Abstract
An immunochromatographic test for the rapid determination of immunoglobulin M (IgM) and IgG antibodies to Burkholderia pseudomallei was evaluated by using sera from bacteriologically confirmed melioidosis patients and high-risk and clinically suspected patients, along with disease control groups. The sensitivities were 100 and 93% for the IgG and IgM tests, respectively, while the specificity was 95% for both assays. The test was rapid and simple to perform, with results obtained in 10 min.
Melioidosis is an infection caused by the bacterium Burkholderia pseudomallei, which is a motile gram-negative bacillus found in soil and water. Infection is obtained through inhalation or ingestion of the bacterium, with most patients presenting with nonspecific symptoms (3). Mortality can be as high as 90% when the disease progresses and acute septicemia develops (6, 10). Due to the nonspecific symptoms and the speed at which death can occur, rapid and specific diagnostic tests are essential.
While the definitive diagnosis for melioidosis is made by bacterial culture (4), the most common method for serodiagnosis is the indirect hemagglutination assay (IHA) (1, 7). Although IHA has the advantage of speed over culture techniques, interference can be observed due to background antibody levels present in patients from areas where melioidosis is endemic, making a distinction between current and past infections difficult (11, 12). The IHA has also been reported to have low sensitivity in serum from patients with acute septicemia (5). Due to these limitations, enzyme-linked immunosorbent assays (ELISAs) and indirect fluorescent-antibody assays (IFAs) are becoming more popular tools for the serodiagnosis of melioidosis. Both the IFA and the ELISA have been reported to have good sensitivities and specificities, though the IFA results can be subjective, and the test requires a fluorescent microscope (2). Indirect immunoglobulin G (IgG) and IgM ELISAs have been reported to be useful in the presumptive diagnosis of melioidosis and in laboratories where large numbers of samples require testing (2, 8). Indirect IgG ELISAs also have the ability to discriminate between past and active infections in areas where the disease is endemic (5), while the IgM ELISA has high sensitivity in patients with acute septicemia (8).
This study investigated the ability of the PanBio IgM and IgG immunochromatographic test to diagnose melioidosis and discriminate between other diseases that present with similar clinical symptoms. Thirty samples were obtained from patients who were culture positive for B. pseudomallei. A further 15 samples from patients who presented with symptoms including prolonged fever, nonhealing wounds, pneumonia, cellulitis, pleural effusions, and unresolved cough were tested along with 14 samples from healthy farmers who are known to be at risk for melioidosis. The disease controls from an area of melioidosis endemicity (Malaysia) included sera from patients with Pseudomonas aeruginosa (n = 4), Chlamydia pneumoniae (n = 4), Legionella spp. (n = 5), and tuberculosis (n = 5). Disease controls from Queensland, Australia, included sera from patients with leptospirosis (n = 10) and Epstein-Barr virus (EBV) infection (n = 10), as well as samples from patients with antibodies to rheumatoid factor (RF; n = 10) and antinuclear antibodies (ANA; n = 10). Blood donors from areas of melioidosis endemicity, such as Malaysia (n = 3) and Thailand (n = 30), were also tested, along with blood donors from an area of nonendemicity, such as Queensland, Australia (n = 40). All samples were frozen at −70°C prior to performing the assay.
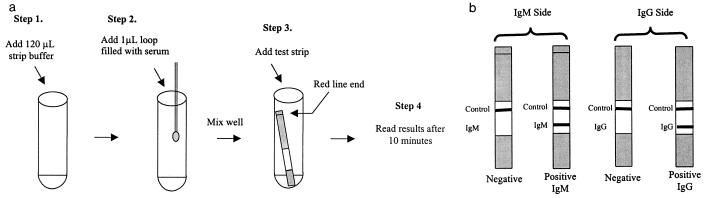
The melioidosis IgM and IgG test was presented as a single strip consisting of the IgM test on one side and the IgG test on the other. The test was performed in glass tubes by adding 1 μl of serum obtained with a measuring loop to 120 μl of strip buffer. Once the serum was mixed by gently shaking the vials, a test strip was added. Exactly 10 min after adding the test strip, the results were read. A control line was included in both sides of the test to ensure that each assay had run correctly. A positive reaction was defined as a visible test and control line, and a negative reaction was defined as a positive control line only (Fig. 1).
FIG. 1.
(a) Diagrammatical representation of the assay procedure. (b) Interpretation of assay results.
The IFA was performed as previously described (2), with modifications. The antigen was coated, air dried, and heat fixed onto wells of Teflon-coated slides. Each serum sample was prepared in doubling dilutions from 1:80 to 1:320 and allowed to incubate at 37°C for 30 min. The slide was washed three times in phosphate-buffered saline (pH 7.4) prior to the addition of fluorescein-labelled anti-human IgM and IgG antibodies. Following an incubation for a further 30 min, the slide was washed three times, air dried, and mounted with buffered glycerol and viewed under a UV microscope. Results were scored as 3+, 2+, 1+, or negative as compared with positive and negative control sera. The lower limit of a positive cutoff was a score of 1+ at a dilution of 1:80. Serum samples demonstrating a fluorescence intensity of 3+ or 2+ at a dilution of 1:320 were considered to have a titer of ≥320.
The sera from confirmed and clinically suspected melioidosis patients were characterized by IFA. All culture-confirmed cases were positive by the IFA, which detected the total amount of specific IgM and IgG present in the sample. Both the IgM and the IgG rapid test results also correlated well with the culture results, producing a sensitivity of 100% for the IgG assay and 93% for the IgM assay (Table 1). There was a significant correlation between the number of positive test results and the IFA titer (IgM assay, chi-square test for trend = 18.798, P < 0.0001; IgG assay, chi-square test for trend = 18.235, P < 0.001). Of the samples from patients with clinically suspected melioidosis, five were negative by both the IFA and the rapid test. Of the 10 IFA-positive samples, five were positive for IgM and five were positive for IgG by the rapid test. When the presence of IgM or IgG was taken as a positive result, 8 of 10 IFA-positive samples were detected.
TABLE 1.
Sensitivity and specificity of the IgM and IgG rapid tests
| Test parameter and serum group | Sensitivity [no. positive/total (%)] and specificity [no. negative/total (%)] of tests
|
||
|---|---|---|---|
| IgG rapid test | IgM rapid test | IgG and IgM rapid test | |
| Sensitivity | |||
| Confirmed melioidosis infection | 30/30 (100) | 28/30 (93) | 30/30 (100) |
| Clinically suspected melioidosis infection | 5/15 (33) | 7/15 (47) | 8/15 (53) |
| ELISA negative or high-risk patient | 2/14 (14) | 4/14 (29) | 4/14 (29) |
| Specificity | |||
| P. aeruginosa | 4/4 (100) | 3/4 (75) | 3/4 (75) |
| Legionella sp. | 5/5 (100) | 4/5 (80) | 4/5(80) |
| Mycobacterium tuberculosis | 5/5 (100) | 4/5 (80) | 4/5 (80) |
| C. pneumoniae | 4/4 (100) | 4/4 (100) | 4/4 (100) |
| Leptospirosis | 10/10 (100) | 10/10 (100) | 10/10 (100) |
| ANA | 10/10 (100) | 10/10 (100) | 10/10 (100) |
| RF | 10/10 (100) | 10/10 (100) | 10/10 (100) |
| EBV | 10/10 (100) | 10/10 (100) | 10/10 (100) |
| Malaysian blood donors | 3/3 (100) | 3/3 (100) | 3/3 (100) |
| Thai blood donors | 16/20 (80) | 17/20 (85) | 16/20 (80) |
| Australian blood donors | 38/40 (95) | 40/40 (100) | 38/40 (95) |
| Total specificity | 115/121 (95) | 115/121 (95) | 113/121 (93) |
The IgG rapid test was found to have 100% specificity, with no cross-reactivity occurring with sera from patients with diseases that present with similar clinical symptoms (18 of 18). The IgM assay showed some cross-reactivity with sera from patients with infections due to Pseudomonas, Legionella, and tuberculosis (3 of 18, 83% specificity). Of the Thai blood donors tested, 20 and 15% were positive for IgG and IgM, respectively. This may reflect subclinical or undiagnosed melioidosis, since the disease is endemic in Thailand (9). In contrast, only 5% of the Australian blood donors were positive for IgG, with no samples showing detectable levels of IgM. All sera tested from patients with antibodies to Leptospira, EBV, and RF and with ANA were negative by both the IgM and IgG assays. The overall specificity of the PanBio IgM and IgG rapid test was 93% (113 of 121 [Table 1]).
This study suggests that the PanBio melioidosis IgM and IgG rapid test had equal sensitivity to both bacterial culture techniques and a reference IFA. The specificity of the rapid test was also high, indicating that it should be a useful alternative for the qualitative diagnosis of melioidosis, especially in laboratories with limited equipment or when a rapid diagnosis is necessary.
REFERENCES
- 1.Alexander A D, Huxsoll D L, Warner A R, Shepler V, Dorsey A. Serological diagnosis of human melioidosis with indirect hemagglutination and complement fixation tests. Appl Microbiol. 1970;20:825–833. doi: 10.1128/am.20.5.825-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashdown L R, Johnson R W, Koehler J M, Cooney C A. Enzyme-linked immunosorbent assay for the diagnosis of clinical and subclinical melioidosis. J Infect Dis. 1989;160:253–260. doi: 10.1093/infdis/160.2.253. [DOI] [PubMed] [Google Scholar]
- 3.Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Looareesuwan T M E, Pitakwatchara N. Melioidosis: a major cause of community acquired septicemia in northern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 4.Dance D A B. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharakul T, Songsivilai S, Anuntagool N, Chaowagul W, Wongbunnate S, Intachote P, Sirisinha S. Diagnostic value of an antibody enzyme-linked immunosorbent assay using affinity-purified antigen in an area endemic for melioidosis. Am J Trop Med Hyg. 1997;56:418–423. doi: 10.4269/ajtmh.1997.56.418. [DOI] [PubMed] [Google Scholar]
- 6.Guard R W, Khafagi F A, Brigden M C, Ashdown L R. Melioidosis in far north Queensland. A clinical and epidemiological review of twenty cases. Am J Trop Med Hyg. 1984;33:467–473. doi: 10.4269/ajtmh.1984.33.467. [DOI] [PubMed] [Google Scholar]
- 7.Hatsadee A, Silpapojakul K R, Wansit R, Pornpatkul M. Diagnostic value of the indirect hemagglutination test for melioidosis in an endemic area. Am J Trop Med Hyg. 1990;42:248–253. doi: 10.4269/ajtmh.1990.42.248. [DOI] [PubMed] [Google Scholar]
- 8.Kunakorn M, Boonma P, Khupulsup K, Petchclai B. Enzyme-linked immunosorbent assay for immunoglobulin M specific antibody for the diagnosis of melioidosis. J Clin Microbiol. 1990;28:1249–1253. doi: 10.1128/jcm.28.6.1249-1253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leelarasamee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 10.Puthucheary S D, Parasakthi N, Lee M K. Septicaemic melioidosis: a review of 50 cases from Malaysia. Trans R Soc Trop Med Hyg. 1992;86:683–685. doi: 10.1016/0035-9203(92)90191-e. [DOI] [PubMed] [Google Scholar]
- 11.Strauss J, Alexander M A D, Rapmond G, Gan E, Dorsey A E. Melioidosis in Malaysia. III. Antibodies to Pseudomonas pseudomallei in the human population. Am J Trop Med Hyg. 1969;18:703–707. [PubMed] [Google Scholar]
- 12.Vadivelu J, Puthucheary S D, Gendeh G S, Parasakthi N. Serodiagnosis of melioidosis in Malaysia. Singap Med J. 1995;36:299–302. [PubMed] [Google Scholar]