for the use of classical blockers in the treatment of breast cancer have been discussed. One interesting finding of this review is that drugs used to treat mental illness are implicated in playing an important role in the treatment of breast cancer, thereby expanding the new uses of classical drugs (Fig. 6).
Fig. 6.
Potential drug development for future treatment of breast cancer.
We should pay more attention to the psychological state of patients and put forth scientific and reasonable stress management strategies. We advocate timely and targeted psychological interventions for patients with breast cancer with depressive symptoms. For example, meditation, exercise, and cognitive-behavioral therapy may become a feasible method for the treatment of patients with cancer and help to improve the psychological state of patients. Paying attention to the clinical manifestations of depressive symptoms in patients with breast cancer and improving the effectiveness of antitumor treatment for patients with breast cancer will undoubtedly provide novel therapeutic strategies for breast cancer treatment. We also want to attract the readers’ attention to the fact that drugs used to treat mental illness are implicated in playing an important role in the treatment of breast cancer, thereby expanding the new uses of classical drugs.
There are still many unsolved problems regarding the role of neurotransmitters in tumor genesis and development: (1) Are the mechanisms of neurotransmitters in different types of breast cancer the same? (2) How do neurotransmitters participate in the regulation of tumor cells through other cells (such as fibroblasts, endothelial cells, and immune cells) in the tumor microenvironment? (3) Glucocorticoids are often clinically used to reduce the side effects of chemotherapy in patients with cancer. Will this have an adverse effect on cancer treatment? (4) Is there any connection between the inflammatory response and neurotransmitters in tumors? The effect of the nervous system on breast cancer is a non-negligible factor, and it is evident that different neurotransmitters have different mechanisms of action in different types of breast cancer, ultimately adding to the complexity of this relationship. This will be the focus of our future studies.
CRediT authorship contribution statement
Hui-min Liu: Writing – review & editing, Visualization, Writing – original draft. Le-le Ma: Writing – review & editing, Visualization. Chunyu Li: Investigation, Writing – review & editing. Bo Cao: Writing – review & editing, Visualization. Yifang Jiang: Investigation, Writing – review & editing. Li Han: Writing – original draft. Runchun Xu: Investigation, Writing – review & editing. Junzhi Lin: Writing – original draft. Dingkun Zhang: Investigation, Writing – review & editing.
CRediT authorship contribution statement
Hui-min Liu: Writing – review & editing, Visualization, Writing – original draft. Le-le Ma: Writing – review & editing, Visualization. Chunyu Li: Investigation, Writing – review & editing. Bo Cao: Writing – review & editing, Visualization. Yifang Jiang: Investigation, Writing – review & editing. Li Han: Writing – original draft. Runchun Xu: Investigation, Writing – review & editing. Junzhi Lin: Writing – original draft. Dingkun Zhang: Investigation, Writing – review & editing.
Declaration of Competing Interest
No author has an actual or perceived conflict of interest with the contents of this article.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China [81873232] and the Innovative Research Team Project of Chinese Medicine Discipline in Chengdu University of Traditional Chinese Medicine [CXTD2018006].
Footnotes
Chemical compounds studied in this article: Fenoldopam (PubChem CID: 3341); l-Stepholidine (PubChem CID: 6,917,970); A77636 (PubChem CID: 16,069,211); Bromocriptine (PubChem CID: 31,101); Thioridazine (PubChem CID: 5452); Sulpiride (PubChem CID: 5355); Doxazosin (PubChem CID: 3157); Rauwolscine (PubChem CID: 643,606); Yohimbine (PubChem CID: 8969); Tramadol (PubChem CID: 33,741); Quercetin 3-O-glucuronide (PubChem CID: 5,274,585); Propranolol (PubChem CID: 4946); ICI 118,551 (PubChem CID: 25,102,594); Atenolol (PubChem CID: 2249); Isoproterenol (PubChem CID: 3779); SB269970 (PubChem CID: 6,604,889); Cevimeline (PubChem CID: 83,898); 4-DAMP (PubChem CID: 3,014,059); Darifenacin (PubChem CID: 444,031); epigallocatechin-3-gallate, EGCG (PubChem CID: 65,064); garcinol (PubChem CID: 5,490,884); luteolin (PubChem CID: 5,280,445); quercetin (PubChem CID: 5,280,343); Riluzole (PubChem CID: 5070); BAY36–7620 (PubChem CID: 9,903,757); YM 298,198 (PubChem CID: 45,073,464); clobenpropit (PubChem CID: 2790); JNJ7777120 (PubChem CID: 4,908,365); Scorpion venom (PubChem CID: 197,701); JMV449 (PubChem CID: 164,415); SR-48,692 (PubChem CID: 119,192)
Contributor Information
Runchun Xu, Email: wsxrch@qq.com.
Junzhi Lin, Email: linjunzhi@cdutcm.edu.cn.
Dingkun Zhang, Email: zhangdingkun@cdutcm.edu.cn.
References
- 1.Acosta J.J., Muñoz R.M., González L., et al. Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol. Endocrinol. 2003;17:2268–2282. doi: 10.1210/me.2002-0422. [DOI] [PubMed] [Google Scholar]
- 2.Ahles A., Engelhardt S. Polymorphic variants of adrenoceptors: pharmacology, physiology, and role in disease. Pharmacol. Rev. 2014;66:598–637. doi: 10.1124/pr.113.008219. [DOI] [PubMed] [Google Scholar]
- 3.Akbari M.E., Kashani F.L., Ahangari G., et al. The effects of spiritual intervention and changes in dopamine receptor gene expression in breast cancer patients. Breast Cancer. 2016;23:893–900. doi: 10.1007/s12282-015-0658-z. [DOI] [PubMed] [Google Scholar]
- 4.Al-Asmari A.K., Islam M., Al-Zahrani A.M. In vitro analysis of the anticancer properties of scorpion venom in colorectal and breast cancer cell lines. Oncol. Lett. 2016;11:1256–1262. doi: 10.3892/ol.2015.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Asmari A.K., Riyasdeen A., Islam M. Scorpion venom causes upregulation of p53 and downregulation of Bcl-x (L) and BID protein expression by modulating signaling proteins Erk (1/2) and STAT3, and DNA damage in breast and colorectal cancer cell lines. Integr. Cancer Ther. 2018;17:271–281. doi: 10.1177/1534735417704949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander S.P., Benson H.E., Faccenda E., et al. The Concise guide to pharmacology 2013/14: g protein-coupled receptors. Br. J. Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen J.K., Armaiz-Pena G.N., Nagaraja A.S., et al. Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res. 2018;78:3233–3242. doi: 10.1158/0008-5472.Can-16-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anbalagan M., Rowan B.G. Estrogen receptor alpha phosphorylation and its functional impact in human breast cancer. Mol. Cell Endocrinol. 2015;418:264–272. doi: 10.1016/j.mce.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Andereggen L., Frey J., Andres R.H., et al. 10-year follow-up study comparing primary medical vs. surgical therapy in women with prolactinomas. Endocrine. 2017;55:223–230. doi: 10.1007/s12020-016-1115-2. [DOI] [PubMed] [Google Scholar]
- 10.Antoni M.H., Dhabhar F.S. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019;125:1417–1431. doi: 10.1002/cncr.31943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araujo R.V., Fernandes A.F.C., Nery I.S., et al. Meditation effect on psychological stress level in women with breast cancer: a systematic review. Rev. Esc. Enferm. USP. 2019;53:3529–3540. doi: 10.1590/s1980-220x2018031303529. [DOI] [PubMed] [Google Scholar]
- 12.Bakhtou H., Olfatbakhsh A., Deezagi A., et al. The expression of dopamine receptors gene and their potential role in targeting breast cancer cells with selective agonist and antagonist drugs. Could it be the novel insight to therapy? Curr. Drug Discov. Technol. 2019;16:184–197. doi: 10.2174/1570163815666180130101421. [DOI] [PubMed] [Google Scholar]
- 13.Bakirtzi K., Hatziapostolou M., Karagiannides I., et al. Neurotensin signaling activates microRNAs-21 and -155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology. 2011;141:1749–1761. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banasr M., Lepack A., Fee C., et al. Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress (Thousand Oaks) 2017;1 doi: 10.1177/2470547017720459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barre P.V., Padmaja G., Rana S., et al. Stress and quality of life in cancer patients: medical and psychological intervention. Indian J. Psychol. Med. 2018;40:232–238. doi: 10.4103/ijpsym.Ijpsym_512_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrenbruch C., Shembrey C., Paquet-Fifield S., et al. Surgical stress response and promotion of metastasis in colorectal cancer: a complex and heterogeneous process. Clin. Exp. Metastasis. 2018;35:333–345. doi: 10.1007/s10585-018-9873-2. [DOI] [PubMed] [Google Scholar]
- 17.Benarroch E.E. Neuropeptide Y: its multiple effects in the CNS and potential clinical significance. Neurology. 2009;72:1016–1020. doi: 10.1212/01.wnl.0000345258.18071.54. [DOI] [PubMed] [Google Scholar]
- 18.Borcherding D.C., Tong W., Hugo E.R., et al. Expression and therapeutic targeting of dopamine receptor-1 (D1R) in breast cancer. Oncogene. 2016;35:3103–3113. doi: 10.1038/onc.2015.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brothers S.P., Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol. Med. 2010;2:429–439. doi: 10.1002/emmm.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brzozowska A., Burdan F., Duma D., et al. γ-amino butyric acid (GABA) level as an overall survival risk factor in breast cancer. Ann. Agric. Environ. Med. 2017;24:435–439. doi: 10.26444/aaem/75891. [DOI] [PubMed] [Google Scholar]
- 21.Cakir Y., Plummer H.K., Tithof P.K., et al. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int. J. Oncol. 2002;21:153–157. [PubMed] [Google Scholar]
- 22.Calvani M., Bruno G., Dal Monte M., et al. β (3) -Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br. J. Pharmacol. 2019;176:2509–2524. doi: 10.1111/bph.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannon D.M., Klaver J.M., Peck S.A., et al. Dopamine type-1 receptor binding in major depressive disorder assessed using positron emission tomography and [11C]NNC-112. Neuropsychopharmacology. 2009;34:1277–1287. doi: 10.1038/npp.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carraway R.E., Plona A.M. Involvement of neurotensin in cancer growth: evidence, mechanisms and development of diagnostic tools. Peptides. 2006;27:2445–2460. doi: 10.1016/j.peptides.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Carroll F.Y., Stolle A., Beart P.M., et al. BAY36-7620: a potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol. Pharmacol. 2001;59:965–973. [PMC free article] [PubMed] [Google Scholar]
- 26.Chang H.J., Yoo B.C., Lim S.B., et al. Metabotropic glutamate receptor 4 expression in colorectal carcinoma and its prognostic significance. Clin. Cancer Res. 2005;11:3288–3295. doi: 10.1158/1078-0432.Ccr-04-1912. [DOI] [PubMed] [Google Scholar]
- 27.Chen C.S., Lee C.H., Hsieh C.D., et al. Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins. Breast Cancer Res. Treat. 2011;125:73–87. doi: 10.1007/s10549-010-0821-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen H., Liu D., Guo L., et al. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J. Pathol. 2018;244:49–60. doi: 10.1002/path.4988. [DOI] [PubMed] [Google Scholar]
- 29.Chen H., Liu D., Yang Z., et al. Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocr. Relat. Cancer. 2014;21:783–795. doi: 10.1530/erc-14-0236. [DOI] [PubMed] [Google Scholar]
- 30.Chen J., Cheuk I.W.Y., Shin V.Y., et al. Acetylcholine receptors: key players in cancer development. Surg. Oncol. 2019;31:46–53. doi: 10.1016/j.suronc.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Cao Q., Liao R., et al. Loss of ABAT-mediated gabaergic system promotes basal-like breast cancer progression by activating Ca2+-NFAT1 Axis. Theranostics. 2019;9:34–47. doi: 10.7150/thno.29407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Childers W.K., Hollenbeak C.S., Cheriyath P. β-Blockers reduce breast cancer recurrence and breast cancer death: a meta-analysis. Clin. Breast Cancer. 2015;15:426–431. doi: 10.1016/j.clbc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Choy C., Raytis J.L., Smith D.D., et al. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: the potential benefit of perioperative β-blockade. Oncol. Rep. 2016;35:3135–3142. doi: 10.3892/or.2016.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 35.Clément-Demange L., Mulcrone P.L., Tabarestani T.Q., et al. β2ARs stimulation in osteoblasts promotes breast cancer cell adhesion to bone marrow endothelial cells in an IL-1β and selectin-dependent manner. J. Bone Oncol. 2018;13:1–10. doi: 10.1016/j.jbo.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole S.W., Sood A.K. Molecular pathways: beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.Ccr-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa R., Santa-Maria C.A., Scholtens D.M., et al. A pilot study of cabergoline for the treatment of metastatic breast cancer. Breast Cancer Res. Treat. 2017;165:585–592. doi: 10.1007/s10549-017-4370-x. [DOI] [PubMed] [Google Scholar]
- 38.Cui B., Luo Y., Tian P., et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Invest. 2019;129:1030–1046. doi: 10.1172/jci121685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Giorgi V., Grazzini M., Benemei S., et al. β-Blocker use and reduced disease progression in patients with thick melanoma: 8 years of follow-up. Melanoma Res. 2017;27:268–270. doi: 10.1097/cmr.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 40.Decressac M., Barker R.A. Neuropeptide Y and its role in CNS disease and repair. Exp. Neurol. 2012;238:265–272. doi: 10.1016/j.expneurol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Dolfi S.C., Medina D.J., Kareddula A., et al. Riluzole exerts distinct antitumor effects from a metabotropic glutamate receptor 1-specific inhibitor on breast cancer cells. Oncotarget. 2017;8:44639–44653. doi: 10.18632/oncotarget.17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubrovina N.I., Zinov'eva D.V. [Effects of activation and blockade of dopamine receptors on extinction of passive avoidance response in mice with depressive-like state] Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova. 2008;58:605–610. [PubMed] [Google Scholar]
- 43.Duman R.S., Sanacora G., Krystal J.H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102:75–90. doi: 10.1016/j.neuron.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dupouy S., Mourra N., Doan V.K., et al. The potential use of the neurotensin high affinity receptor 1 as a biomarker for cancer progression and as a component of personalized medicine in selective cancers. Biochimie. 2011;93:1369–1378. doi: 10.1016/j.biochi.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Dupouy S., Viardot-Foucault V., Alifano M., et al. The neurotensin receptor-1 pathway contributes to human ductal breast cancer progression. PLoS ONE. 2009;4:4223–4234. doi: 10.1371/journal.pone.0004223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Marroun H., White T.J., Fernandez G., et al. Prenatal exposure to selective serotonin reuptake inhibitors and non-verbal cognitive functioning in childhood. J. Psychopharmacol. 2017;31:346–355. doi: 10.1177/0269881116665335. [DOI] [PubMed] [Google Scholar]
- 47.Gad A.A., Balenga N. The emerging role of adhesion GPCRs in cancer. ACS Pharmacol. Transl. Sci. 2020;3:29–42. doi: 10.1021/acsptsci.9b00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galarza T.E., Táquez Delgado M.A., Mohamad N.A., et al. Histamine H4 receptor agonists induce epithelial-mesenchymal transition events and enhance mammosphere formation via Src and TGF-β signaling in breast cancer cells. Biochem. Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114177. [DOI] [PubMed] [Google Scholar]
- 49.Gankhuyag N., Lee K.H., Cho J.Y. The role of nitrosamine (NNK) in breast cancer carcinogenesis. J. Mammary Gland Biol. Neoplas. 2017;22:159–170. doi: 10.1007/s10911-017-9381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautam J., Banskota S., Regmi S.C., et al. Tryptophan hydroxylase 1 and 5-HT (7) receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol. Cancer. 2016;15:75. doi: 10.1186/s12943-016-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giessler C., Wangemann T., Silber R.E., et al. Noradrenaline-induced contraction of human saphenous vein and human internal mammary artery: involvement of different alpha-adrenoceptor subtypes. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:104–109. doi: 10.1007/s00210-002-0582-6. [DOI] [PubMed] [Google Scholar]
- 52.Grando S.A. Connections of nicotine to cancer. Nat. Rev. Cancer. 2014;14:419–429. doi: 10.1038/nrc3725. [DOI] [PubMed] [Google Scholar]
- 53.Gumireddy K., Li A., Kossenkov A.V., et al. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat. Commun. 2016;7:10715. doi: 10.1038/ncomms10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harikumar K.B., Kunnumakkara A.B., Ochi N., et al. A novel small-molecule inhibitor of protein kinase D blocks pancreatic cancer growth in vitro and in vivo. Mol. Cancer Ther. 2010;9:1136–1146. doi: 10.1158/1535-7163.Mct-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatzikirou H., Basanta D., Simon M., et al. Go or grow': the key to the emergence of invasion in tumour progression? Math. Med. Biol. 2012;29:49–65. doi: 10.1093/imammb/dqq011. [DOI] [PubMed] [Google Scholar]
- 56.Havrda M. The purpose of galen's treatise on demonstration. Early Sci. Med. 2015;20:265–287. doi: 10.1163/15733823-00203p03. [DOI] [PubMed] [Google Scholar]
- 57.Hecht I., Natan S., Zaritsky A., et al. The motility-proliferation-metabolism interplay during metastatic invasion. Sci. Rep. 2015;5:13538. doi: 10.1038/srep13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Hirata N., Sekino Y., Kanda Y. Nicotine increases cancer stem cell population in MCF-7 cells. Biochem. Biophys. Res. Commun. 2010;403:138–143. doi: 10.1016/j.bbrc.2010.10.134. [DOI] [PubMed] [Google Scholar]
- 60.Hoyer D. Targeting the 5-HT system: potential side effects. Neuropharmacology. 2020;179 doi: 10.1016/j.neuropharm.2020.108233. [DOI] [PubMed] [Google Scholar]
- 61.Hu L., Wang B., Zhang Y. Serotonin 5-HT6 receptors affect cognition in a mouse model of Alzheimer's disease by regulating cilia function. Alzheimers Res. Ther. 2017;9:76. doi: 10.1186/s13195-017-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Q., Tan Q., Mao K., et al. The role of adrenergic receptors in lung cancer. Am. J. Cancer Res. 2018;8:2227–2237. [PMC free article] [PubMed] [Google Scholar]
- 63.Hui H., Fernando M.A., Heaney A.P. The alpha1-adrenergic receptor antagonist doxazosin inhibits EGFR and NF-kappaB signalling to induce breast cancer cell apoptosis. Eur. J. Cancer. 2008;44:160–166. doi: 10.1016/j.ejca.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Hylander B.L., Eng J.W., Repasky E.A. The impact of housing temperature-induced chronic stress on preclinical mouse tumor models and therapeutic responses: an important role for the nervous system. Adv. Exp. Med. Biol. 2017;1036:173–189. doi: 10.1007/978-3-319-67577-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Idorn M., Hojman P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol. Med. 2016;22:565–577. doi: 10.1016/j.molmed.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Iwabayashi M., Taniyama Y., Sanada F., et al. Role of serotonin in angiogenesis: induction of angiogenesis by sarpogrelate via endothelial 5-HT1B/Akt/eNOS pathway in diabetic mice. Atherosclerosis. 2012;220:337–342. doi: 10.1016/j.atherosclerosis.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 67.Jansen M.P., Sas L., Sieuwerts A.M., et al. Decreased expression of ABAT and STC2 hallmarks ER-positive inflammatory breast cancer and endocrine therapy resistance in advanced disease. Mol. Oncol. 2015;9:1218–1233. doi: 10.1016/j.molonc.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang X., Su L., Zhang Q., et al. GABAB receptor complex as a potential target for tumor therapy. J. Histochem. Cytochem. 2012;60:269–279. doi: 10.1369/0022155412438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiménez E., Montiel M. Activation of MAP kinase by muscarinic cholinergic receptors induces cell proliferation and protein synthesis in human breast cancer cells. J. Cell Physiol. 2005;204:678–686. doi: 10.1002/jcp.20326. [DOI] [PubMed] [Google Scholar]
- 70.Jin X., Zou B., Luo L., et al. Codelivery of thioridazine and doxorubicin using nanoparticles for effective breast cancer therapy. Int. J. Nanomed. 2016;11:4545–4552. doi: 10.2147/ijn.S104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jobling P., Pundavela J., Oliveira S.M., et al. Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res. 2015;75:1777–1781. doi: 10.1158/0008-5472.Can-14-3180. [DOI] [PubMed] [Google Scholar]
- 72.Kafetzopoulou L.E., Boocock D.J., Dhondalay G.K., et al. Biomarker identification in breast cancer: beta-adrenergic receptor signaling and pathways to therapeutic response. Comput. Struct. Biotechnol. J. 2013;6 doi: 10.5936/csbj.201303003. 201303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang F., Ma W., Ma X., et al. Propranolol inhibits glucose metabolism and 18F-FDG uptake of breast cancer through posttranscriptional downregulation of hexokinase-2. J. Nucl. Med. 2014;55:439–445. doi: 10.2967/jnumed.113.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S.Y., Kang K.L., Lee J.C., et al. Nicotinic acetylcholine receptor α7 and β4 subunits contribute nicotine-induced apoptosis in periodontal ligament stem cells. Mol. Cells. 2012;33:343–350. doi: 10.1007/s10059-012-2172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krizanova O., Babula P., Pacak K. Stress, catecholaminergic system and cancer. Stress. 2016;19:419–428. doi: 10.1080/10253890.2016.1203415. [DOI] [PubMed] [Google Scholar]
- 76.Kruk J., Aboul-Enein B.H., Bernstein J., et al. Psychological stress and cellular aging in cancer: a meta-analysis. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1270397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labbé-Jullié C., Barroso S., Nicolas-Etève D., et al. Mutagenesis and modeling of the neurotensin receptor NTR1. Identification of residues that are critical for binding SR 48692, a nonpeptide neurotensin antagonist. J. Biol. Chem. 1998;273:16351–16357. doi: 10.1074/jbc.273.26.16351. [DOI] [PubMed] [Google Scholar]
- 78.Le C.P., Nowell C.J., Kim-Fuchs C., et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lebbe E.K., Peigneur S., Wijesekara I., et al. Conotoxins targeting nicotinic acetylcholine receptors: an overview. Mar. Drugs. 2014;12:2970–3004. doi: 10.3390/md12052970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee A., Djamgoz M.B.A. Triple negative breast cancer: emerging therapeutic modalities and novel combination therapies. Cancer Treat. Rev. 2018;62:110–122. doi: 10.1016/j.ctrv.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 81.Lee C.H., Huang C.S., Chen C.S., et al. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J. Natl. Cancer Inst. 2010;102:1322–1335. doi: 10.1093/jnci/djq300. [DOI] [PubMed] [Google Scholar]
- 82.Li J., Yao Q.Y., Xue J.S., et al. Dopamine D2 receptor antagonist sulpiride enhances dexamethasone responses in the treatment of drug-resistant and metastatic breast cancer. Acta Pharmacol. Sin. 2017;38:1282–1296. doi: 10.1038/aps.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu D., Yang Z., Wang T., et al. β2-AR signaling controls trastuzumab resistance-dependent pathway. Oncogene. 2016;35:47–58. doi: 10.1038/onc.2015.58. [DOI] [PubMed] [Google Scholar]
- 84.Liu H., Wang C., Xie N., et al. Activation of adrenergic receptor β2 promotes tumor progression and epithelial mesenchymal transition in tongue squamous cell carcinoma. Int. J. Mol. Med. 2018;41:147–154. doi: 10.3892/ijmm.2017.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y., Zhang H., Wang Z., et al. 5-Hydroxytryptamine1a receptors on tumour cells induce immune evasion in lung adenocarcinoma patients with depression via autophagy/pSTAT3. Eur. J. Cancer. 2019;114:8–24. doi: 10.1016/j.ejca.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 86.Lombardi M.G., Negroni M.P., Pelegrina L.T., et al. Autoantibodies against muscarinic receptors in breast cancer: their role in tumor angiogenesis. PLoS ONE. 2013;8:e57572. doi: 10.1371/journal.pone.0057572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma H., Huang Y., Zhang B., et al. Association between neurotensin receptor 1 (NTR1) gene polymorphisms and schizophrenia in a Han Chinese population. J. Mol. Neurosci. 2013;50:345–352. doi: 10.1007/s12031-013-9988-5. [DOI] [PubMed] [Google Scholar]
- 88.Maass S., Boerman L.M., Verhaak P.F.M., et al. Long-term psychological distress in breast cancer survivors and their matched controls: a cross-sectional study. Maturitas. 2019;130:6–12. doi: 10.1016/j.maturitas.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Mahar I., Bambico F.R., Mechawar N., et al. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 90.Martinel Lamas D.J., Rivera E.S., Medina V.A. Histamine H4 receptor: insights into a potential therapeutic target in breast cancer. Front. Biosci. 2015;7:1–9. doi: 10.2741/S420. Schol Ed. [DOI] [PubMed] [Google Scholar]
- 91.Medina V.A., Rivera E.S. Histamine receptors and cancer pharmacology. Br. J. Pharmacol. 2010;161:755–767. doi: 10.1111/j.1476-5381.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minami K., Liu S., Liu Y., et al. Inhibitory Effects of Dopamine Receptor D (1) Agonist on Mammary Tumor and Bone Metastasis. Sci. Rep. 2017;7:45686. doi: 10.1038/srep45686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mizoguchi K., Shoji H., Ikeda R., et al. Persistent depressive state after chronic stress in rats is accompanied by HPA axis dysregulation and reduced prefrontal dopaminergic neurotransmission. Pharmacol. Biochem. Behav. 2008;91:170–175. doi: 10.1016/j.pbb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Mohammad-Zadeh L.F., Moses L., Gwaltney-Brant S.M. Serotonin: a review. J. Vet. Pharmacol. Ther. 2008;31:187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 95.Montejo Á.L., Arango C., Bernardo M., et al. Spanish consensus on the risks and detection of antipsychotic drug-related hyperprolactinaemia. Rev. Psiquiatr. Salud Mental. 2016;9:158–173. doi: 10.1016/j.rpsm.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Montoya A., Varela-Ramirez A., Dickerson E., et al. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed. J. 2019;42:155–165. doi: 10.1016/j.bj.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mustain W.C., Rychahou P.G., Evers B.M. The role of neurotensin in physiologic and pathologic processes. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18:75–82. doi: 10.1097/MED.0b013e3283419052. [DOI] [PubMed] [Google Scholar]
- 98.Myers R.M., Shearman J.W., Kitching M.O., et al. Cancer, chemistry, and the cell: molecules that interact with the neurotensin receptors. ACS Chem. Biol. 2009;4:503–525. doi: 10.1021/cb900038e. [DOI] [PubMed] [Google Scholar]
- 99.Negroni M.P., Fiszman G.L., Azar M.E., et al. Immunoglobulin G from breast cancer patients in stage I stimulates muscarinic acetylcholine receptors in MCF7 cells and induces proliferation. Participation of nitric oxide synthase-derived nitric oxide. J. Clin. Immunol. 2010;30:474–484. doi: 10.1007/s10875-010-9370-0. [DOI] [PubMed] [Google Scholar]
- 100.Nicoud M.B., Sterle H.A., Massari N.A., et al. Study of the antitumour effects and the modulation of immune response by histamine in breast cancer. Br. J. Cancer. 2020;122:348–360. doi: 10.1038/s41416-019-0636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Obeid E.I., Conzen S.D. The role of adrenergic signaling in breast cancer biology. Cancer Biomark. 2013;13:161–169. doi: 10.3233/cbm-130347. [DOI] [PubMed] [Google Scholar]
- 102.Ossowska G., Nowa G., Kata R., et al. Brain monoamine receptors in a chronic unpredictable stress model in rats. J. Neural Transm. (Vienna) 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- 103.Pavlov V.A., Ochani M., Yang L.H., et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007;35:1139–1144. doi: 10.1097/01.Ccm.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 104.Pedersen L., Idorn M., Olofsson G.H., et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 105.Peixoto R., Pereira M.L., Oliveira M. Beta-blockers and cancer: where are we? Pharmaceuticals (Basel) 2020;13 doi: 10.3390/ph13060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pelegrina L.T., Lombardi M.G., Fiszman G.L., et al. Immunoglobulin g from breast cancer patients regulates MCF-7 cells migration and MMP-9 activity by stimulating muscarinic acetylcholine receptors. J. Clin. Immunol. 2013;33:427–435. doi: 10.1007/s10875-012-9804-y. [DOI] [PubMed] [Google Scholar]
- 107.Phadke S., Clamon G. Beta blockade as adjunctive breast cancer therapy: a review. Crit. Rev. Oncol. Hematol. 2019;138:173–177. doi: 10.1016/j.critrevonc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 108.Pornour M., Ahangari G., Hejazi S.H., et al. Dopamine receptor gene (DRD1-DRD5) expression changes as stress factors associated with breast cancer. Asian Pac. J. Cancer Prev. 2014;15:10339–10343. doi: 10.7314/apjcp.2014.15.23.10339. [DOI] [PubMed] [Google Scholar]
- 109.Pornour M., Ahangari G., Hejazi S.H., et al. New perspective therapy of breast cancer based on selective dopamine receptor D2 agonist and antagonist effects on MCF-7 cell line. Recent Patents Anticancer Drug Discov. 2015;10:214–223. doi: 10.2174/1574892810666150416111831. [DOI] [PubMed] [Google Scholar]
- 110.Rangarajan S., Enserink J.M., Kuiperij H.B., et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J. Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rasheed N., Ahmad A., Pandey C.P., et al. Differential response of central dopaminergic system in acute and chronic unpredictable stress models in rats. Neurochem. Res. 2010;35:22–32. doi: 10.1007/s11064-009-0026-5. [DOI] [PubMed] [Google Scholar]
- 112.Reubi J.C., Gugger M., Waser B., et al. Y (1)-mediated effect of neuropeptide Y in cancer: breast carcinomas as targets. Cancer Res. 2001;61:4636–4641. [PubMed] [Google Scholar]
- 113.Ring H., Mendu S.K., Shirazi-Fard S., et al. GABA maintains the proliferation of progenitors in the developing chick ciliary marginal zone and non-pigmented ciliary epithelium. PLoS ONE. 2012;7:36874. doi: 10.1371/journal.pone.0036874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rondou P., Haegeman G., Van Craenenbroeck K. The dopamine D4 receptor: biochemical and signalling properties. Cell. Mol. Life Sci. 2010;67:1971–1986. doi: 10.1007/s00018-010-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rush S.E., Sharma M. Mindfulness-based stress reduction as a stress management intervention for cancer care: a systematic review. J. Evid. Based Complement. Altern. Med. 2017;22:348–360. doi: 10.1177/2156587216661467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sabbe T., Detraux J., De Hert M. [Prolactin, antipsychotics and breast cancer: is there a connection?] Tijdschr. Psychiatr. 2016;58:641–649. [PubMed] [Google Scholar]
- 117.Sachlos E., Risueño R.M., Laronde S., et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 118.Safer D.J. Raising the minimum effective dose of serotonin reuptake inhibitor antidepressants: adverse drug events. J. Clin. Psychopharmacol. 2016;36:483–491. doi: 10.1097/jcp.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 119.Sai B., Dai Y., Fan S., et al. Cancer-educated mesenchymal stem cells promote the survival of cancer cells at primary and distant metastatic sites via the expansion of bone marrow-derived-PMN-MDSCs. Cell Death Dis. 2019;10:941. doi: 10.1038/s41419-019-2149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sales M.E. Muscarinic receptors as targets for metronomic therapy in breast cancer. Curr. Pharm. Des. 2016;22:2170–2177. doi: 10.2174/1381612822666160229115317. [DOI] [PubMed] [Google Scholar]
- 121.Schaal C., Chellappan S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014;12:14–23. doi: 10.1158/1541-7786.Mcr-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schellekens M.P.J., van den Hurk D.G.M., Prins J.B., et al. Mindfulness-based stress reduction added to care as usual for lung cancer patients and/or their partners: a multicentre randomized controlled trial. Psychooncology. 2017;26:2118–2126. doi: 10.1002/pon.4430. [DOI] [PubMed] [Google Scholar]
- 123.Schmitt J.M., Abell E., Wagner A., et al. ERK activation and cell growth require CaM kinases in MCF-7 breast cancer cells. Mol. Cell. Biochem. 2010;335:155–171. doi: 10.1007/s11010-009-0252-9. [DOI] [PubMed] [Google Scholar]
- 124.Sehgal I., Powers S., Huntley B., et al. Neurotensin is an autocrine trophic factor stimulated by androgen withdrawal in human prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 1994;91:4673–4677. doi: 10.1073/pnas.91.11.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shende P., Desai D. Physiological and Therapeutic Roles of Neuropeptide Y on Biological Functions. Adv. Exp. Med. Biol. 2020;1237:37–47. doi: 10.1007/5584_2019_427. [DOI] [PubMed] [Google Scholar]
- 126.Shin K.J., Lee Y.J., Yang Y.R., et al. Molecular mechanisms underlying psychological stress and cancer. Curr. Pharm. Des. 2016;22:2389–2402. doi: 10.2174/1381612822666160226144025. [DOI] [PubMed] [Google Scholar]
- 127.Sizemore G.M., Sizemore S.T., Seachrist D.D., et al. GABA (A) receptor pi (GABRP) stimulates basal-like breast cancer cell migration through activation of extracellular-regulated kinase 1/2 (ERK1/2) J. Biol. Chem. 2014;289:24102–24113. doi: 10.1074/jbc.M114.593582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sloan E.K., Priceman S.J., Cox B.F., et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.Can-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Slotkin T.A., Zhang J., Dancel R., et al. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2000;60:153–166. doi: 10.1023/a:1006338232150. [DOI] [PubMed] [Google Scholar]
- 130.Sola-Penna M., Paixão L.P., Branco J.R., et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br. J. Cancer. 2020;122:194–208. doi: 10.1038/s41416-019-0640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Somaï S., Gompel A., Rostène W., et al. Neurotensin counteracts apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2002;295:482–488. doi: 10.1016/s0006-291x(02)00703-9. [DOI] [PubMed] [Google Scholar]
- 132.Song Y., Gan Y., Wang Q., et al. Enriching the housing environment for mice enhances their NK cell antitumor immunity via sympathetic nerve-dependent regulation of NKG2D and CCR5. Cancer Res. 2017;77:1611–1622. doi: 10.1158/0008-5472.Can-16-2143. [DOI] [PubMed] [Google Scholar]
- 133.Sood A.K., Armaiz-Pena G.N., Halder J., et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J. Clin. Invest. 2010;120:1515–1523. doi: 10.1172/jci40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Souazé F., Dupouy S., Viardot-Foucault V., et al. Expression of neurotensin and NT1 receptor in human breast cancer: a potential role in tumor progression. Cancer Res. 2006;66:6243–6249. doi: 10.1158/0008-5472.Can-06-0450. [DOI] [PubMed] [Google Scholar]
- 135.Speyer C.L., Hachem A.H., Assi A.A., et al. Metabotropic glutamate receptor-1 as a novel target for the antiangiogenic treatment of breast cancer. PLoS ONE. 2014;9:e88830. doi: 10.1371/journal.pone.0088830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Speyer C.L., Nassar M.A., Hachem A.H., et al. Riluzole mediates anti-tumor properties in breast cancer cells independent of metabotropic glutamate receptor-1. Breast Cancer Res. Treat. 2016;157:217–228. doi: 10.1007/s10549-016-3816-x. [DOI] [PubMed] [Google Scholar]
- 137.Speyer C.L., Smith J.S., Banda M., et al. Metabotropic glutamate receptor-1: a potential therapeutic target for the treatment of breast cancer. Breast Cancer Res. Treat. 2012;132:565–573. doi: 10.1007/s10549-011-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Strell C., Niggemann B., Voss M.J., et al. Norepinephrine promotes the β1-integrin-mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROα release. Mol. Cancer Res. 2012;10:197–207. doi: 10.1158/1541-7786.Mcr-11-0130. [DOI] [PubMed] [Google Scholar]
- 139.Su F., Ouyang N., Zhu P., et al. Psychological stress induces chemoresistance in breast cancer by upregulating mdr1. Biochem. Biophys. Res. Commun. 2005;329:888–897. doi: 10.1016/j.bbrc.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 140.Sun Z., Zhangsun M., Dong S., et al. Differential Expression of Nicotine Acetylcholine Receptors Associates with Human Breast Cancer and Mediates Antitumor Activity of αO-Conotoxin GeXIVA. Mar. Drugs. 2020;18 doi: 10.3390/md18010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Surman M., Janik M.E. Stress and its molecular consequences in cancer progression. Postepy Hig. Med. Dosw. (Online) 2017;71:485–499. doi: 10.5604/01.3001.0010.3830. [DOI] [PubMed] [Google Scholar]
- 142.Tanaka S., Sakaguchi M., Yoneyama H., et al. Histamine H (3) receptor antagonist OUP-186 attenuates the proliferation of cultured human breast cancer cell lines. Biochem. Biophys. Res. Commun. 2016;480:479–485. doi: 10.1016/j.bbrc.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 143.Tang J., Li Z., Lu L., et al. β-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin. Cancer Biol. 2013;23:533–542. doi: 10.1016/j.semcancer.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 144.Tu S.H., Ku C.Y., Ho C.T., et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits nicotine- and estrogen-induced α9-nicotinic acetylcholine receptor upregulation in human breast cancer cells. Mol. Nutr. Food Res. 2011;55:455–466. doi: 10.1002/mnfr.201000254. [DOI] [PubMed] [Google Scholar]
- 145.Vázquez S.M., Pignataro O., Luthy I.A. Alpha2-adrenergic effect on human breast cancer MCF-7 cells. Breast Cancer Res. Treat. 1999;55:41–49. doi: 10.1023/a:1006196308001. [DOI] [PubMed] [Google Scholar]
- 146.Wang A.W., Bouchard L.C., Gudenkauf L.M., et al. Differential psychological effects of cognitive-behavioral stress management among breast cancer patients with high and low initial cancer-specific distress. J. Psychosom. Res. 2018;113:52–57. doi: 10.1016/j.jpsychores.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang L.P., Jin J., Lv F.F., et al. Norepinephrine attenuates CXCR4 expression and the corresponding invasion of MDA-MB-231 breast cancer cells via β2-adrenergic receptors. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1170–1181. [PubMed] [Google Scholar]
- 148.Wang S., Mou Z., Ma Y., et al. Dopamine enhances the response of sunitinib in the treatment of drug-resistant breast cancer: involvement of eradicating cancer stem-like cells. Biochem. Pharmacol. 2015;95:98–109. doi: 10.1016/j.bcp.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 149.Wang T., Li Y., Lu H.L., et al. β-Adrenergic receptors: new target in breast cancer. Asian Pac. J. Cancer Prev. 2015;16:8031–8039. doi: 10.7314/apjcp.2015.16.18.8031. [DOI] [PubMed] [Google Scholar]
- 150.Watters J.J., Dorsa D.M. Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J. Neurosci. 1998;18:6672–6680. doi: 10.1523/jneurosci.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu C.H., Lee C.H., Ho Y.S. Nicotinic acetylcholine receptor-based blockade: applications of molecular targets for cancer therapy. Clin. Cancer Res. 2011;17:3533–3541. doi: 10.1158/1078-0432.Ccr-10-2434. [DOI] [PubMed] [Google Scholar]
- 152.Xia M., Ji N.N., Duan M.L., et al. Dexmedetomidine regulate the malignancy of breast cancer cells by activating α2-adrenoceptor/ERK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3500–3506. [PubMed] [Google Scholar]
- 153.Xia M., Tong J.H., Zhou Z.Q., et al. Tramadol inhibits proliferation, migration and invasion via α2-adrenoceptor signaling in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016;20:157–165. [PubMed] [Google Scholar]
- 154.Xie W.Y., He R.H., Zhang J., et al. β‑blockers inhibit the viability of breast cancer cells by regulating the ERK/COX‑2 signaling pathway and the drug response is affected by ADRB2 single‑nucleotide polymorphisms. Oncol. Rep. 2019;41:341–350. doi: 10.3892/or.2018.6830. [DOI] [PubMed] [Google Scholar]
- 155.Yamazaki S., Miyoshi N., Kawabata K., et al. Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β₂-adrenergic signaling. Arch. Biochem. Biophys. 2014;557:18–27. doi: 10.1016/j.abb.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 156.Yang L., Yao Y., Yong L., et al. Dopamine D (1) receptor agonists inhibit lung metastasis of breast cancer reducing cancer stemness. Eur. J. Pharmacol. 2019;859 doi: 10.1016/j.ejphar.2019.172499. [DOI] [PubMed] [Google Scholar]
- 157.Yin T., He S., Shen G., et al. Dopamine receptor antagonist thioridazine inhibits tumor growth in a murine breast cancer model. Mol. Med. Rep. 2015;12:4103–4108. doi: 10.3892/mmr.2015.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zambello E., Fuchs E., Abumaria N., et al. Chronic psychosocial stress alters NPY system: different effects in rat and tree shrew. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:122–130. doi: 10.1016/j.pnpbp.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 159.Zhang L., Bijker M.S., Herzog H. The neuropeptide Y system: pathophysiological and therapeutic implications in obesity and cancer. Pharmacol. Ther. 2011;131:91–113. doi: 10.1016/j.pharmthera.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 160.Zhang L., Pan J., Chen W., et al. Chronic stress-induced immune dysregulation in cancer: implications for initiation, progression, metastasis, and treatment. Am. J. Cancer Res. 2020;10:1294–1307. [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang Z., Liu Y., Wang K., et al. Activation of type 4 metabotropic glutamate receptor promotes cell apoptosis and inhibits proliferation in bladder cancer. J. Cell Physiol. 2019;234:2741–2755. doi: 10.1002/jcp.27089. [DOI] [PubMed] [Google Scholar]
- 162.Zhao D., Bakirtzi K., Zhan Y., et al. Insulin-like growth factor-1 receptor transactivation modulates the inflammatory and proliferative responses of neurotensin in human colonic epithelial cells. J. Biol. Chem. 2011;286:6092–6099. doi: 10.1074/jbc.M110.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao D., Zhan Y., Zeng H., et al. Neurotensin stimulates expression of early growth response gene-1 and EGF receptor through MAP kinase activation in human colonic epithelial cells. Int. J. Cancer. 2007;120:1652–1656. doi: 10.1002/ijc.22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao H., Lin Y., Chen S., et al. 5-HT3 receptors: a potential therapeutic target for epilepsy. Curr. Neuropharmacol. 2018;16:29–36. doi: 10.2174/1570159x15666170508170412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhao Y., Wang Q., Zhao X., et al. Effect of antihypertensive drugs on breast cancer risk in female hypertensive patients: evidence from observational studies. Clin. Exp. Hypertens. 2018;40:22–27. doi: 10.1080/10641963.2017.1288736. [DOI] [PubMed] [Google Scholar]