A report of extended longevity in mice homozygous for a mutation producing growth hormone (GH) deficiency (Brown-Borg et al., 1996) was quickly followed by the demonstration of extensive homology between one of the key longevity genes in a worm, Caenorhabditis elegans, and genes coding for insulin and insulin-like growth factor-1 (IGF-1) receptors in mammals (Kimura et al., 1997). Since GH is the key determinant of hepatic IGF-1 expression and circulating IGF-1 levels, and has major impact on insulin signaling (Figure 1), these findings led to an exciting conclusion that the insulin/insulin-like growth factor signaling (IIS) is an evolutionarily conserved mechanism which controls aging in organisms ranging from yeast and worms to insects and mammals. Subsequent work provided much evidence in support of this exciting realization (Tissenbaum and Ruvkun, 1998; Fabrizio et al., 2001; Tatar et al., 2001; Tatar et al., 2003; Piper et al., 2008; Finch and Ruvkun, 2001), and this has led to a focus on IIS, rather than GH signaling, in analyzing genetic control of mammalian aging. This is an important distinction. Although biosynthesis and blood plasma levels of GH and IGF-1 are closely linked, the actions of these hormones are not identical and, in some cases, opposite. For example, IGF-1 mimics some of the insulin actions and promotes insulin sensitivity, while GH is anti-insulinemic and promotes insulin resistance; IGF-1 promotes fat deposition, while GH is lipolytic (Figure 2) (Scavo et al., 2004; Veldhuis et al., 2005; Hu et al., 2009). Actions of GH not shared with IGF-1 include other effects relevant to aging such as impact on reactive radicals production and anti-oxidative defenses (Brown-Borg et al., 2002; Bokov et al., 2009), DNA damage and repair (Chesnokova et al., 2019; Chesnokova and Melmed, 2019), macrophage reprogramming (Schneider et al., 2019), ovarian primordial follicle reserve (Saccon et al., 2017), bone resorption and turnover (Thomas and Monson, 2009), kidney dysfunction (Soliman et al., 2019), and cognitive functioning (Nyberg and Hallberg, 2013).
FIGURE 1.
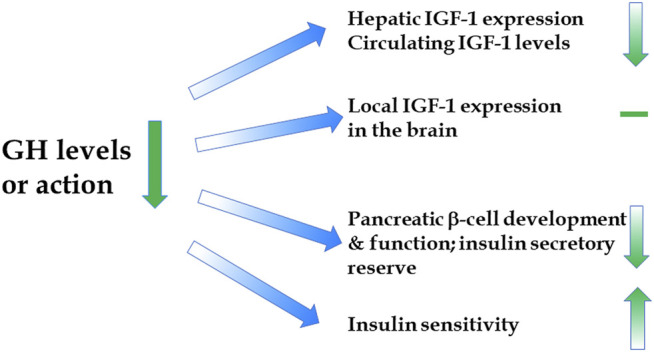
Impact of reduced GH signaling on the levels and actions of IGF-1 and insulin.
FIGURE 2.
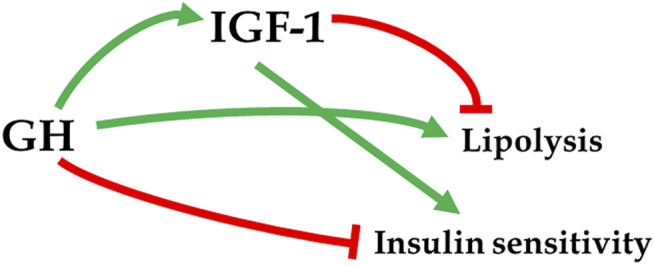
Divergent actions of GH and IGF-1 on metabolic parameters. → stimulation; –| inhibition.
Evidence for the ability of GH to influence healthspan and lifespan of laboratory mice is very strong and includes significant extension of longevity in both sexes of mice with hypopituitarism (combined deficiency of GH, prolactin, and TSH) (Brown-Borg et al., 1996; Flurkey et al., 2001), in mice with isolated GH deficiency due to mutation of Ghrhr gene or deletion of Ghrh (Flurkey et al., 2001; Sun et al., 2013), and in mice with GH resistance due to Ghr gene disruption (Zhou et al., 1997; Coschigano et al., 2003). This evidence for association of genetically reduced GH signaling with extended longevity was obtained in different laboratories and included animals with different genetic background (Bartke and Turyn, 2001; Coschigano et al., 2003; Aguiar-Oliveira and Bartke, 2019). Importantly, extended longevity of hypopituitary Ames dwarf mice can be reduced by GH replacement therapy during the period of rapid peri-pubertal growth (Panici et al., 2010; Sun et al., 2017). This provides evidence that the association of GH deficiency and increased lifespan in Ames dwarf mice is causal (mechanistic).
In contrast to the remarkable extension of longevity in female and male mice lacking GH or GH receptors, the impact of reduced IGF-1 signaling on longevity of IGF1R ± mice and mice treated with an antibody to IGF-1 receptor is modest and seen only in one sex (Holzenberger et al., 2003; Mao et al., 2018; Garratt et al., 2017) (Table 1). This difference between the effects of reduced IGF-1 and GH signaling is likely related to IGF-1 exerting both beneficial and detrimental effects on aging and age-related disease (including opposite effects on the risk of type 2 diabetes vs cardiovascular disease and cognitive decline) and GH having primarily “pro-aging” effects. Both hormones impact growth, but the metabolic effects of GH are significantly greater. Growth hormone has different and more potent effects on glucose regulation when compared to IGF-1. Growth hormone is a regulator of IGF-1 by controlling much of its production and release from the liver and other tissues, and thus regulating plasma concentrations of IGF-1 (Haluzik et al., 2003; Vijayakumar et al., 2011). Liver-derived IGF-1 represents >75% of the circulating hormone (Haluzik et al., 2003; Aguirre et al., 2016). In contrast to the effects on somatic growth, the effects of GH and IGF-1 on glucose homeostasis are markedly different. Growth hormone promotes insulin resistance acting as a counterregulatory mechanism for hypoglycemia (protection during fasting, food deprivation). While GH counteracts insulin action, IGF-1 enhances insulin sensitivity and mimics some of its actions. Both GH and IGF-1 influence insulin production. When GH levels are reduced, insulin levels are also reduced, whereas IGF-1 inhibits insulin secretion (Haluzik et al., 2003). Another complexity is suggested by the evidence that most of IGF-1’s actions on glucose homeostasis and insulin sensitivity are mediated indirectly (through GH suppression), while circulating IGF-1 is bound to high-affinity binding proteins and has low affinity for insulin receptors (Vijayakumar et al., 2011). Direct effects of IGF-1 on glucose management occur mostly in skeletal muscle by increasing glucose uptake (Haluzik et al., 2003; Vijayakumar et al., 2011). Growth hormone influences insulin signaling in liver and adipocytes, whereas no IGF-1 receptors are present in these tissues (Haluzik et al., 2003). Other actions of GH that impact lifespan are also not shared by IGF-1, and thus GH deficiency promotes health and lifespan extension more profoundly than suppression of the levels or action of IGF-1 (Haluzik et al., 2003). Sex-specific responses to suppressing IGF-1 signaling in mice (Ashpole et al., 2017) add to the emerging evidence that, in this species, aging of males is related primarily to the insulin arm of IIS while in females effects of the IGF-1 arm predominate.
TABLE 1.
Effects of reduced IIS and GH signaling on healthspan and lifespan in different taxonomic groups.
| Yeast | Worms | Insects | Mammals | |||
|---|---|---|---|---|---|---|
| Mice | Humans | |||||
| IIS | healthspan | ? | ↑ | ↑ | ? | ? |
| lifespan | ↑ | ↑ | ↑ | ↑ (♀ ♀) | — | |
| GH | healthspan | NA | NA | NA | ↑ | ↑ |
| lifespan | NA | NA | NA | ↑ | — | |
In contrast to the findings of extended longevity of IGF-1R heterozygous mice by Holzenberger et al. (Holzenberger et al., 2003), Bokov and his colleagues reported that such animals had very small lifespan extension, no indications of delayed aging, and no changes in end-of-life pathology (Bokov et al., 2011). Discrepancies between the results of a loss of one IGF-1R allele in these two studies were subsequently shown to be related to differences in constitutive IGF-1 signaling and in endocrine responses to reducing the number of IGF-1 receptors in the employed strains of mice (Xu et al., 2014). In further contrast between the effects of suppressing GH and IGF-1 signaling, complete (homologous) disruption of Igf1 or Igf1r genes can have severe detrimental effects on development, postnatal survival and fertility (Liu et al., 1993; Powell-Braxton et al., 1993; Yakar et al., 1999), while GH-deficient and GH-resistant mice are viable and fertile.
Reduced insulin levels and improved insulin sensitivity are associated with extension of longevity in response to calorie restriction or disruption of GH signaling. However, the effects of genetic alterations of insulin levels, global or organ-specific insulin sensitivity, or early steps of intracellular insulin signaling on longevity of laboratory mice are not consistent. Interpretation of the available data is complicated by the negative regulation of expression of the insulin receptors by insulin and by indications that insulin resistance can have both detrimental and protective effects (Barzilai et al., 2012). Templeman and her colleagues reported an 11 percent increase in median longevity of female Ins2+/− Ins1−/− mice in which insulin levels are reduced by approximately 30 percent (Templeman et al., 2017). This association of improved insulin sensitivity and longevity was also seen in other mutants (Masternak et al., 2009; Zhang et al., 2012), but was absent or reversed in others (Shimizu et al., 2011; Nelson et al., 2012; Takeda et al., 2017). Deletion of Insulin receptor substrate 1 (Irs1) extended longevity, but the effects of Irs2 deletion were not consistent in different studies, likely due to difference in the composition of the diet used in the two laboratories (Taguchi et al., 2007; Selman et al., 2008).
Reports of GH signaling and lifespan in rats are very limited. Spontaneous dwarf rats exhibit reduced GH and IIS signaling and longer lifespans compared to controls (Kuramoto et al., 2010; Sasaki et al., 2013). GH-deficient rats generated by antisense GH gene suppression (±) also live 7% longer, but −/− animals do not (Shimokawa et al., 2002). Lewis dwarf rats do not live longer, but are not profoundly GH/IGF-1 deficient (∼55% reduced), exhibit additional endocrine abnormalities (i.e. hyporesponsive HPA axis), and a general tendency towards pro-inflammation resulting in nephropathy and intracerebral hemorrhage, among other issues (Perretti et al., 1993; Oitzl et al., 1995; Sonntag et al., 2005; Ungvari et al., 2010; Groeneweg et al., 2011; Ungvari et al., 2011; Podlutsky et al., 2017).
Collectively, the available evidence suggests that in addition to the evolutionarily conserved role of IIS in the control of aging, GH (which has no known homologs in invertebrates) emerges as a major regulator of aging and longevity in mammals. Alterations in IIS in long-lived GH signaling-related mutants represent some of the multiple mechanisms believed to link GH deficiency or resistance with increases in the healthspan and lifespan (Aguiar-Oliveira and Bartke, 2019).
In humans, the impact of GH and growth/anabolic processes on longevity is more subtle than in laboratory mice, likely reflecting major differences in the pace-of-life including the reproductive strategies (Aguiar-Oliveira and Bartke, 2019; Bartke, 2020). Genetic syndromes of GH deficiency or resistance do not extend human longevity, even though some individuals with these mutations can reach very advanced age (Aguiar-Oliveira and Bartke, 2019). However, pathological excess of GH reduces life expectancy in both humans and mice (Bengtsson et al., 1988; Steger et al., 1993; Wolf et al., 1993) and familial longevity was shown to be associated with reduced GH secretion (van der Spoel et al., 2016). Intriguingly, there is considerable overlap of phenotypic and metabolic consequences of genetic disruption of GH signaling in mice and humans (Aguiar-Oliveira and Bartke, 2019), and humans with these syndromes show a remarkable degree of protection from several age-associated chronic diseases along with indications of extended healthspan, that is “healthy aging” (Guevara-Aguirre et al., 2011; Aguiar-Oliveira and Bartke, 2019).
Author Contributions
Article concept and design, AB. Writing of manuscript, AB, HB-B. Approval of final manuscript, AB.
Funding
William E. McElroy Charitable Foundation, NIH R21AG062985, and American Diabetes Association 1-19-IBS-126 to AB and NIH R56AG067724 to HB-B.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aguiar-Oliveira M. H., Bartke A. (2019). Growth Hormone Deficiency: Health and Longevity. Endocr. Rev. 40 (2), 575–601. 10.1210/er.2018-00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre G. A., De Ita J. R., de la Garza R. G., Castilla-Cortazar I. (2016). Insulin-like Growth Factor-1 Deficiency and Metabolic Syndrome. J. Transl Med. 14 (1), 3. 10.1186/s12967-015-0762-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole N. M., Logan S., Yabluchanskiy A., Mitschelen M. C., Yan H., Farley J. A., et al. (2017). IGF-1 Has Sexually Dimorphic, Pleiotropic, and Time-dependent Effects on Healthspan, Pathology, and Lifespan. Geroscience 39 (2), 129–145. 10.1007/s11357-017-9971-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A. (2020). Growth Hormone and Aging. Rev. Endocr. Metab. Disord. 22 (1), 71–80. 10.1007/s11154-020-09593-2 [DOI] [PubMed] [Google Scholar]
- Bartke A., Turyn D. (2001). Mechanisms of Prolonged Longevity: Mutants, Knock-Outs, and Caloric Restriction. J. Anti-Aging Med. 4 (3), 197–203. 10.1089/109454501753249966 [DOI] [Google Scholar]
- Barzilai N., Huffman D. M., Muzumdar R. H., Bartke A. (2012). The Critical Role of Metabolic Pathways in Aging. Diabetes 61 (6), 1315–1322. 10.2337/db11-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson B. A., Edén S., Ernest I., Odén A., Sjögren B. (1988). Epidemiology and Long-Term Survival in Acromegaly. A Study of 166 Cases Diagnosed between 1955 and 1984. Acta Med. Scand. 223 (4), 327–335. 10.1111/j.0954-6820.1988.tb15881.x [DOI] [PubMed] [Google Scholar]
- Bokov A. F., Garg N., Ikeno Y., Thakur S., Musi N., DeFronzo R. A., et al. (2011). Does Reduced IGF-1R Signaling in Igf1r+/− Mice Alter Aging. PLoS One 6 (11), e26891. 10.1371/journal.pone.0026891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov A. F., Lindsey M. L., Khodr C., Sabia M. R., Richardson A. (2009). Long-lived ames dwarf Mice Are Resistant to Chemical Stressors. J. Gerontol. A. Biol. Sci. Med. Sci. 64A (8), 819–827. 10.1093/gerona/glp052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg H. M., Borg K. E., Meliska C. J., Bartke A. (1996). Dwarf Mice and the Ageing Process. Nature 384, 33. 10.1038/384033a0 [DOI] [PubMed] [Google Scholar]
- Brown-Borg H. M., Rakoczy S. G., Romanick M. A., Kennedy M. A. (2002). Effects of Growth Hormone and Insulin-like Growth Factor-1 on Hepatocyte Antioxidative Enzymes. Exp. Biol. Med. (Maywood) 227 (2), 94–104. 10.1177/153537020222700203 [DOI] [PubMed] [Google Scholar]
- Chesnokova V., Melmed S. (2019). Growth Hormone in the Tumor Microenvironment. Arch. Endocrinol. Metab. 63 (6), 568–575. 10.20945/2359-3997000000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V., Zonis S., Barrett R. J., Gleeson J. P., Melmed S. (2019). Growth Hormone Induces Colon DNA Damage Independent of IGF-1. Endocrinology 160 (6), 1439–1447. 10.1210/en.2019-00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano K. T., Holland A. N., Riders M. E., List E. O., Flyvbjerg A., Kopchick J. J. (2003). Deletion, but Not Antagonism, of the Mouse Growth Hormone Receptor Results in Severely Decreased Body Weights, Insulin, and Insulin-like Growth Factor I Levels and Increased Life Span. Endocrinology 144 (9), 3799–3810. 10.1210/en.2003-0374 [DOI] [PubMed] [Google Scholar]
- Fabrizio P., Pozza F., Pletcher S. D., Gendron C. M., Longo V. D. (2001). Regulation of Longevity and Stress Resistance by Sch9 in Yeast. Science 292 (5515), 288–290. 10.1126/science.1059497 [DOI] [PubMed] [Google Scholar]
- Finch C. E., Ruvkun G. (2001). The Genetics of Aging. Annu. Rev. Genom. Hum. Genet. 2, 435–462. 10.1146/annurev.genom.2.1.435 [DOI] [PubMed] [Google Scholar]
- Flurkey K., Papaconstantinou J., Miller R. A., Harrison D. E. (2001). Lifespan Extension and Delayed Immune and Collagen Aging in Mutant Mice with Defects in Growth Hormone Production. Proc. Natl. Acad. Sci. 98 (12), 6736–6741. 10.1073/pnas.111158898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt M., Nakagawa S., Simons M. J. P. (2017). Life-span Extension with Reduced Somatotrophic Signaling: Moderation of Aging Effect by Signal Type, Sex, and Experimental Cohort. J. Gerontol. A. Biol. Sci. Med. Sci. 72 (12), 1620–1626. 10.1093/gerona/glx010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg F. L., Karst H., de Kloet E. R., Joëls M. (2011). Rapid Non-genomic Effects of Corticosteroids and Their Role in the central Stress Response. J. Endocrinol. 209 (2), 153–167. 10.1530/joe-10-0472 [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J., Balasubramanian P., Guevara-Aguirre M., Wei M., Madia F., Cheng C. W., et al. (2011). Growth Hormone Receptor Deficiency Is Associated with a Major Reduction in Pro-aging Signaling, Cancer, and Diabetes in Humans. Sci. Transl Med. 3 (70), 70ra13. 70ra13. 10.1126/scitranslmed.3001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluzik M., Yakar S., Gavrilova O., Setser J., Boisclair Y., LeRoith D. (2003). Insulin Resistance in the Liver-specific IGF-1 Gene-Deleted Mouse Is Abrogated by Deletion of the Acid-Labile Subunit of the IGF-Binding Protein-3 Complex: Relative Roles of Growth Hormone and IGF-1 in Insulin Resistance. Diabetes 52 (10), 2483–2489. 10.2337/diabetes.52.10.2483 [DOI] [PubMed] [Google Scholar]
- Holzenberger M., Dupont J., Ducos B., Leneuve P., Géloën A., Even P. C., et al. (2003). IGF-1 Receptor Regulates Lifespan and Resistance to Oxidative Stress in Mice. Nature 421 (6919), 182–187. 10.1038/nature01298 [DOI] [PubMed] [Google Scholar]
- Hu D., Pawlikowska L., Kanaya A., Hsueh W.-C., Colbert L., Newman A. B., et al. (2009). Serum Insulin-like Growth Factor-1 Binding Proteins 1 and 2 and Mortality in Older Adults: the Health, Aging, and Body Composition Study. J. Am. Geriatr. Soc. 57 (7), 1213–1218. 10.1111/j.1532-5415.2009.02318.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G. (1997). daf-2 , an Insulin Receptor-like Gene that Regulates Longevity and Diapause in Caenorhabditis elegans . Science 277 (5328), 942–946. 10.1126/science.277.5328.942 [DOI] [PubMed] [Google Scholar]
- Kuramoto K., Tahara S., Sasaki T., Matsumoto S., Kaneko T., Kondo H., et al. (2010). Spontaneous dwarf Rat: a Novel Model for Aging Research. Geriatr. Gerontol. Int. 10 (1), 94–101. 10.1111/j.1447-0594.2009.00559.x [DOI] [PubMed] [Google Scholar]
- Liu J.-P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. (1993). Mice Carrying Null Mutations of the Genes Encoding Insulin-like Growth Factor I (Igf-1) and Type 1 IGF Receptor (Igf1r). Cell 75 (1), 59–72. 10.1016/s0092-8674(05)80084-4 [DOI] [PubMed] [Google Scholar]
- Mao K., Quipildor G. F., Tabrizian T., Novaj A., Guan F., Walters R. O., et al. (2018). Late-life Targeting of the IGF-1 Receptor Improves Healthspan and Lifespan in Female Mice. Nat. Commun. 9 (1), 2394. 10.1038/s41467-018-04805-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak M. M., Panici J. A., Bonkowski M. S., Hughes L. F., Bartke A. (2009). Insulin Sensitivity as a Key Mediator of Growth Hormone Actions on Longevity. Journals Gerontol. Ser. A: Biol. Sci. Med. Sci. 64A (5), 516–521. 10.1093/gerona/glp024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. F., Strong R., Bokov A., Diaz V., Ward W. (2012). Probing the Relationship between Insulin Sensitivity and Longevity Using Genetically Modified Mice. J. Gerontol. A. Biol. Sci. Med. Sci. 67 (12), 1332–1338. 10.1093/gerona/gls199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg F., Hallberg M. (2013). Growth Hormone and Cognitive Function. Nat. Rev. Endocrinol. 9 (6), 357–365. 10.1038/nrendo.2013.78 [DOI] [PubMed] [Google Scholar]
- Oitzl M. S., van Haarst A. D., Sutanto W., Ron de Kloet E. (1995). Corticosterone, Brain Mineralocorticoid Receptors (MRs) and the Activity of the Hypothalamic-Pituitary-Adrenal (HPA) axis: the Lewis Rat as an Example of Increased central MR Capacity and a Hyporesponsive HPA axis. Psychoneuroendocrinology 20 (6), 655–675. 10.1016/0306-4530(95)00003-7 [DOI] [PubMed] [Google Scholar]
- Panici J. A., Harper J. M., Miller R. A., Bartke A., Spong A., Masternak M. M. (2010). Early Life Growth Hormone Treatment Shortens Longevity and Decreases Cellular Stress Resistance in Long-Lived Mutant Mice. FASEB J. 24 (12), 5073–5079. 10.1096/fj.10-163253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M., Duncan G. S., Flower R. J., Peers S. H. (1993). Serum Corticosterone, Interleukin-1 and Tumour Necrosis Factor in Rat Experimental Endotoxaemia: Comparison between Lewis and Wistar Strains. Br. J. Pharmacol. 110 (2), 868–874. 10.1111/j.1476-5381.1993.tb13893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. D. W., Selman C., McElwee J. J., Partridge L. (2008). Separating Cause from Effect: How Does Insulin/IGF Signalling Control Lifespan in Worms, Flies and Mice. J. Intern. Med. 263 (2), 179–191. 10.1111/j.1365-2796.2007.01906.x [DOI] [PubMed] [Google Scholar]
- Podlutsky A., Valcarcel-Ares M. N., Yancey K., Podlutskaya V., Nagykaldi E., Gautam T., et al. (2017). The GH/IGF-1 axis in a Critical Period Early in Life Determines Cellular DNA Repair Capacity by Altering Transcriptional Regulation of DNA Repair-Related Genes: Implications for the Developmental Origins of Cancer. Geroscience 39 (2), 147–160. 10.1007/s11357-017-9966-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Braxton L., Hollingshead P., Warburton C., Dowd M., Pitts-Meek S., Dalton D., et al. (1993). IGF-I Is Required for normal Embryonic Growth in Mice. Genes Dev. 7 (12B), 2609–2617. 10.1101/gad.7.12b.2609 [DOI] [PubMed] [Google Scholar]
- Saccon T. D., Moreira F., Cruz L. A., Mondadori R. G., Fang Y., Barros C. C., et al. (2017). Ovarian Aging and the Activation of the Primordial Follicle reserve in the Long-Lived Ames dwarf and the Short-Lived bGH Transgenic Mice. Mol. Cell Endocrinol. 455, 23–32. 10.1016/j.mce.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Tahara S., Shinkai T., Kuramoto K., Matsumoto S., Yanabe M., et al. (2013). Lifespan Extension in the Spontaneous dwarf Rat and Enhanced Resistance to Hyperoxia-Induced Mortality. Exp. Gerontol. 48 (5), 457–463. 10.1016/j.exger.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Scavo L. M., Karas M., Murray M., Leroith D. (2004). Insulin-like Growth Factor-I Stimulates Both Cell Growth and Lipogenesis during Differentiation of Human Mesenchymal Stem Cells into Adipocytes. J. Clin. Endocrinol. Metab. 89 (7), 3543–3553. 10.1210/jc.2003-031682 [DOI] [PubMed] [Google Scholar]
- Schneider A., Wood H. N., Geden S., Greene C. J., Yates R. M., Masternak M. M., et al. (2019). Growth Hormone-Mediated Reprogramming of Macrophage Transcriptome and Effector Functions. Sci. Rep. 9 (1), 19348. 10.1038/s41598-019-56017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C., Lingard S., Choudhury A. I., Batterham R. L., Claret M., Clements M., et al. (2008). Evidence for Lifespan Extension and Delayed Age-Related Biomarkers in Insulin Receptor Substrate 1 Null Mice. FASEB j. 22 (3), 807–818. 10.1096/fj.07-9261com [DOI] [PubMed] [Google Scholar]
- Shimizu T., Baba T., Ogawara M., Shirasawa T. (2011). Lifespan and Glucose Metabolism in Insulin Receptor Mutant Mice. J. Aging Res. 2011, 315640. 10.4061/2011/315640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I., Higami Y., Utsuyama M., Tuchiya T., Komatsu T., Chiba T., et al. (2002). Life Span Extension by Reduction in Growth Hormone-insulin-like Growth Factor-1 axis in a Transgenic Rat Model. Am. J. Pathol. 160 (6), 2259–2265. 10.1016/s0002-9440(10)61173-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A. R., Soliman M. A., Sadek K. M. (2019). Association of Insulin Growth Factor-1 and Growth Hormone Levels in Elderly Renal Transplant Recipients with Cardiac Dysfunction. Saudi J. Kidney Dis. Transpl. 30 (1), 62–67. [PubMed] [Google Scholar]
- Sonntag W. E., Carter C. S., Ikeno Y., Ekenstedt K., Carlson C. S., Loeser R. F., et al. (2005). Adult-onset Growth Hormone and Insulin-like Growth Factor I Deficiency Reduces Neoplastic Disease, Modifies Age-Related Pathology, and Increases Life Span. Endocrinology 146 (7), 2920–2932. 10.1210/en.2005-0058 [DOI] [PubMed] [Google Scholar]
- Steger R. W., Bartke A., Cecim M. (1993). Premature Ageing in Transgenic Mice Expressing Different Growth Hormone Genes. J. Reprod. Fertil. Suppl. 46, 61–75. [PubMed] [Google Scholar]
- Sun L. Y., Fang Y., Patki A., Koopman J. J., Allison D. B., Hill C. M., et al. (2017). Longevity Is Impacted by Growth Hormone Action during Early Postnatal Period. Elife 6. 10.7554/eLife.24059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. Y., Spong A., Swindell W. R., Fang Y., Hill C., Huber J. A., et al. (2013). Growth Hormone-Releasing Hormone Disruption Extends Lifespan and Regulates Response to Caloric Restriction in Mice. Elife 2, e01098. 10.7554/eLife.01098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A., Wartschow L. M., White M. F. (2007). Brain IRS2 Signaling Coordinates Life Span and Nutrient Homeostasis. Science 317 (5836), 369–372. 10.1126/science.1142179 [DOI] [PubMed] [Google Scholar]
- Takeda E., Suzuki Y., Yamada T., Katagiri H., Sato Y. (2017). Knockout of Vasohibin-1 Gene in Mice Results in Healthy Longevity with Reduced Expression of Insulin Receptor, Insulin Receptor Substrate 1, and Insulin Receptor Substrate 2 in Their White Adipose Tissue. J. Aging Res. 2017, 9851380. 10.1155/2017/9851380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M., Bartke A., Antebi A. (2003). The Endocrine Regulation of Aging by Insulin-like Signals. Science 299 (5611), 1346–1351. 10.1126/science.1081447 [DOI] [PubMed] [Google Scholar]
- Tatar M., Kopelman A., Epstein D., Tu M.-P., Yin C.-M., Garofalo R. S. (2001). A Mutant Drosophila Insulin Receptor Homolog that Extends Life-Span and Impairs Neuroendocrine Function. Science 292 (5514), 107–110. 10.1126/science.1057987 [DOI] [PubMed] [Google Scholar]
- Templeman N. M., Flibotte S., Chik J. H. L., Sinha S., Lim G. E., Foster L. J., et al. (2017). Reduced Circulating Insulin Enhances Insulin Sensitivity in Old Mice and Extends Lifespan. Cel Rep. 20 (2), 451–463. 10.1016/j.celrep.2017.06.048 [DOI] [PubMed] [Google Scholar]
- Thomas J. D., Monson J. P. (2009). Adult GH Deficiency throughout Lifetime. Eur. J. Endocrinol. 161 Suppl 1 (Suppl. 1), S97–S106. 10.1530/EJE-09-0258 [DOI] [PubMed] [Google Scholar]
- Tissenbaum H. A., Ruvkun G. (1998). An Insulin-like Signaling Pathway Affects Both Longevity and Reproduction in Caenorhabditis elegans . Genetics 148 (2), 703–717. 10.1093/genetics/148.2.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Gautam T., Koncz P., Henthorn J. C., Pinto J. T., Ballabh P., et al. (2010). Vasoprotective Effects of Life Span-Extending Peripubertal GH Replacement in Lewis dwarf Rats. Journals Gerontol. Ser. A: Biol. Sci. Med. Sci. 65A (11), 1145–1156. 10.1093/gerona/glq147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Sosnowska D., Podlutsky A., Koncz P., Sonntag W. E., Csiszar A. (2011). Free Radical Production, Antioxidant Capacity, and Oxidative Stress Response Signatures in Fibroblasts from Lewis dwarf Rats: Effects of Life Span-Extending Peripubertal GH Treatment. Journals Gerontol. Ser. A: Biol. Sci. Med. Sci. 66A (5), 501–510. 10.1093/gerona/glr004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Spoel E., Jansen S. W., Akintola A. A., Ballieux B. E., Cobbaert C. M., Slagboom P. E., et al. (2016). Growth Hormone Secretion Is Diminished and Tightly Controlled in Humans Enriched for Familial Longevity. Aging Cell 15 (6), 1126–1131. 10.1111/acel.12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis J. D., Iranmanesh A., Bowers C. Y. (2005). Joint Mechanisms of Impaired Growth-Hormone Pulse Renewal in Aging Men. J. Clin. Endocrinol. Metab. 90 (7), 4177–4183. 10.1210/jc.2005-0336 [DOI] [PubMed] [Google Scholar]
- Vijayakumar A., Yakar S., Leroith D. (2011). The Intricate Role of Growth Hormone in Metabolism. Front. Endocrin. 2, 32. 10.3389/fendo.2011.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E., Kahnt E., Ehrlein J., Hermanns W., Brem G., Wanke R. (1993). Effects of Long-Term Elevated Serum Levels of Growth Hormone on Life Expectancy of Mice: Lessons from Transgenic Animal Models. Mech. Ageing Dev. 68 (1-3), 71–87. 10.1016/0047-6374(93)90141-d [DOI] [PubMed] [Google Scholar]
- Xu J., Gontier G., Chaker Z., Lacube P., Dupont J., Holzenberger M. (2014). Longevity Effect of IGF-1R+/−mutation Depends on Genetic Background-specific Receptor Activation. Aging Cell 13 (1), 19–28. 10.1111/acel.12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S., Liu J.-L., Stannard B., Butler A., Accili D., Sauer B., et al. (1999). Normal Growth and Development in the Absence of Hepatic Insulin-like Growth Factor I. Proc. Natl. Acad. Sci. 96 (13), 7324–7329. 10.1073/pnas.96.13.7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xie Y., Berglund E. D., Coate K. C., He T. T., Katafuchi T., et al. (2012). The Starvation Hormone, Fibroblast Growth Factor-21, Extends Lifespan in Mice. Elife 1, e00065. 10.7554/eLife.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xu B. C., Maheshwari H. G., He L., Reed M., Lozykowski M., et al. (1997). A Mammalian Model for Laron Syndrome Produced by Targeted Disruption of the Mouse Growth Hormone Receptor/binding Protein Gene (The Laron Mouse). Proc. Natl. Acad. Sci. 94 (24), 13215–13220. 10.1073/pnas.94.24.13215 [DOI] [PMC free article] [PubMed] [Google Scholar]


