Abstract
Objective
The advanced lung cancer inflammation index (ALI) predicts overall survival (OS) in patients with advanced lung cancer. However, few studies have tested ALI’s prognostic effect in patients with non-small cell lung cancer (NSCLC) following video-assisted thoracic surgery (VATS), especially patients at stage III. This study investigated the relationship between ALI and outcomes of patients with NSCLC following VATS.
Methods
We retrospectively examined 339 patients with NSCLC who underwent VATS at Hebei General Hospital, China. Preoperative clinical and laboratory parameters were collected and analyzed. Optimal cutoff values of potential prognostic factors, including ALI, were determined. Kaplan–Meier and Cox regression analyses were used to determine each factor’s prognostic value.
Results
The median OS was 31 months. The optimal cutoff value for ALI was 41.20. Patients with high ALI (≥41.20) displayed increased OS (33.87 vs. 30.24 months), higher survival rates, and milder clinical characteristics. Univariate and multivariate analyses showed a significant correlation between ALI and the prognosis of patients with NSCLC, including those at stage IIIA, who underwent VATS.
Conclusions
Low ALI correlated with poor outcomes in patients with NSCLC following VATS. Preoperative ALI might be a potential prognostic biomarker for patients with NSCLC following VATS, including patients at stage IIIA.
Keywords: Non-small cell lung cancer, advanced lung cancer inflammation index, video-assisted thoracic surgery, overall survival, stage IIIA, prognostic indicator
Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide. 1 Despite progress in treatment, patients with lung cancer still have poor prognoses. Because the outcomes of individual patients are difficult to predict, it is important to explore accurate prognostic biomarkers for lung cancer. The TNM Classification of Malignant Tumors (TNM) is one of the most important predictors of overall survival (OS) for patients with lung cancer. 2 Inflammation status also correlates with cancer growth and can be used to estimate prognosis in several malignancies.3–5 Jafri et al. 6 developed an inflammation-based prognostic index known as the advanced lung cancer inflammation index (ALI), which is calculated as follows: body mass index (BMI, kg/m2) × serum albumin (ALB, g/dL) ÷ the neutrophil to lymphocyte ratio (NLR). The prognostic accuracy of ALI has been tested in patients with different malignancies including lung cancer.7–9 Non-small cell lung cancer (NSCLC) is the most common type of lung cancer. 10 However, the prognostic significance of ALI has not been adequately investigated in patients with NSCLC following video-assisted thoracic surgery (VATS) as the resection technique, especially patients at stage III. In this study, we explored whether ALI is associated with the prognosis of patients with NSCLC, especially patients at stage IIIA, who underwent VATS. Additionally, several clinical indicators and laboratory parameters such as sex, age, smoking status, performance status (PS), Charlson comorbidity index (CCI), C-creative protein (CRP), red blood cell distribution width (RDW), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR) are reported to be correlated with the prognosis of patients with malignancies including those with lung cancer.10–14 Using univariable and multivariable analyses, we discuss the prognostic effect of ALI and some of the clinical indexes for patients with NSCLC, especially patients at stage IIIA, following VATS.
Methods
Patients
For this study, we enrolled patients with NSCLC who underwent VATS, which refers to local resection of lung tissue with curative intent, at Hebei General Hospital from January 2017 to January 2019. Before surgery, all patients underwent positron emission tomography (PET)/computed tomography (CT), magnetic resonance imaging, emission CT, and other examinations to rule out distant metastasis. All patients were pathologically diagnosed with NSCLC. No patients had clinical evidence of any inflammatory conditions. Patients received no preoperative chemoradiotherapy and had no other malignancies. Anatomic pulmonary resection was preferred for the majority of patients with NSCLC. Some patients choose sublobar resection, segmentectomy, or wedge resection for the following reasons: 1) parenchymal resection margin ≥2 cm or greater than the nodule size; 2) appropriate N1 and N2 lymph node station sampling; 3) poor pulmonary reserve or other major comorbidities that contraindicated lobectomy; and 4) peripheral nodule ≤2 cm, with at least one of the following: 4a) pure adenocarcinoma in situ histology; 4b) ≥50% ground-glass appearance on CT; and 4c) radiologic surveillance confirmed a long doubling time (>400 days). After completing definitive therapy, all patients at stage I/II underwent follow-up consisting of medical history, physical examination, and chest CT ± contrast every 6 months for the first 2 to 3 years, and then medical history, physical examination, and low-dose non-contrast-enhanced chest CT were performed annually. For patients at stage IIIA, follow-up consisted of medical history, physical examination, and chest CT ± contrast every 3 to 6 months for 3 years, then medical history, physical examination, and chest CT ± contrast every 6 months for 2 years, and finally medical history, physical examination, and low-dose non-contrast-enhanced chest CT annually until death or the last follow-up. The primary end point was OS, which was defined as the duration of time from the date of surgery to the date of all-cause death or the last follow-up. For subjects who had missed follow-up visits prior to death, the time of the last follow-up was counted as the time of death. The Ethical Committee of Hebei General Hospital approved this retrospective study (No. 202155). Informed consent was waived due to the retrospective nature of the study. We confirm that data confidentiality has been maintained and compliance with the Declaration of Helsinki and its later amendments.
Variables
Clinical indicators including age, sex, BMI, smoking status, PS, CCI, pathological type, and postoperative treatment were recorded by the electronic medical records system. Laboratory parameters including red blood cell count (RBC), mean cell volume (MCV), RDW, hemoglobin count (HGB), white blood cell count (WBC), neutrophil count (NEUT), lymphocyte count (LYMPH), monocyte count (MONO), eosinophil count (EO), basophil count (BASO), platelet count (PLT), ALB, lactate dehydrogenase (LDH), and CRP were tested before surgery. ALI was calculated as BMI (kg/m2) ×ALB (g/dL) ÷ NLR. 8 Preoperative serum carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC), cytokeratin-19 fragment (CYFRA 21-1), and neuron-specific enolase (NSE) levels were measured by enzyme immunoassays at the same hospital laboratory.
Statistical analysis
Receiver operating characteristic (ROC) curve analysis was used to determine cutoff values for ALI, ALB, WBC, NEUT, LYMPH, RBC, MCV, RDW, HGB, PLT, MONO, EO, BASO, NLR, PLR, LMR, CRP, CEA, NSE, SCC, and CYFRA21-1. By estimating the optimal sensitivity, specificity, and area under the curve (AUC), ROC curves were used to predict all-cause death. Comparisons of continuous and categorical variables were performed using Pearson’s correlation, the Chi-square test, and Fisher’s exact test. Cumulative cancer-specific survival curves after surgery were calculated using the Kaplan–Meier method. Differences in OS according to ALI and other parameters were assessed using the log-rank test. The Cox proportional hazard model was used to evaluate the prognostic potential of the variables, from which hazard ratios were estimated and reported as relative risks with corresponding 95% confidence intervals. All statistical analyses were performed using IBM SPSS statistics software program, version 21.0 (IBM Corporation, Armonk, NY, USA). P < 0.05 was used to indicate statistically significant differences.
Results
Patient statistics
In total, 339 patients with NSCLC who underwent VATS at Hebei General Hospital were reviewed and analyzed according to the parameters shown in Table 1. Among the patients, 167 (49.26%) were male, and the median age was 60-years-old. There were 110 (32.45%) current or ever smokers. According to the clinical information, 315 were diagnosed with lung adenocarcinoma and 24 with squamous cell carcinoma. Eighty-six patients were determined to be stage 0, 166 to be stage IA, 31 to be stage IB, 15 to be stage II, and 41 to be IIIA. Regarding surgical procedures, 168 patients received wedge resection, 85 underwent segmentectomy, and 86 received lobectomy. Postoperative pathology of all patients revealed negative margins. The PS and CCI scores of 263 (77.58%) and 159 (46.90%) patients, respectively, were 0 or 1. The median OS of the patients was 31 months. Eighteen patients (5.31%) died during the observation period, and 36 (10.62%) were lost to follow-up. Seventy-seven patients (22.71%) received postoperative treatments including chemotherapy, radiotherapy, targeted therapy, and immunotherapy. Thirty-three patients had recurrence, among which 16 died (Figure 1).
Table 1.
Relationship between patient characteristics and ALI.
| Baseline | ALI < 41.20 | ALI ≥ 41.20 | P value | |
|---|---|---|---|---|
| n=125 | n=214 | |||
| Age | 0.460 | |||
| <65 years | 84 | 152 | ||
| ≥65 years | 41 | 62 | ||
| Sex | <0.001 | |||
| Male | 81 | 86 | ||
| Female | 44 | 128 | ||
| Pathology | 0.167 | |||
| Adenocarcinoma | 113 | 202 | ||
| Squamous cell carcinoma | 12 | 12 | ||
| Death | <0.001 | |||
| Yes | 32 | 22 | ||
| No | 93 | 192 | ||
| Stage | 0.045 | |||
| 0 | 28 | 58 | ||
| IA | 54 | 112 | ||
| IB-II | 22 | 24 | ||
| IIIA | 21 | 20 | ||
| Surgical Procedure | 0.104 | |||
| Wedge resection | 57 | 111 | ||
| Segmentectomy | 28 | 57 | ||
| Lobectomy | 40 | 46 | ||
| Smoking | 0.012 | |||
| Never | 74 | 155 | ||
| Current/ever | 51 | 59 | ||
| BMI | 0.015 | |||
| <18.5 | 5 | 3 | ||
| ≥18.5 to <24 | 71 | 95 | ||
| ≥24 | 49 | 116 | ||
| Recurrence | 0.001 | |||
| Yes | 21 | 12 | ||
| No | 104 | 202 | ||
| PS | 0.585 | |||
| 0–1 | 99 | 164 | ||
| ≥2 | 26 | 50 | ||
| CCI | 0.222 | |||
| 0–1 | 61 | 98 | ||
| 2–3 | 13 | 13 | ||
| ≥4 | 51 | 103 | ||
| Postoperative Treatment | 0.520 | |||
| Yes | 26 | 51 | ||
| No | 99 | 163 | ||
| CRP (mg/L) | 0.270 | |||
| <0.05 | 1 | 2 | ||
| ≥0.05 | 56 | 114 | ||
| WBC (×109/L) | 0.619 | |||
| <5.63 | 66 | 107 | ||
| ≥5.63 | 59 | 107 | ||
| RBC (×1012/L) | 0.320 | |||
| <5.11 | 107 | 191 | ||
| ≥5.11 | 18 | 23 | ||
| NEUT (×109/L) | 0.079 | |||
| <3.12 | 54 | 72 | ||
| ≥3.12 | 71 | 142 | ||
| LYMPH (×109/L) | 0.540 | |||
| <2.47 | 112 | 196 | ||
| ≥2.47 | 13 | 18 | ||
| HGB (g/L) | 0.225 | |||
| <151.50 | 96 | 176 | ||
| ≥151.50 | 29 | 38 | ||
| PLT (×109/L) | 0.148 | |||
| <459 | 124 | 207 | ||
| ≥459 | 1 | 7 | ||
| MONO (×109/L) | 0.029 | |||
| <0.57 | 125 | 206 | ||
| ≥0.57 | 0 | 8 | ||
| EO (×109/L) | 0.934 | |||
| <0.11 | 80 | 136 | ||
| ≥0.11 | 45 | 78 | ||
| BASO (×109/L) | 0.056 | |||
| <0.08 | 120 | 212 | ||
| ≥0.08 | 5 | 2 | ||
| MCV (fL) | 0.973 | |||
| <90.55 | 31 | 47 | ||
| ≥90.55 | 30 | 46 | ||
| RDW (%) | 0.262 | |||
| <11.65 | 2 | 7 | ||
| ≥11.65 | 60 | 86 | ||
| ALB (g/dL) | 0.385 | |||
| <4.24 | 46 | 89 | ||
| ≥4.24 | 79 | 125 | ||
| LDH (U/L) | 0.901 | |||
| <187.25 | 89 | 151 | ||
| ≥187.25 | 36 | 63 | ||
| CEA (ng/mL) | 0.759 | |||
| <4.21 | 80 | 133 | ||
| ≥4.21 | 18 | 27 | ||
| NSE (ng/mL) | 0.624 | |||
| <10.18 | 22 | 40 | ||
| ≥10.18 | 59 | 92 | ||
| CYFRA21-1 (ng/mL) | 0.197 | |||
| <3.56 | 69 | 103 | ||
| ≥3.56 | 13 | 31 | ||
| SCC (ng/mL) | 0.391 | |||
| <0.69 | 15 | 18 | ||
| ≥0.69 | 71 | 118 | ||
| NLR | 0.265 | |||
| <1.89 | 52 | 76 | ||
| ≥1.89 | 73 | 138 | ||
| LMR | 0.048 | |||
| <3.74 | 10 | 33 | ||
| ≥3.74 | 115 | 151 | ||
| PLR | 0.758 | |||
| <87.26 | 11 | 21 | ||
| ≥87.26 | 114 | 193 |
Note: The number of deaths in the death group is the total number of deaths and loss to follow-up.
ALI, advanced lung cancer inflammation index; BMI, body mass index; PS, performance status; CCI, Charlson comorbidity index; CRP, C-reactive protein; WBC, white blood cell; RBC, red blood cell; NEUT, Neutrophil; LYMPH, Lymphocyte; HGB, hemoglobin; PLT, platelet; MONO, monocyte; EO, eosinophil; BASO, basophil; MCV, mean corpuscular volume; RDW, red cell distribution width; ALB, albumin; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; NSE, neuron specific enolase; CYFRA 21-1, cytokeratin-19 fragment; SCC, squamous cell carcinoma antigen; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PLR, platelet to lymphocyte ratio.
Figure 1.
The flowchart for patients included in the study group.
Comparison between groups
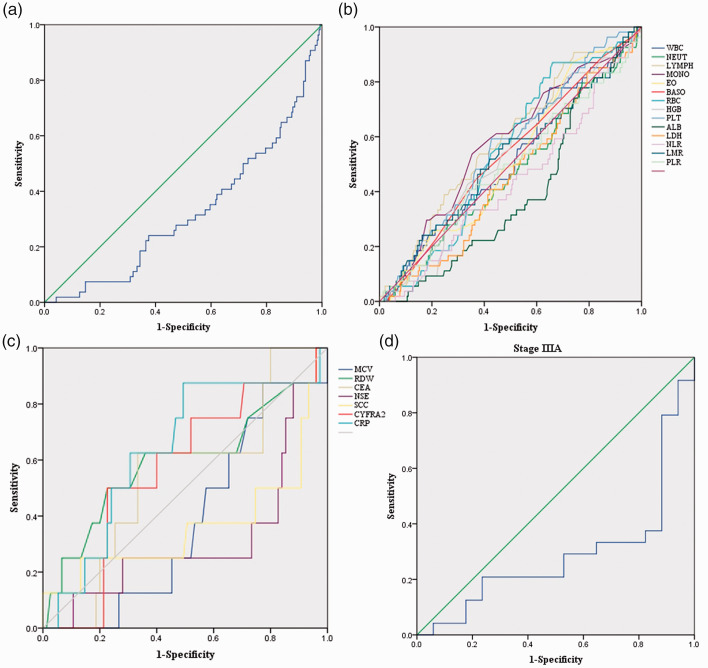
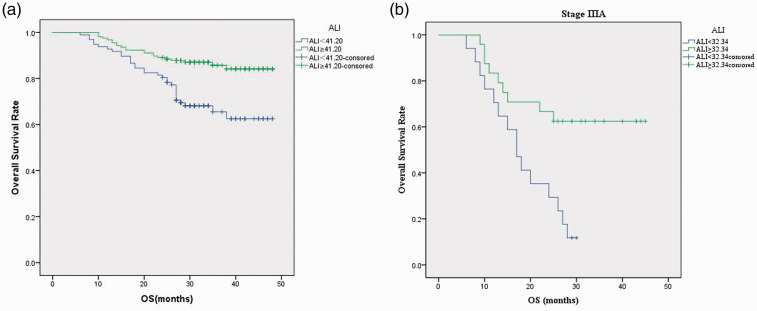
Relationships between baseline characteristics and ALI are shown in Table 1. ROC curve analysis showed the cutoff value of ALI for the layering of OS was 41.20 (Figure 2a). The optimal cutoff points analyzed by ROC curves for other parameters are shown in Figure 2b and 2c. Patients were divided into two groups according to the ALI cutoff value: the low ALI group (ALI < 41.20, n = 125) and the high ALI group (ALI≥41.20, n = 214). Postoperative survival curves of the two groups are shown in Figure 3a. The curves showed a significant difference in patient survival rates (P < 0.001). Patients in the high ALI group had longer OS (33.87 vs. 30.24 months, P < 0.001) and displayed milder clinical and laboratory characteristics than those in the low ALI group.
Figure 2.
Receiver operating characteristic (ROC) curves. (a) ROC curve for the advanced lung cancer inflammation index (ALI). The area under the curve (AUC) is 0.324, and the corresponding optimal cutoff value of ALI is 41.20. (b) ROC curves for white blood cell (WBC), neutrophil (NEUT), lymphocyte (LYMPH), monocyte (MONO), eosinophil (EO), basophil (BASO), red blood cell (RBC), hemoglobin (HGB), platelet (PLT), albumin (ALB), lactate dehydrogenase (LDH), neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), and platelet to lymphocyte ratio (PLR). The optimal cutoff points for WBC (×109/L), NEUT (×109/L), LYMPH (×109/L), MONO (×109/L), EO (×109/L), BASO (×109/L), RBC (×1012/L), HGB (g/L), PLT (×109/L), ALB (g/dL), LDH (U/L), NLR, LMR, and PLR are 5.63, 3.12, 2.47, 0.57, 0.11, 0.08, 5.11, 151.50, 459.00, 4.24, 187.25, 1.89, 3.74, and 87.26, respectively; the corresponding AUCs were 0.517, 0.472, 0.585, 0.574, 0.518, 0.532, 0.545, 0.526, 0.564, 0.391, 0.458, 0.432, 0.522, and 0.489, respectively. (c) ROC curves for mean corpuscular volume (MCV), red cell distribution width (RDW), carcinoembryonic antigen (CEA), neuron specific enolase (NSE), cytokeratin-19 fragment (CYFRA 21-1), squamous cell carcinoma antigen (SCC), and C-reactive protein (CRP). The optimal cutoff values for MCV (fL), RDW (%), CEA (ng/mL), NSE (ng/mL), SCC (ng/mL), CYFRA21-1 (ng/mL), and CRP (mg/L) are 90.55, 11.65, 4.21, 10.18, 0.69, 3.56, and 0.05, respectively, with AUCs of 0.382, 0.594, 0.543, 0.315, 0.364, 0.568, and 0.638, respectively. (d) ROC curve for ALI in patients at stage IIIA. The AUC is 0.289, and the corresponding optimal cutoff value for ALI is 32.34.
Figure 3.
Overall survival (OS) curves for patients at stage I to IIIA following video-assisted thoracic surgery (VATS). (a) Curve indicating that the high advanced lung cancer inflammation index (ALI) group (ALI ≥ 41.20) had increased survival rates and survival times (P < 0.05). (b) Curve indicating that among patients with stage IIIA NSCLC, the high ALI group (ALI ≥ 41.20) had increased survival rates and survival times (P < 0.05).
OS, sex, death rate, smoking status, BMI, recurrence rate, stage, MONO, and LMR were significantly different between the two groups (P < 0.05). Univariate analyses of each parameter are shown in Table 2 (performed in regard to OS). In univariable analysis of OS, low ALI (<41.20), squamous cell carcinoma, male sex, age >65 years, smoking, cancer recurrence, PS≥2, CCI≥3, and BMI<18.5 were significant factors for poor survival (P < 0.05, Table 2). Additionally, patients with low ALB (<4.24 g/dL), high LDH (≥187.25 U/L), high PLR (≥87.26), high SCC (≥0.69 ng/mL), and later stage disease (>IB) had worse OS than those with high ALB, low LDH, low PLR, low SCC, and earlier stage disease (P < 0.05, Table 2). Results of multivariable analyses using the Cox hazard model are shown in Table 2. The results indicated that low ALI (<41.20), smoking, high LDH (≥187.25 U/L), high PLR (≥87.26), high RDW (≥11.65%), and high MCV (≥90.55 fL) were independent predictors of poor prognosis (P < 0.05).
Table 2.
Univariable and multivariable analyses.
| Univariable analysis |
Multivariable analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI |
P value | HR | 95% CI |
|||
| LL | UL | LL | UL | |||||
| ALI | <0.0001 | 2.748 | 1.595 | 4.734 | 0.041 | 4.779 | 1.068 | 21.380 |
| Age | 0.008 | 0.483 | 0.283 | 0.824 | 0.736 | 1.252 | 0.340 | 4.613 |
| Sex | <0.0001 | 0.247 | 0.130 | 0.469 | 0.252 | 0.165 | 0.008 | 3.584 |
| Pathology | <0.0001 | 0.214 | 0.115 | 0.400 | ||||
| Smoking | <0.0001 | 0.166 | 0.092 | 0.298 | 0.006 | 0.046 | 0.005 | 0.413 |
| BMI < 18.5 | 0.139 | 0.213 | ||||||
| BMI 18.5–24 | 0.048 | 3.379 | 1.013 | 11.270 | 0.100 | 35.686 | 0.504 | 2525.755 |
| BMI ≥ 24 | 0.517 | 1.200 | 0.691 | 2.084 | 0.691 | 0.751 | 0.182 | 3.089 |
| Recurrence | <0.0001 | 0.034 | 0.019 | 0.060 | ||||
| Stage | <0.0001 | 0.019 | ||||||
| Stage IA | 0.920 | 0.000 | 0.000 | 6.661E + 114 | 0.954 | 0.000 | 0.000 | 1.240E + 206 |
| Stage IB–II | <0.0001 | 0.063 | 0.030 | 0.132 | 0.002 | 0.069 | 0.013 | 0.364 |
| Stage IIIA | 0.037 | 0.531 | 0.293 | 0.963 | 0.106 | 0.250 | 0.046 | 1.343 |
| PS | <0.0001 | 0.305 | 0.179 | 0.521 | 0.713 | 0.719 | 0.124 | 4.172 |
| CCI=0–1 | 0.006 | 0.263 | ||||||
| CCI=2 | 0.003 | 0.269 | 0.114 | 0.635 | 0.103 | 12.227 | 0.604 | 247.356 |
| CCI=2–3 | 0.161 | 0.574 | 0.263 | 1.249 | 0.171 | 7.615 | 0.416 | 139.271 |
| Postoperative treatment | 0.564 | 0.836 | 0.455 | 1.537 | ||||
| CRP (mg/L) | 0.267 | 3.129 | 0.417 | 23.492 | ||||
| WBC (×109/L) | 0.516 | 1.194 | 0.698 | 2.043 | ||||
| RBC (×1012/L) | 0.138 | 2.416 | 0.754 | 7.742 | 0.564 | 1.922 | 0.209 | 17.663 |
| NEUT (×109/L) | 0.255 | 1.366 | 0.798 | 2.336 | ||||
| LYMPH (×109/L) | 0.338 | 1.767 | 0.552 | 5.662 | ||||
| HGB (g/L) | 0.055 | 2.462 | 0.981 | 6.181 | 0.266 | 3.359 | 0.397 | 28.460 |
| PLT (×109/L) | 0.437 | 20.862 | 0.010 | 44278.292 | ||||
| MONO (×109/L) | 0.424 | 20.911 | 0.012 | 36194.887 | ||||
| EO (×109/L) | 0.331 | 1.336 | 0.745 | 2.397 | ||||
| BASO (×109/L) | 0.469 | 20.756 | 0.006 | 76791.105 | ||||
| MCV (fL) | 0.068 | 2.291 | 0.942 | 5.570 | 0.004 | 19.577 | 2.612 | 146.740 |
| RDW (%) | 0.086 | 2.894 | 0.860 | 9.738 | 0.033 | 9.028 | 1.197 | 68.071 |
| ALB (g/dL) | 0.002 | 2.396 | 1.392 | 4.126 | 0.399 | 2.105 | 0.374 | 11.853 |
| LDH (U/L) | 0.036 | 2.151 | 1.052 | 4.401 | 0.019 | 9.568 | 1.449 | 63.177 |
| CEA (ng/mL) | 0.110 | 2.611 | 0.805 | 8.471 | ||||
| NSE (ng/mL) | 0.188 | 1.598 | 0.795 | 3.215 | ||||
| CYFRA21-1 (ng/mL) | 0.259 | 1.828 | 0.641 | 5.215 | ||||
| SCC (ng/mL) | <0.0001 | 3.526 | 1.748 | 7.127 | ||||
| NLR | 0.054 | 1.689 | 0.990 | 2.879 | 0.369 | 0.474 | 0.093 | 2.416 |
| LMR | 0.311 | 1.448 | 0.708 | 2.962 | 0.331 | 0.351 | 0.043 | 2.894 |
| PLR | 0.033 | 2.180 | 1.064 | 1.465 | 0.012 | 27.658 | 2.102 | 363.873 |
ALI, advanced lung cancer inflammation index; BMI, body mass index; PS, performance status; CCI, Charlson comorbidity index; CRP, C-reactive protein; WBC, white blood cell; RBC, red blood cell; NEUT, Neutrophil; LYMPH, Lymphocyte; HGB, hemoglobin; PLT, platelet; MONO, monocyte; EO, eosinophil; BASO, basophil; MCV, mean corpuscular volume; RDW, red cell distribution width; ALB, albumin; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; NSE, neuron specific enolase; CYFRA21-1, cytokeratin-19 fragment; SCC, squamous cell carcinoma antigen; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PLR, platelet to lymphocyte ratio; HR, hazard ratio; LL, lower limit; UL, upper limit.
For patients at stage IIIA with an ALI cutoff value of 32.34 (Figure 2d), 13 (31.71%) died during the observation period and 11 (26.83%) were lost to follow-up. Six patients were staged with N0 disease by the TNM system, while nine were staged with N1 and 26 with N2 malignancies. Kaplan–Meier analysis showed that ALI was an prognostic indicator for patients with stage IIIA NSCLC following curative VATS as the lobectomy technique (Figure 3b, P < 0.05). Additionally, Kaplan–Meier analysis showed that the prognosis of each N stage for patients with stage IIIA NSCLC was not statistically different between the high ALI group and the low ALI group.
Discussion
The correlation between systemic inflammation and cancer has been widely explored for decades. Systemic inflammation and immune responses are thought to affect carcinogenesis by regulating angiogenesis and cellular proliferation. 3 Inflammatory markers have been found to be expressed in the tumor microenvironment, and their importance in cancer etiology has been proven, which indicates the prognostic significance of inflammatory factors in different cancers.15–17 It has been reported that absolute inflammatory cell counts in peripheral blood including WBC, NEUT, LYMPH and ratios based on those cell counts such as NLR, PLR, and LMR may provide valuable information for the prognosis of patients with cancer.18–20 Inflammation is involved in multiple pathological conditions. Our study investigated several inflammatory factors and indicated the prognostic power of some parameters.
Lymphopenia may serve as a biomarker of poor prognosis in patients with terminal malignancies. Lymphocytes play important roles in regulating cancer progression through tumor immune surveillance and cytotoxic activity, which kills cancer cells. 21 Neutrophils were reported to participate in tumor proliferation and metastasis by remodeling the extracellular matrix and releasing reactive oxygen species and nitric oxide.22,23 The prognostic power of NLR in patients with cancer has been reported by various studies,14,19,20 consistently, elevated NLR may predict poor outcomes in lung cancer.
Cancer cachexia is common in advanced malignancies and is a multi-factorial syndrome that impairs patients’ quality of life and response to treatment. Cachexia is considered to be the clinical consequence of interactions between tumor, metabolic, and inflammatory factors. 24 Cachexia rarely appears in patients with early-stage cancer. However, estimations of body composition are widely considered to be important prognostic indicators in patients with operable tumors. Body weight loss in advanced cancer may increase the risk of mortality. 25 Muscle and fat burden are decreased as cancer advances, which results in body weight loss and increases the risk of mortality. 26 BMI and ALB have been shown to be useful indicators for evaluating nutritional status. Both parameters are closely related to survival in patients with operable lung cancers.27–29 Therefore, BMI and ALB are likely to have prognostic power in patients with NSCLC who underwent VATS. As shown in previous studies,6,7,19,30–33 ALI has prognostic power for patients with lung cancer. As a result, ALI, which is calculated from NEUT, LYMPH, BMI, and ALB, may serve as a prognostic factor for patients with NSCLC following VATS.
We studied other factors that may affect the prognosis of patients with NSCLC. Platelets can secret growth factors that stimulate tumor angiogenesis and contribute to the adhesion and transmigration of tumor cells, which can promote the metastatic potential of tumor cells. 34 Monocytes can facilitate the progression and dissemination of tumor cells by promoting tumor growth and survival. 35 Studies have shown that peripheral monocyte levels are negatively associated with prognosis in patients with cancer.23,36 Moreover, as reported by various studies, elevated PLR and reduced LMR may predict poor outcomes in lung cancer.14,18–20 In this study, PLR showed a strong prognostic power for patients with NSCLC following VATS. WBC, NEUT, LYMPH, PLT, MONO, CRP, NLR, and LMR showed no prognostic value, which may be because the majority of our samples were at the early stages (stage 0–I) and did not show obvious inflammatory responses.
Inflammation may cause changes in erythrocyte maturation. 13 RDW is often used to diagnose anemia and evaluates changes in the size of peripheral red blood cells in circulation. Recent studies have indicated a correlation between MCV and RDW, and cancer prognosis.37–39 Increased RDW has been found to be related to an increase in various inflammatory markers, oxidative stress responses, and malnutrition.40,41 Because RDW and MCV may be related to inflammation and malnutrition, there is a great possibility that they could have prognostic power in lung cancer.
LDH is associated with tumor hypoxia, neoangiogenesis, and metastasis and has been shown to be correlated with poor outcomes in several malignancies including NSCLC.42,43 Our findings indicated the prognostic power of LDH in patients with NSCLC who underwent VATS. The underlying mechanism will need to be further characterized, as there is no evidence of a direct between LDH and NSCLC development.
Our findings demonstrate the importance of systemic inflammatory status and malnutrition in the prognosis of patients with NSCLC undergoing VATS. We determined a cutoff value of 41.20 for ALI with ROC curve analysis. In previous studies, Jafri et al. 6 reported an ALI cutoff value of 18 for advanced NSCLC, Kobayashi et al. 31 reported an ALI cutoff value of 22.2 for early-stage NSCLC, and Tomita et al. 32 reported an ALI cutoff value of 37.66 for patients with resected NSCLC. Additionally, Kim et al. 30 reported an ALI cutoff value of 31.1 for patients with SCLC, while Zhou et al. 33 reported a cutoff value of 47.0; the cut-off was determined to be 19.5 by He et al. 7 Our study resulted in a cutoff value within the range of those reports. OS, sex, death rate, smoking status, BMI, stage, recurrence rate, MONO, and LMR were significantly different between patients in the high and low ALI groups. Our analysis strongly indicated that ALI could be a prognostic factor for patients with NSCLC following VATS. For patients at stage IIIA in our study, ALI was associated with OS and living status, indicating its prognostic power for patients with stage IIIA NSCLC who underwent curative VATS.
Our analysis suggests that preoperative ALI is a prognostic factor for operable NSCLC, along with smoking, pathological type, male sex, age >65 years, late stage, cancer recurrence, BMI, PS, CCI, ALB, LDH, PLR, and SCC. Furthermore, ALI, smoking, stage, PLR, LDH, RDW, and MCV could be independent prognostic factors. The other inflammatory indicators we evaluated were not significantly associated with prognosis, which may be because our data contain several patients at the early stage who did not show obvious inflammatory responses. Radical resection was associated with significantly longer OS in this NSCLC cohort. Our data showed that ALI could be a prognostic indicator for patients with stage IIIA NSCLC following VATS, which has never been reported. By characterizing the prognostic factors of patients with NSCLC, we can better assess their prognostic risk and give appropriate postoperative treatments to effectively extend OS.
Limitations
The study had limitations in the following aspects: 1) this was a single-center retrospective study with no postoperative ALI data, which may have resulted in selection bias; and 2), there was a small number of patients at stage IIIA. As ALI could be an independent prognostic factor, a large-scale multicenter prospective validation study is required to establish more reliable and independent parameters.
Conclusions
Inflammation and nutrition status are vital markers for cancer progression and prognosis. Preoperative ALI, along with several clinical factors, might have prognostic power for patients with NSCLC who undergo VATS. Furthermore, multivariate analysis indicated that ALI may be an independent and effective prognostic factor for patients with NSCLC following VATS. For patients with stage IIIA NSCLC who underwent curative lobectomy via VATS, ALI could be an effective prognostic factor. The prognostic effect of ALI for patients with NSCLC can be adapted into clinical practice to stratify patients for future trials.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by the Scientific Research Program of Traditional Chinese Medicine (No. 2019139).
ORCID iD: Guochen Duan https://orcid.org/0000-0001-9608-4066
References
- 1.Siegel RL Miller KD andJemal A.. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. DOI: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015; 33: 861–869. DOI: 10.1200/jco.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 3.Diakos CI, Charles KA, McMillan DC, et al . Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014; 15: e493–e503. DOI: 10.1016/s1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 4.Grange JM Krone B andMastrangelo G.. Infection, inflammation and cancer. Int J Cancer 2011; 128: 2240–2241. DOI: 10.1002/ijc.25533. [DOI] [PubMed] [Google Scholar]
- 5.Tomita M, Shimizu T, Ayabe T, et al. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res 2012; 32: 3535–3538. [PubMed] [Google Scholar]
- 6.Jafri SH Shi R andMills G.. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013; 13: 158. DOI: 10.1186/1471-2407-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Zhou T, Yang Y, et al. Advanced Lung Cancer Inflammation Index, a New Prognostic Score, Predicts Outcome in Patients With Small-Cell Lung Cancer. Clin Lung Cancer 2015; 16: e165–e171. DOI: 10.1016/j.cllc.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Kusunoki K, Toiyama Y, Okugawa Y, et al. Advanced Lung Cancer Inflammation Index Predicts Outcomes of Patients With Colorectal Cancer After Surgical Resection. Dis Colon Rectum 2020; 63: 1242–1250. DOI: 10.1097/dcr.0000000000001658. [DOI] [PubMed] [Google Scholar]
- 9.Topkan E, Ozdemir Y, Kucuk A, et al. Low Advanced Lung Cancer Inflammation Index Predicts Poor Prognosis in Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Definitive Concurrent Chemoradiotherapy. J Oncol 2020; 2020: 3127275. DOI: 10.1155/2020/3127275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torre LA Siegel RL andJemal A.. Lung Cancer Statistics. Adv Exp Med Biol 2016; 893: 1–19. DOI: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Wu W, Zhang X, et al. Advanced Lung Cancer Inflammation Index is a Prognostic Factor of Patients with Small-Cell Lung Cancer Following Surgical Resection. Cancer Manag Res 2021; 13: 2047–2055. DOI: 10.2147/cmar.S295952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francescone R Hou V andGrivennikov SI.. Microbiome, inflammation, and cancer. Cancer J 2014; 20: 181–189. DOI: 10.1097/ppo.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirahara N, Tajima Y, Fujii Y, et al. Comprehensive Analysis of Red Blood Cell Distribution Width as a Preoperative Prognostic Predictor in Gastric Cancer. Anticancer Res 2019; 39: 3121–3130. DOI: 10.21873/anticanres.13448. [DOI] [PubMed] [Google Scholar]
- 14.Kim EY, Lee JW, Yoo HM, et al. The Platelet-to-Lymphocyte Ratio Versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Ann Surg Oncol 2015; 22: 4363–4370. DOI: 10.1245/s10434-015-4518-z. [DOI] [PubMed] [Google Scholar]
- 15.Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med 2019; 18: 121–126. DOI: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkwill F andMantovani A.. Inflammation and cancer: back to Virchow? Lancet 2001; 357: 539–545. DOI: 10.1016/s0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 17.Proctor MJ, Morrison DS, Talwar D, et al . A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011; 47: 2633–2641. DOI: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Lin S, Fang Y, Mo Z, et al. Prognostic value of lymphocyte to monocyte ratio in pancreatic cancer: a systematic review and meta-analysis including 3338 patients. World J Surg Oncol 2020; 18: 186. DOI: 10.1186/s12957-020-01962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019; 8: 886–894. DOI: 10.21037/tlcr.2019.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao QT, Yang Y, Xu S, et al. Prognostic role of neutrophil to lymphocyte ratio in lung cancers: a meta-analysis including 7,054 patients. Onco Targets Ther 2015; 8: 2731–2738. DOI: 10.2147/ott.S90875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ocana A, Nieto-Jiménez C, Pandiella A, et al. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer 2017; 16: 137. DOI: 10.1186/s12943-017-0707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rapoport BL, Steel HC, Theron AJ, et al. Role of the Neutrophil in the Pathogenesis of Advanced Cancer and Impaired Responsiveness to Therapy. Molecules 2020; 25: 1618. DOI: 10.3390/molecules25071618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valero C, Pardo L, López M, et al. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck 2017; 39: 219–226. DOI: 10.1002/hed.24561. [DOI] [PubMed] [Google Scholar]
- 24.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–495. DOI: 10.1016/s1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 25.McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc 2008; 67: 257–262. DOI: 10.1017/s0029665108007131. [DOI] [PubMed] [Google Scholar]
- 26.Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr 2017; 36: 1187–1196. DOI: 10.1016/j.clnu.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y Zhao L andPeng F.. Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics (Sao Paulo) 2013; 68: 686–693. DOI: 10.6061/clinics/2013(05)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa T, Toyazaki T, Chiba N, et al. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Thorac Surg 2016; 23: 560–566. DOI: 10.1093/icvts/ivw175. [DOI] [PubMed] [Google Scholar]
- 29.Tewari N, Martin-Ucar AE, Black E, et al. Nutritional status affects long term survival after lobectomy for lung cancer. Lung Cancer 2007; 57: 389–394. DOI: 10.1016/j.lungcan.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Kim EY, Kim N, Kim YS, et al. Prognostic Significance of Modified Advanced Lung Cancer Inflammation Index (ALI) in Patients with Small Cell Lung Cancer_ Comparison with Original ALI. PLoS One 2016; 11: e0164056. DOI: 10.1371/journal.pone.0164056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi S, Karube Y, Inoue T, et al. Advanced Lung Cancer Inflammation Index Predicts Outcomes of Patients with Pathological Stage IA Lung Adenocarcinoma Following Surgical Resection. Ann Thorac Cardiovasc Surg 2019; 25: 87–94. DOI: 10.5761/atcs.oa.18-00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomita M Ayabe T andNakamura K.. The advanced lung cancer inflammation index is an independent prognostic factor after surgical resection in patients with non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2018; 26: 288–292. DOI: 10.1093/icvts/ivx329. [DOI] [PubMed] [Google Scholar]
- 33.Zhou T, Zhao Y, Zhao S, et al. Comparison of the Prognostic Value of Systemic Inflammation Response Markers in Small Cell Lung Cancer Patients. J Cancer 2019; 10: 1685–1692. DOI: 10.7150/jca.29319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Li Z, Sun L, et al. Platelets enhance the ability of bone-marrow mesenchymal stem cells to promote cancer metastasis. Onco Targets Ther 2018; 11: 8251–8263. DOI: 10.2147/ott.S181673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YQ, Zhu YJ, Pan JH, et al. Peripheral monocyte count: an independent diagnostic and prognostic biomarker for prostate cancer - a large Chinese cohort study. Asian J Androl 2017; 19: 579–585. DOI: 10.4103/1008-682x.186185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han L, Jia Y, Song Q, et al. Prognostic significance of preoperative absolute peripheral monocyte count in esophageal squamous cell carcinoma. Dis Esophagus 2016; 29: 740–746. DOI: 10.1111/dote.12401. [DOI] [PubMed] [Google Scholar]
- 37.Han F, Liu Y, Cheng S, et al. Diagnosis and survival values of neutrophil-lymphocyte ratio (NLR) and red blood cell distribution width (RDW) in esophageal cancer. Clin Chim Acta 2019; 488: 150–158. DOI: 10.1016/j.cca.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Sun P, Zhang F, Chen C, et al. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from southern China. Oncotarget 2016; 7: 42650–42660. DOI: 10.18632/oncotarget.9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang D, Quan W, Wu J, et al. The value of red blood cell distribution width in diagnosis of patients with colorectal cancer. Clin Chim Acta 2018; 479: 98–102. DOI: 10.1016/j.cca.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Wang PF, Song SY, Guo H, et al. Prognostic role of pretreatment red blood cell distribution width in patients with cancer: A meta-analysis of 49 studies. J Cancer 2019; 10: 4305–4317. DOI: 10.7150/jca.31598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao T Cui L andLi A.. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark 2016; 16: 507–512. DOI: 10.3233/cbm-160591. [DOI] [PubMed] [Google Scholar]
- 42.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer 2003; 89: 877–885. DOI: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langhammer S, Najjar M, Hess-Stumpp H, et al. LDH-A influences hypoxia-inducible factor 1α (HIF1 α) and is critical for growth of HT29 colon carcinoma cells in vivo. Target Oncol 2011; 6: 155–162. DOI: 10.1007/s11523-011-0184-7. [DOI] [PubMed] [Google Scholar]