Abstract
Over the past 30 years, arthroscopic rotator cuff repair (ARCR) has evolved to become the gold standard in treating rotator cuff pathology. As procedural concepts of ARCR continue to improve, it is also continually compared with the open rotator cuff repair as the historical standard of care. This review highlights the evolution of ARCR, including a historical perspective; the anatomic, clinical, and surgical implications of the development of an arthroscopic approach; how arthroscopy improved some of the problems of the open approach; adaptations in techniques and technologies associated with ARCR; future perspectives in orthobiologics as they pertain to ARCR; and lastly, the clinical improvements, or lack of improvements, with all of these adaptations.
Keywords: shoulder arthroscopy, arthroscopic rotator cuff repair, orthobiologics
Over the past 30 years, arthroscopic rotator cuff repair (ARCR) has evolved to become the gold standard in treating rotator cuff pathology. 14 In fact, between 2004 and 2009, the percentage of rotator cuff repairs (RCRs) performed arthroscopically increased from 48.8% to 74.3%, whereas the percentage of open cases decreased from 51.2% to 25.7%. 94 While today’s techniques and technologies appear to make open rotator cuff repair (ORCR) seem antiquated, the paradigm shift did not occur overnight. The ARCR procedure has evolved and continues to improve; however, it is also continually compared with the ORCR as the historical standard of care. 21 This review highlights the evolution of ARCR, including a historical perspective; the anatomic, clinical, and surgical implications of the development of an arthroscopic approach; how arthroscopy improved some of the problems of the open approach; adaptations in techniques and technologies associated with ARCR; and lastly, future perspectives in orthobiologics as they pertain to ARCR.
History of ARCR
The endoscope was first described by a German urologist named Philipp Bozzini in 1806, and the arthroscope did not follow until 1912, when a Danish surgeon named Severin Nordentoft examined the knee joint. 20 The arthroscope was introduced to the glenohumeral joint in a cadaveric study by Burman 19 in 1931 when he compared arthroscopic and open findings. In 1967, the arthroscopic technique took a giant leap when Masaki Watanabe published his Atlas of Arthroscopy, as well as introduced fiber optics to the arthroscope, thus allowing the technique to spread more widely. 27,74 Watanabe is additionally credited for describing the posterior viewing portal and founding the International Arthroscopy Association, both in the mid-1970s. 74 It would not be until 1989 when the various anterior arthroscopy working portals would be additionally described by Eugene Wolf. 74
In an effort to better diagnose and repair rotator cuff injuries, in 1990, Levy et al 46 reported on the results of a combined mini-open and arthroscopically assisted technique in 25 patients with a minimum of 1-year follow-up. However, arthroscopic margin convergence of rotator cuff tears was being performed as early as the late 1980s by pioneers such as Steve Burkhart. 14 By 1993, a preliminary report by Stephen Snyder 80 and a technical note by Raymond Thal 84 officially introduced ARCR to the United States and paved the way for modern ARCR techniques.
In the mid-1990s, ARCR evolved through the hands of several young leaders, including Jim Esch, Stephen Snyder, Dick Caspari, Lanny Johnson, Eugene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. 14 Margin convergence for massive tears was formally introduced by Burkhart et al 16 in 1996, and shortly after, in 1998, Tauro 83 reported on 2- and 3-year follow-ups for ARCR using a single-row technique with both simple and mattress sutures. Tauro 82 would add to the growing knowledge of the field by describing the anterior interval slide technique in 1999.
As ARCR surgery transitioned into the 2000s, the techniques and technologies would only continue to expand with the goal of improved surgeon efficiency and accuracy and improved rotator cuff healing, all while matching the anatomic restoration of the footprint achieved using an open technique. Double-row methods had been used in mini-open techniques for some time, but in 2003, Lo and Burkhart 48 adapted the double-row repair technique to ARCR to improve healing by reestablishing the footprint of the rotator cuff. Various surgeons would follow suit aiming to maximize efficiency, apposition, biomechanical stability, and healing.
Millett et al 61 in 2004 were the first to describe linking the medial and lateral rows and also creating a crisscross method that has now become the gold standard method of ARCR. Park et al 68 called this method of linking the medial and lateral row the “transosseous-equivalent” RCR technique in 2006. Burkhart et al 14 would further discuss the self-reinforcing properties of such a linked ARCR. Many future endeavors at optimizing technique and implant designs would stem from the linked concept, and clinical trials with controls would validate the efficacy of these new fixation methods. 17,24 Entering into the 2010s, it was noted that there was an increase in the number of relevant articles that pertained to ARCR, particularly in how to optimize the biomechanics of the repair to advance clinical outcomes. 54 As the biomechanical repair constructs were refined, it was postulated that attention should be turned from the technological enhancement of repair toward the biological enhancement, and the focus shifted to clinical investigations into the addition of orthobiologics with ARCR. Herein, a more detailed description of the evolution these techniques, implants, and technologies is given.
Evolution of ARCR Techniques
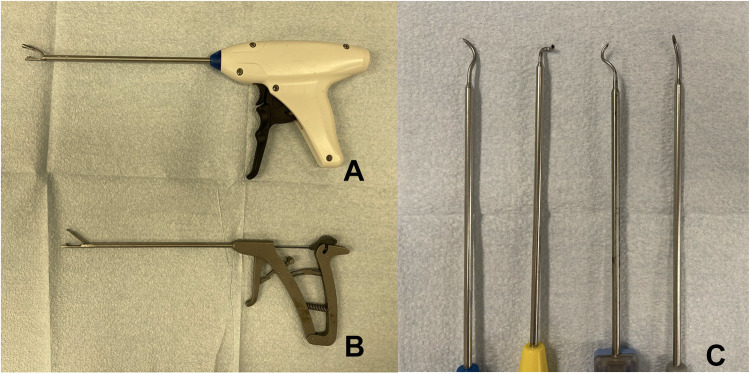
The development of ARCR would stem from some of the issues historically attributed to ORCR, 1 including poor pain control, stiffness, deltoid violation, infection risk, delayed recovery, and adequate visualization and characterization of the rotator cuff tear, which, in turn, correlate with proper anatomic repair technique. 21,36 Several critical prerequisites addressed these issues, including the 30° arthroscope, the arthroscopic pump for fluid management, bipolar radiofrequency ablation, arthroscopic instrumentation (probe, scissors, graspers, crochet hooks, suture retrievers, tissue graspers, knot pushers, guillotine knot cutters), suture passers, cannulas, and switching sticks (Figure 1). 52 Research and development of devices and techniques were closely tied to innovations from the medical device industry. 47,52 With regard to soft tissue management, deltoid takedown and repair posed unique risks to the patients and precluded them from an accelerated rehabilitation program. There was also the small but real risk for deltoid avulsion. 36,51,64,91,93 Several studies 1,33 –35,75,76 have reported increased pain with open techniques, which delays recovery, limits patient satisfaction, and affects progression of rehabilitation. Additionally, visualization and characterization of tears, particularly articular-sided partial tears, are challenging during an open procedure. 33 Arthroscopy has allowed better visualization and has improved understanding of the various tear patterns. This allows surgeons to achieve the most accurate, functional, and stable ARCR possible. 25,62
Figure 1.
Examples of suture-passing devices created to facilitate ease of suture passage during arthroscopic rotator cuff repair. (A) Smith & Nephew FIRSTPASS ST. (B) Arthrex Scorpion. (C) Various SutureLasso devices (Arthrex).
After improved visualization and improved tear pattern recognition and understanding, the next technical evolutions were aimed at arthroscopic fixation methods. An enormous amount of time and effort was aimed at the development of suture anchors, suture material and fixation methods, and surgical techniques. These developmental steps aimed to restore and recreate the anatomic footprint of the rotator cuff to enhance healing of the tendon to the bone. 87
Suture Anchors
With ORCR, surgeons would commonly drill bone tunnels using a transosseous technique to attach the tendon to the humeral head via knotted sutures. As this was not as feasible with arthroscopy, suture anchors were developed. These suture anchors varied in terms of fixation technique, shape, size, composition, radiopacity, and suture material. 6 Moreover, the anchors initially were not specifically designed for ARCR and the unique features needed to provide stable fixation at the greater tuberosity and to enhance healing. Although the attributes were variable, all anchors had the same general aim to reattach torn tendons to the bone of the greater tuberosity. The anchors needed to secure the tendon for the different phases of healing (inflammation, vascularization, and tissue remodeling) over the course of at least 12 weeks. 6 Several research studies 7,8,10 have outlined the developments in the biomechanical features of numerous anchors over the past 3 decades.
Anchor design has evolved over time and included anchors with fins, partially threaded anchors, and eventually fully threaded anchors, with the last being proclaimed as advantageous in osteoporotic bone and fixation at the tuberosity. 9 Additionally, anchors can be screw-in or non–screw-in. In 2011, Barber et al 9 investigated the biomechanical properties of various anchor types and found that a fully threaded screw-in anchor demonstrated a significantly greater load to failure versus smaller, non–screw-in anchors. Interestingly, in this same study 9 with certain anchor designs, the size of screw-in anchors was not predictive of load to failure, with both larger (6.5 mm) and smaller (5.5 mm) screw-in anchors having similar strengths.
Anchor composition has evolved from metal anchors to bioabsorbable; to permanent polyether ether ketone (PEEK); and, more recently, to soft, all-suture anchors (ASAs). 8 Metal anchors have historically been simple to utilize and easy to visualize radiographically postoperatively, but because they are permanent, they present challenges in revision surgery and cause image artifact on postsurgical magnetic resonance imaging (MRI) scans (Figure 2). One of the weaknesses that was recognized was the anchor eyelets, which could make tying sliding knots difficult and, in some instances, could actually abrade or damage the suture material. 6 This was especially true in metal anchors where potential sharp edges seemed to be a risk factor for ARCR failure due to suture breakage. Researchers learned that the rotation of the anchor eyelet in relation to the line of force could be important to protect the sutures and to affect load to failure. 49 Bioabsorbable anchors composed of polyglyconate or poly-l-lactic acid (PLA) were developed and had the benefit of dissolving over time and also had better biomechanical profiles with less risk of damaging the repair sutures (Figure 3). Clinically, further benefits included their minimal effect on revision surgery and lack of artifact on MRI scans. 8,49 Downfalls of bioabsorbable anchors included the fact that PLA anchors need up to 4 years to resorb and some cases of an inflammatory reaction to polyglyconate anchors have been described. 4 The addition of biocomposite anchors with a combination of PLA and calcium triphosphate has helped the anchors to resorb more quickly while maintaining the advantages of resorption, postoperative imaging, and ease of revision.
Figure 2.

Examples of metal suture anchors in various sizes from Smith & Nephew, the TWINFIX Ti.
Figure 3.

Examples of biocomposite suture anchors used for arthroscopic rotator cuff repair. From left to right: Arthrex BioComposite Corkscrew FT, Smith & Nephew Helicoil Regenasorb, and Arthrex BioComposite SwiveLock.
PEEK anchors were also developed as an alternative to metallic ones and included radiolucent, nonabsorbable, nonmetallic anchors with biomechanically similar pull-out strengths to the abovementioned materials (Figure 4). 7 In theory, PEEK anchors should offer some design advantages, as PEEK is stronger than most absorbable materials. To further improve the perfusion of the medial row, vented suture anchors were developed in both bioabsorbable and PEEK anchors. 90
Figure 4.

Examples of polyether ether ketone (PEEK) anchors used in arthroscopic rotator cuff repair. From left to right: Smith & Nephew Helicoil PK, Arthrex PEEK SwiveLock SP, and Smith & Nephew Footprint PK.
Recently, ASA designs have become available for use in ARCR (Figure 5). 5,66 ASAs are composed of suture material and differ from prior anchor systems in the primary fixation. 5,66 In general, the intracortical suture material expands and fixes the anchor in the bone. The upside includes smaller drill holes that lead to less bone loss, especially after pullout. 66 The anchor pull-out strength and the fixation angles have been shown to be comparable to those of conventional anchors in biomechanical testing, as demonstrated by a recent randomized prospective clinical trial. 66 However, while analyzing in vitro studies, one should keep in mind they do not necessarily translate into enhancement in clinical outcomes.
Figure 5.

Example of an all-suture anchor used in arthroscopic rotator cuff repair, the Arthrex FiberTak.
Suture Material
Suture material varies and includes braided, nonbraided, and cord-like sutures. Braided polyester sutures and monofilament polydioxanone sutures were initially utilized (eg, PDS II; Ethicon). These braided sutures frequently broke during knot tying and did not withstand cyclical loading well because of their poor resistance to abrasion. The development of high-strength sutures for ARCR was a major development. The high-strength sutures had markedly improved load-to-failure values as compared with polyblend polyethylene sutures in biomechanical testing (eg, FiberWire, Arthrex; Ultrabraid, Smith & Nephew; Orthocord, Depuy Synthes) (Figure 6). 81 The polyblend polyethylene sutures also demonstrated less fraying at the edge of the anchor eyelets. 92 Furthermore, the polyblend polyethylene material was developed further in recent years and now uses ultra–high-molecular-weight polyethylene, which has been beneficial regarding fraying and failure through submaximal strength slipping in vitro. 6
Figure 6.

Examples of high-strength sutures and tape used in arthroscopic rotator cuff repair that contain ultra–high-molecular-weight polyethylene. Top: Arthrex Fiber Tape. Bottom: Smith & Nephew Ultrabraid.
As opposed to conventional sutures, suture tapes were also developed to dissipate forces over a greater surface area of the tendon, leading to less “cut-through.” They also act as biological scaffolds with biomechanical advantages in load to failure and stiffness because of the wider dimension of the material. 11 However, few to no randomized clinical trials have been performed to verify the effect of these mostly biomechanical findings.
Fixation Techniques
Fixation techniques with ARCR are centered around restoring anatomy, recreating biomechanics with strong fixation, promoting healing, and improving function. 87 To fulfill these aims, numerous fixation methods have been developed over time, differing in terms of the quantity of anchors, suture material, region of fixation, and biomechanical qualities. 65 Fixation techniques include single-row repair, 87 double-row repair, 48 linked versus unlinked double-row repair, 71 conventional sutures versus suture tapes, 71 and knotted versus knotless constructs. 56
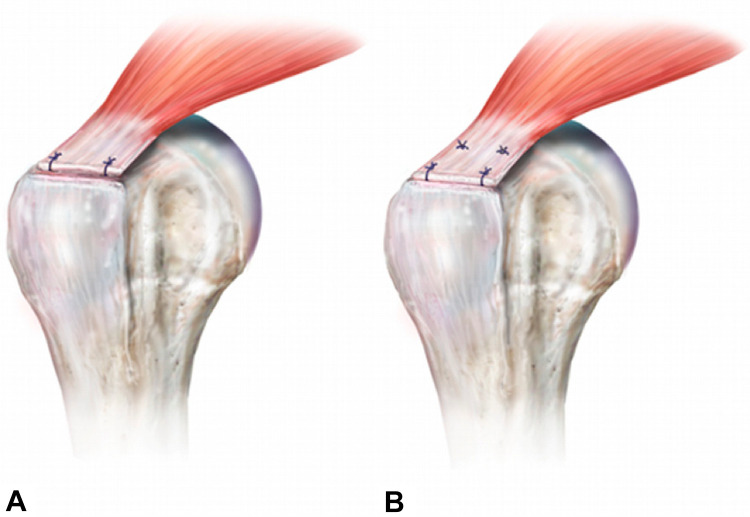
At the turn of the millennium, ARCR was performed either using a single anchor in the center of the tear or via the placement of anchors in a row from anterior to posterior on the greater tuberosity, referred to as a single-row repair (Figure 7). 83 After diagnostic arthroscopy, identification of the tear pattern, and mobilization of the tendon, anchor placement is achieved through an additional superolateral portal at an inclination of 45°. 78 Severud et al 78 were the first to show comparable results in ARCR and an arthroscopy-assisted mini-open repair with less fibrosis in the ARCR group. However, after these promising results, Galatz et al 31 reported a disturbing study in which ultrasound testing showed recurrent defects, after single-row ARCR, in approximately 94% of repairs. Furthermore, the study group reported a significant deterioration of the functional outcomes between the 12- and 24-month follow-ups. 31 Dugas et al 28 showed that the surface area of the tendon on the greater tuberosity included a medial-lateral dimension of up to 15 mm and that single-row repairs did not reproduce this well, thus creating spot welds for healing. Some have speculated that lack of healing with ARCR was possibly due to reduced restoration of the anatomic surface area (“footprint”). In 1 study, 1 only 67% of the anatomic footprint was restored using certain ARCR techniques compared with 85% surface area restoration when using more traditional ORCR methods. Many surgeons had been using double-row techniques with mini-ORCR to enhance the contact area between tendon to bone, so Lo and Burkhart 48 adapted this to ARCR and introduced the double-row ARCR in 2003 (Figure 7). When utilized, the medial anchors are placed just lateral to the articular margin, while the lateral row anchors are placed at the lateral aspect of the greater tuberosity in front of the “drop-off.” 48,74 This anchor position takes advantage of the best bone quality in the humeral head. 86
Figure 7.
Arthroscopic rotator cuff repair constructs: (A) single row and (B) double row. Reprinted with permission from Roth KM, Warth RJ, Lee JT, Millett PJ, ElAttrache NS. Arthroscopic single-row versus double-row repair for full-thickness posterosuperior rotator cuff tears: a critical analysis review. JBJS Rev. 2014;2(7):e6. Wolters Kluwer Health.
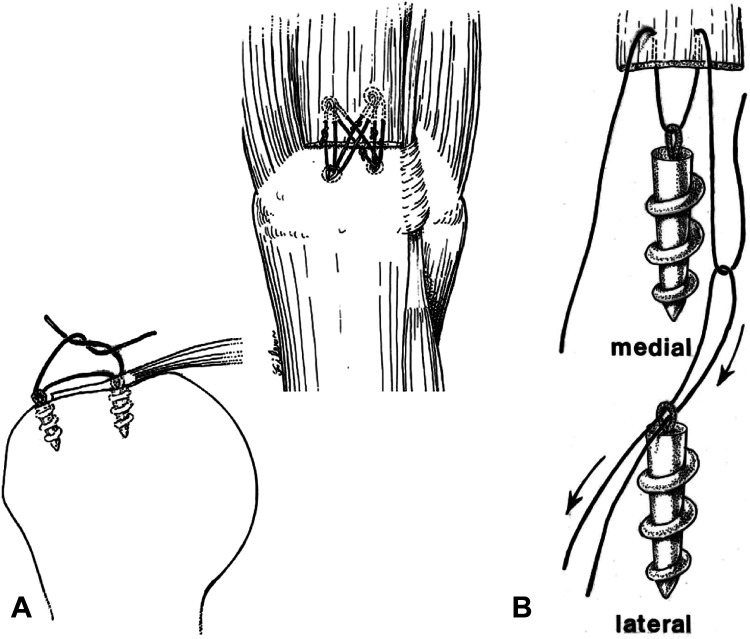
Millett et al 61 were the first to describe linking the medial and lateral rows and also creating a crisscross method that has now become the gold standard method of ARCR (Figure 8). This method optimized anatomy, biomechanics, and biology by maximizing the surface area of the tendon that was in contact with the bone, by placing the anchors in the best bone quality of the greater tuberosity, by compressing the tendon down onto the bone with sutures that were on the bursal surface, by minimizing foreign material at the cuff bone interface where healing occurred, by allowing for resistance to multivector loads because of the crisscrossing suture configuration, and by maximizing resistance of bone to tendon failure because the forces were dissipated over the whole construct. Two years later, Park et al 68 called this method of linking the medial and lateral row the transosseous equivalent. Burkhart et al 14 would further discuss the self-reinforcing properties (the greater the load, the more the tendon is compressed onto the footprint) of such a linked ARCR construct. Park et al 67,69 also validated these biomechanical concepts in the laboratory.
Figure 8.
(A) Early descriptions of the linking medial and lateral rows. (B) Displaying how the suture anchors are linked together. Image A reprinted with permission from Vaishnav S, Millett PJ. Arthroscopic rotator cuff repair: scientific rationale, surgical technique, and early clinical and functional results of a knotless self-reinforcing double-row rotator cuff repair system. J Shoulder Elbow Surg. 2010;19(2 suppl):83-90. ©2010, Elsevier Ltd. Image B reprinted with permission from Millett PJ, Mazzocca A, Guanche CA. Mattress double anchor footprint repair: a novel, arthroscopic rotator cuff repair technique. Arthroscopy. 2004;20(8):875-879. ©2004, Elsevier Ltd.
Depending on the tear pattern, different suture configurations were proposed. 53,61,87 In 2005, Mazzocca et al 53 compared the single-row with the double-row technique and found improved surface area restoration with similar strength in load-to-failure, cyclic displacement, and gap formation tests. Biomechanical testing alone is not the only factor, as the biological quality of a repair through a larger tendon-to-footprint surface is also highly important. Through different meta-analyses, Duquin et al 29 and Millett et al 63 were able to show comparable clinical results and significantly lower retear rates with the double-row technique. Duquin et al 29 defined failure as a retear in a follow-up MRI and analyzed 23 studies comparing open procedures with arthroscopic single- and double-row fixation techniques in mostly single patient cohorts without a control group. On the contrary, Millett et al 60 strictly considered 7 randomized controlled trials, each comparing single-row with double-row fixation. MRI was used to confirm retear rates, and the study groups were evaluated using the American Shoulder and Elbow (ASES) score and the Constant score. There were no significant differences between the groups in terms of clinical scores, but there was a significantly decreased retear rate in the double-row repairs (14.2%) compared with the single-row repairs (25.9%), with a relative risk for the single-row technique of 1.76. 60 However, the study reported that the MRI-based analysis was a limitation because of its being unable to distinguish between a retear and a lack of healing from the initial repair. 60
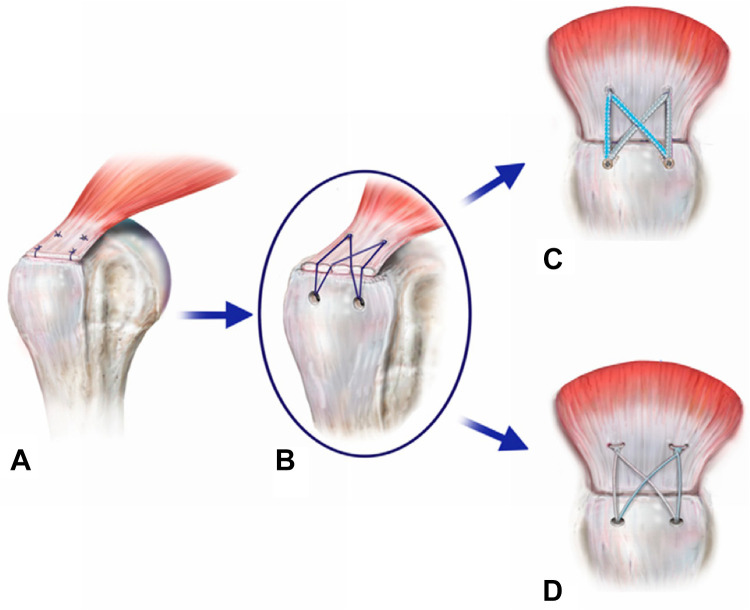
Despite promising clinical results, Cho et al 23 illustrated that the medial row can fail at the musculotendinous junction. Possible reasons described for retearing at the musculotendinous junction were decreased blood flow due to the high contact pressure at the greater tuberosity and increased tension at the medial row. 23 Medial knots have also been proposed as contributing to this by concentrating the force and not dissipating it over the 2 rows (Figure 9). In an effort to address the increased medial tension and reduced tendon perfusion, the suture limbs of the medial and lateral rows were connected to form an anatomic suture bridge construct 61,87,88 with decreased tension of the medial knots, decreased suture cut-through, improved vascular flow, and anchor placement away from the tendon-bone interface. In 2017, Park et al 70 showed benefits for a knotless construct regarding load sharing in biomechanical testing (Figure 9). Furthermore, there was less focal tissue strangulation and significantly more self-reinforcement with equivalent load-to-failure values as compared with knotted configurations. Additionally, Park et al 70 showed no enhancement in the tendon-to-bone contact area via medial knots, leading the study group to question the necessity of knotted medial anchors. In 2017, Millett et al 57 compared knotless double-row suture tape constructs to knotted double-row constructs with conventional sutures in a clinical retrospective comparative level 3 study. While both configurations showed excellent results at the 2-year follow-up, the knotless suture tape variant showed lower retear rates on postoperative MRI scans. 57 Again, the MRI-based follow-up analysis was without an arthroscopic second look and used as an ethically sustainable approximation. Further, Park et al 69 showed reduced operating time as a result of fewer steps, and Huang et al 41 described fewer revision surgeries and a faster recovery time. For this study, Huang et al 41 performed economic and decision analyses using data obtained from a previously published randomized controlled trial that compared single-row to double-row fixation. While the single-row repair had less cost initially (CAD $1654.76 vs $2134.41), the double-row technique was found to be most cost-effective in terms of incremental cost per quality-adjusted life-year gained.
Figure 9.
Evolution of the double-row arthroscopic rotator cuff repair. (A) Double-row repair. (B) Transosseous equivalent. (C) Knotless suture bridge construct with suture tapes. (D) Medial knotted suture bridge construct with sutures. Reprinted with permission from Roth KM, Warth RJ, Lee JT, Millett PJ, ElAttrache NS. Arthroscopic single-row versus double-row repair for full-thickness posterosuperior rotator cuff tears: a critical analysis review. JBJS Rev. 2014;2(7):e6. Wolters Kluwer Health.
Further advancement of the double-row construct included describing the self-reinforcement effect where the strength of the repair construct is increased via movement of the humerus and loading of the tendon. 15 Furthermore, self-linked constructs have shown superior results in biomechanical testing, especially in rotational load sharing, footprint coverage, footprint compression, gap formation, and ultimately load to failure as compared with previous techniques. 15,88 In 2010, Voigt et al 88 showed excellent clinical results in a prospective clinical trial for linked double-row constructs with steady improvement after 4, 12, and 24 months. However, the study group showed retear rates of up to 28% on follow-up MRI scans with medial tendon rerupture rates of 46%, and there was no control group with which to compare these findings. 88 Various authors have identified a weakness in the cuff tissue at the suture tendon interface as the most likely cause of retear. 23,60,90
As surgeons became more experienced in the arthroscopic repair of rotator cuff injuries, they also learned to employ arthroscopic techniques to facilitate the repair of more challenging tear patterns. These techniques are aimed at mobilization of the tissue and simplifying the tear pattern so that maximal tissue apposition to bone can occur. This includes the principle of margin convergence for large U-shaped tears or peripheral L-shaped tears, where the longitudinal aspect of the tear is sutured side to side first before repairing the residual crescent tear down to the bone. For chronic or retracted tears, interval slides can be performed to mobilize tissue for tension-free repair. With an anterior interval slide, the supraspinatus and rotator interval is released, allowing further mobility of the retracted tissue. Often, interval slides and convergence techniques are combined in these complex or chronic/retracted tear patterns. 18
Clinical Outcomes
The evolution of the ARCR not only provided excellent clinical outcomes but also enhanced the survival of the repair constructs compared with the previous ORCR. In 2011, Millett et al 59 exhibited a survival rate of 94% after 5 years and 83% after 10 years in 233 patients treated with ORCR using an arthroscopy-assisted mini-open repair technique. It seems that the evolution of the ARCR further decreased the failure rate of repair constructs even in the long-term follow-up. Plachel et al 72 mirrored these results, describing an 11% revision rate in 56 cases treated with ARCR after 10 years. Additionally, Millett’s study group showed excellent short-term (2 years) 58 , midterm (5 years) 73 , and long-term (10 years) 42 results. This was demonstrated by significantly improved clinical outcomes and a low revision rate of 5.5% with 95.4% survivorship at 10 years in a Kaplan-Meier analysis. 42,58,73 Interestingly, the authors distinguished between a knotted suture bridge and a knotless (medial row) suture tape technique (Figure 9). Both techniques showed excellent clinical results without any significant differences in the clinical outcome. 42,58,73 After nearly 3 decades of evolution from both industry and surgeon innovation, the double-row, knotless, self-reinforcing repair construct with suture tape and optimized anchors has become a highly utilized modern construct (Figure 10). Nevertheless, most clinical studies should be interpreted with the perspective that there are limitations because of the lack of control groups or second looks via arthroscopy and that innovation has industry and economic drivers that are difficult to quantify.
Figure 10.
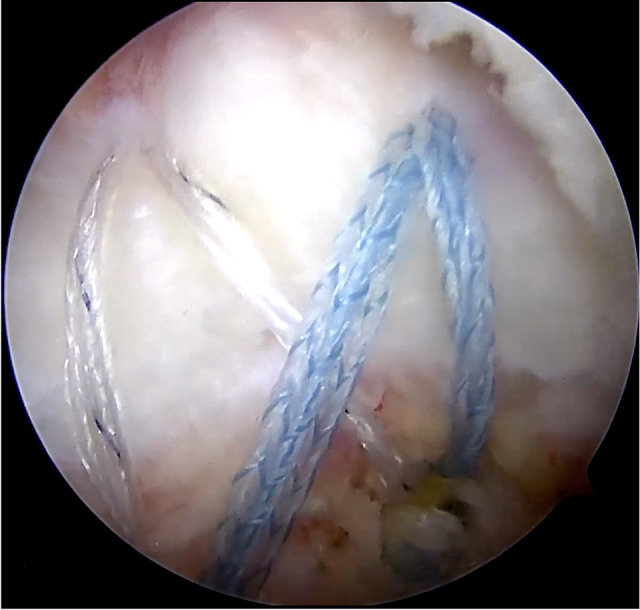
The double-row, knotless, suture bridge arthroscopic rotator cuff repair using suture tapes and biocomposite suture anchors.
Physical Therapy
The postoperative influences of healing have also evolved as it pertains to the transition from ORCR to ARCR and through the evolution of ARCR. Physical therapy, in particular, is probably the most influential postoperative factor as it pertains to healing and clinical outcomes with ARCR. Without the need to protect the deltoid origin, timing, progression, and program type have all been considered in the healing of an ARCR. Typically, the 2 schools of thought that have emerged are early and delayed motion protocols. 26,85 Early motion theories have been implemented with the intentions of increased mobility and earlier return to activity, while delayed motion theories are predicated on protecting the early healing phase of ARCR. 32 The advent of ARCR had been suggested to lower the predisposition to stiffness when compared with ORCR and thus may obviate the need for an early progressive range of motion protocol. Several authors have attempted to compare early and delayed range of motion protocols as they pertain to outcomes, pain, range of motion, and retear rates after ARCR. 32 A 2015 systematic review of evidence level 1 and level 2 studies by Gallagher et al 32 compared these rehabilitation protocols and demonstrated early improvements in range of motion and function with early range of motion protocols; however, similar clinical and anatomic outcomes were observed in delayed range of motion protocols at 1 year. There were no studies that demonstrated differences in retear rates between the 2 schools of thought, although the trend was toward slower mobilization with larger tears.
Future Perspectives
Orthobiologics
As ARCR has continued to evolve, a main focus of research has been directed toward improving the biology at the repair site. Researchers initially turned to platelet-rich plasma (PRP) because of its concentration of growth factors implicated in healing musculoskeletal tissue such as platelet-derived growth factor, basic fibroblast growth factor, transforming growth factor beta-1, and vascular endothelial growth factor. 13 These findings have resulted in numerous studies 38,39,50,79,89 investigating the role of PRP in rotator cuff surgery. Malavolta et al 50 reported that at 60 months after arthroscopic single-row repair, both the control and PRP groups had improved University of California, Los Angeles (UCLA), Constant, and visual analog scale (VAS) scores from baseline; however, there was no difference between the groups’ clinical outcome scores or retear rates evaluated via MRI. Another study on 87 total patients assessed outcomes after ARCR with either a delayed injection of leukocyte-rich PRP or normal saline at 10 to 14 days. Similarly, at the 1-year follow-up, there was overall improvement from preoperative baseline but no significant difference between the groups regarding retear rate or the Western Ontario Rotator Cuff Index (WORC), ASES, and Constant scores. 79 Arthroscopic supraspinatus tendon repair with PRP injections on day 0 and day 7 resulted in no significant difference in patient-reported outcomes at 24.6 months when compared with control repair. 38 Notably, Warth et al 89 conducted a meta-analysis that revealed an improvement in Constant scores with tendon-bone interface PRP injections as opposed to superficial administration on top of the tendon. Additionally, PRP usage in the setting of arthroscopic double-row repair for a tear >3 cm in the anterior-posterior direction resulted in a decreased retear rate. A review by Han et al 39 on 13 studies with 880 total patients found that PRP utilized in rotator cuff surgery resulted in significantly decreased retear rate and increased Constant shoulder scores, simple shoulder test scores, UCLA scores, and VAS scores when compared with repair without PRP. While promising results with regard to retear rates and patient-reported outcomes have been demonstrated in certain studies, the variability of PRP formulations and heterogeneity of studies make the overall data regarding the use of PRP in ARCR questionable.
Additional efforts to augment healing at the site of ARCR have been attempted via the administration of mesenchymal stem cells. An animal study by Kida et al 44 explored the effect of drill holes according to the double-row repair technique and discovered that this group had increased infiltration of bone marrow–derived cells within the tendon and higher strength to failure when compared with the control repair. Conversely, Gulotta et al 37 found no difference in collagen formation or repair strength in rats between the control repair group and a repair plus mesenchymal stem cell group. A separate animal study examined supraspinatus repair without supplementation to repair plus adipose-derived stem cells (ASCs), tendon hydrogel (tHG), or combined tHG-ASC. While the tHG significantly enhanced repair strength, the combination of tHG-ASC did not afford any additional strength. 43 In general, there is a paucity of research evaluating the clinical outcomes of stem cell use in ARCR. One outcome study investigated the effect of adipose-derived mesenchymal stem cells (ADMSC) after ARCR versus a matched control in 70 total patients. Patient-reported outcome measures at 28 months were not significantly different; however, the retear rate of 14.3% in the ADMSC group was significantly lower than the retear rate of 28.5% in the control group. 45 Hernigou et al 40 performed a matched-control study in 90 total patients to assess healing after single-row ARCR with and without iliac crest bone marrow–derived cells. At 6 months, all patients had complete healing at the repair site in the bone marrow–derived MSC group compared with 67% of patients in the control group. Furthermore, 87% of rotator cuffs were intact at 10 years in the bone marrow–derived stem cell group, whereas 44% of rotator cuffs were intact in the control group. These studies indicate that the use of stem cells in ARCR may enhance the resiliency of the repair, but heterogeneity in stem cell type and administration methods make it challenging to elucidate the most effective use of stem cells in ARCR.
Structure-Based Therapies
In addition to plasma-based and cell-based orthobiologics, structure-based therapies have also been a topic of recent interest. The role of patch augmentation in RCR has expanded in recent history to include xenografts, synthetic grafts, allografts, and autografts. 22 Additionally, these patches have been utilized in a variety of repair techniques in an attempt to improve repair strength and/or promote healing at the repair site. These factors are of critical importance in ARCR, as a systematic review by McElvany et al 54 on 108 articles covering 8011 repairs revealed a retear rate of 26.6% at an average of 23.7 months. Several studies 2,12,55,77 have examined the outcomes after bursa-sided patch placement. A histologic analysis on a highly porous, highly oriented, reconstituted bovine collagen implant (Rotation Medical) applied during ARCR found that at 5 weeks after repair, the host tissue’s fibroblasts were linearly oriented along the implant and were creating new collagen. At 6 months, biopsy demonstrated a complete absence of the collagen implant, and the host tissue resembled that of a tendon. 2 A multicenter trial on 33 patients conducted by Schlegel et al 77 evaluated partial-thickness tears of the supraspinatus tendon treated with subacromial decompression and bursa-sided patch placement without tendon repair. MRI scans at 12 months revealed that the tendon tear site was an average of 2 mm thicker, complete resolution of the tear occurred in 8 patients, and reduction of tear size was noted in 23 patients. Similar results were demonstrated in a study by Bokor et al 12 who found an average increase in tendon thickness of 2.2 mm with a significant increase in ASES and Constant scores at the 24-month follow-up. McIntyre et al 55 assessed outcomes after arthroscopic application of a highly porous collagen scaffold (REGENETEN; Smith & Nephew) in 90 partial- and 83 full-thickness rotator cuff tears. Patient-reported WORC, ASES, VAS, Single Assessment Numeric Evaluation, and Veterans RAND 12 scores all significantly improved over the year of follow-up (Figure 11). Despite bursa-sided patch placement for full- and partial-thickness tears resulting in excellent short-term outcomes with limited tear progress, additional studies are needed to assess mid- and long-term outcomes. Additionally, the literature is still lacking in randomized trails comparing patch augmentation with standard ARCR, and thus it is unclear as to whether clinical improvements are observed with augmented ARCR.
Figure 11.
An example of a biologic scaffold used in the augmentation of arthroscopic rotator cuff repair wherein a biologic onlay is used to promote collagen formation and healing (REGENETEN; Smith & Nephew).
While the above-described patches have been implemented to improve repair quality, additional allograft patch augmentation techniques were implemented in an effort to improve strength or bridge tissue gaps in ARCR. Petri et al 71 reported on the 2-year clinical outcomes after open revision biologic patch augmentation in 12 patients with massive rotator cuff retears who had deficient rotator cuff tendons with healthy rotator cuff muscles. There was a 90% satisfaction rate with no reoperations and significantly improved ASES functional outcomes. A meta-analysis by Bailey et al 3 even suggested that patch augmentation or interposition may be as reliable as or better than ARCR alone. Ferguson et al 30 found similar results in a systematic review assessing the structural integrity and functional results of allografts when used for patch augmentation versus conventional primary repair of large to massive rotator cuff tears. Additionally, they suggested that xenografts were structurally inferior and were prone to inflammatory reactions while polypropylene patches were superior in their structural and functional characteristics; however, they lacked randomized comparative data to conclusively say so.
Conclusion
ARCR has become a reproducible and reliable orthopaedic procedure over the past 3 decades. The transition from open to arthroscopic rotator cuff surgery was made possible by a bidirectional collaboration between surgeons and industry. The developmental process has included technological advancements in surgical equipment such as suture materials, anchor materials, and instrumentation, in addition to repair configurations that allow surgeons to effectively address a variety of different rotator cuff tear patterns. As these technical factors were continually refined, they aimed to recreate the anatomic footprint of the rotator cuff, leading to the current double-row, knotless, self-reinforcing repair construct with suture tape and optimized anchors. Years of innovation in optics, instrumentation, devices, and technique have led to the development and evolution of the current ARCR. As the biomechanical and technical aspects of the procedure are continually enhanced, the future of ARCR seems to be centered on elucidating the best use of orthobiologics as a relevant enhancement for tendon healing and improved patient outcomes.
Footnotes
Final revision submitted May 12, 2021; accepted July 19, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: This research was supported by the Steadman Philippon Research Institute (SPRI), which is a 501(c)(3) nonprofit institution supported financially by private donations and corporate support. SPRI exercises special care to identify any financial interests or relationships related to research conducted here. During the past calendar year, SPRI has received grant funding or in-kind donations from Arthrex, Ossur, Siemens, Smith & Nephew, DoD, DJO, MLB, and XTRE. R.O.D.H.’s position at SPRI was supported by AGA via Arthrex for 1 calendar year. J.J.E. has received consulting fees from Medical Device Business Services. R.E.B. has received consulting fees from Smith & Nephew, nonconsulting fees from Arthrex and Smith & Nephew, and hospitality payments from Exactech. P.J.M. has received consulting fees from Arthrex and royalties from MedBridge and Springer and has stock/stock options in VuMedi. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Apreleva M, Ozbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519–526. [DOI] [PubMed] [Google Scholar]
- 2. Arnoczky SP, Bishai SK, Schofield B, et al. Histologic evaluation of biopsy specimens obtained after rotator cuff repair augmented with a highly porous collagen implant. Arthroscopy. 2017;33(2):278–283. [DOI] [PubMed] [Google Scholar]
- 3. Bailey JR, Kim C, Alentorn-Geli E, et al. Rotator cuff matrix augmentation and interposition: a systematic review and meta-analysis. Am J Sports Med. 2019;47(6):1496–1506. [DOI] [PubMed] [Google Scholar]
- 4. Barber FA, Dockery WD, Cowden CH III. The degradation outcome of biocomposite suture anchors made from poly L-lactide-co-glycolide and β-tricalcium phosphate. Arthroscopy. 2013;29(11):1834–1839. [DOI] [PubMed] [Google Scholar]
- 5. Barber FA, Herbert MA. All-suture anchors: biomechanical analysis of pullout strength, displacement, and failure mode. Arthroscopy. 2017;33(6):1113–1121. [DOI] [PubMed] [Google Scholar]
- 6. Barber FA, Herbert MA. Cyclic loading biomechanical analysis of the pullout strengths of rotator cuff and glenoid anchors: 2013 update. Arthroscopy. 2013;29(5):832–844. [DOI] [PubMed] [Google Scholar]
- 7. Barber FA, Herbert MA, Beavis RC, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859–867. [DOI] [PubMed] [Google Scholar]
- 8. Barber FA, Herbert MA, Coons DA, Boothby MH. Sutures and suture anchors—update 2006. Arthroscopy. 2006;22(10):1063.e1-e9. [DOI] [PubMed] [Google Scholar]
- 9. Barber FA, Herbert MA, Hapa O, et al. Biomechanical analysis of pullout strengths of rotator cuff and glenoid anchors: 2011 update. Arthroscopy. 2011;27(7):895–905. [DOI] [PubMed] [Google Scholar]
- 10. Barber FA, Herbert MA, Richards DP. Sutures and suture anchors: update 2003. Arthroscopy. 2003;19(9):985–990. [DOI] [PubMed] [Google Scholar]
- 11. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463–1468. [DOI] [PubMed] [Google Scholar]
- 12. Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscles Ligaments Tendons J. 2016;6(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429–439. [DOI] [PubMed] [Google Scholar]
- 14. Burkhart SS. The burden of craft in arthroscopic rotator cuff repair: where have we been and where we are going. Am J Orthop (Belle Mead NJ). 2015;44(8):353–358. [PubMed] [Google Scholar]
- 15. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274–281. [DOI] [PubMed] [Google Scholar]
- 16. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996;12(3):335–338. [DOI] [PubMed] [Google Scholar]
- 17. Burkhart SS, Cole BJ. Bridging self-reinforcing double-row rotator cuff repair: we really are doing better. Arthroscopy. 2010;26(5):677–680. [DOI] [PubMed] [Google Scholar]
- 18. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333–346. [DOI] [PubMed] [Google Scholar]
- 19. Burman MS. Arthroscopy or the direct visualization of joints: an experimental cadaver study. 1931. Clin Orthop Relat Res. 2001;390:5–9. [DOI] [PubMed] [Google Scholar]
- 20. Bush RB, Leonhardt H, Bush IV, Landes RR. Dr. Bozzini’s Lichtleiter: a translation of his original article (1806). Urology. 1974;3(1):119–123. [DOI] [PubMed] [Google Scholar]
- 21. Carr AJ. Open surgery still plays a substantial role in modern rotator cuff repair. Orthopedics Today . April 14, 2016. Accessed September 30, 2020. https://www.healio.com/news/orthopedics/20160414/open-surgery-still-plays-a-substantial-role-in-modern-rotator-cuff-repair
- 22. Chalmers PN, Tashjian RZ. Patch augmentation in rotator cuff repair. Curr Rev Musculoskelet Med. 2020;13(5):561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cho NS, Yi JW, Lee BG, Rhee YG. Retear patterns after arthroscopic rotator cuff repair: single-row versus suture bridge technique. Am J Sports Med. 2010;38(4):664–671. [DOI] [PubMed] [Google Scholar]
- 24. Cicak N, Klobucar H, Bicanic G, Trsek D. Arthroscopic transosseous suture anchor technique for rotator cuff repairs. Arthroscopy. 2006;22(5):565.e1-e6. [DOI] [PubMed] [Google Scholar]
- 25. Davidson J, Burkhart SS. The geometric classification of rotator cuff tears: a system linking tear pattern to treatment and prognosis. Arthroscopy. 2010;26(3):417–424. [DOI] [PubMed] [Google Scholar]
- 26. Debeyre J, Patie D, Elmelik E. Repair of ruptures of the rotator cuff of the shoulder. J Bone Joint Surg Br. 1965;47:36–42. [PubMed] [Google Scholar]
- 27. DeMaio M. Giants of orthopaedic surgery: Masaki Watanabe MD. Clin Orthop Relat Res. 2013;471(8):2443–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dugas JR, Campbell DA, Warren RF, Robie BH, Millett PJ. Anatomy and dimensions of rotator cuff insertions. J Shoulder Elbow Surg. 2002;11(5):498–503. [DOI] [PubMed] [Google Scholar]
- 29. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38(4):835–841. [DOI] [PubMed] [Google Scholar]
- 30. Ferguson DP, Lewington MR, Smith TD, Wong IH. Graft utilization in the augmentation of large-to-massive rotator cuff repairs: a systematic review. Am J Sports Med. 2016;44(11):2984–2992. [DOI] [PubMed] [Google Scholar]
- 31. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219–224. [DOI] [PubMed] [Google Scholar]
- 32. Gallagher BP, Bishop ME, Tjoumakaris FP, Freedman KB. Early versus delayed rehabilitation following arthroscopic rotator cuff repair: a systematic review. Phys Sportsmed. 2015;43(2):178–187. [DOI] [PubMed] [Google Scholar]
- 33. Gartsman GM. Arthroscopic management of rotator cuff disease. J Am Acad Orthop Surg. 1998;6(4):259–266. [DOI] [PubMed] [Google Scholar]
- 34. Gartsman GM, Brinker MR, Khan M. Early effectiveness of arthroscopic repair for full-thickness tears of the rotator cuff: an outcome analysis. J Bone Joint Surg Am. 1998;80(1):33–40. [PubMed] [Google Scholar]
- 35. Gartsman GM, Hammerman SM. Full-thickness tears: arthroscopic repair. Orthop Clin North Am. 1997;28(1):83–98. [DOI] [PubMed] [Google Scholar]
- 36. Ghodadra NS, Provencher MT, Verma NN, Wilk KE, Romeo AA. Open, mini-open, and all-arthroscopic rotator cuff repair surgery: indications and implications for rehabilitation. J Orthop Sports Phys Ther. 2009;39(2):81–89. [DOI] [PubMed] [Google Scholar]
- 37. Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126–2133. [DOI] [PubMed] [Google Scholar]
- 38. Gwinner C, Gerhardt C, Haneveld H, Scheibel M. Two-staged application of PRP in arthroscopic rotator cuff repair: a matched-pair analysis. Arch Orthop Trauma Surg. 2016;136(8):1165–1171. [DOI] [PubMed] [Google Scholar]
- 39. Han C, Na Y, Zhu Y, et al. Is platelet-rich plasma an ideal biomaterial for arthroscopic rotator cuff repair? A systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2019;14(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811–1818. [DOI] [PubMed] [Google Scholar]
- 41. Huang AL, Thavorn K, van Katwyk S, MacDonald P, Lapner P. Double-row arthroscopic rotator cuff repair is more cost-effective than single-row repair. J Bone Joint Surg Am. 2017;99(20):1730–1736. [DOI] [PubMed] [Google Scholar]
- 42. Johannsen AM, Arner JW, Elrick B, Nolte PC, Horan MP, Millett PJ. Minimum 10-year outcomes of primary arthroscopic transosseous-equivalent double row rotator cuff repairs. Am J Sports Med. 2021;49(8):2035–2041. [DOI] [PubMed] [Google Scholar]
- 43. Kaizawa Y, Franklin A, Leyden J, et al. Augmentation of chronic rotator cuff healing using adipose-derived stem cell-seeded human tendon-derived hydrogel. J Orthop Res. 2019;37(4):877–886. [DOI] [PubMed] [Google Scholar]
- 44. Kida Y, Morihara T, Matsuda K, et al. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J Shoulder Elbow Surg. 2013;22(2):197–205. [DOI] [PubMed] [Google Scholar]
- 45. Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. 2017;45(9):2010–2018. [DOI] [PubMed] [Google Scholar]
- 46. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55–60. [DOI] [PubMed] [Google Scholar]
- 47. Lichtenberg S, Magosch P, Loew M, Habermeyer P. Historie und Entwicklung der arthroskopischen Rotatorenmanschettennaht. Obere Extremität. 2015;10(1):3–9. [Google Scholar]
- 48. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035–1042. [DOI] [PubMed] [Google Scholar]
- 49. Longo UG, Petrillo S, Loppini M, et al. Metallic versus biodegradable suture anchors for rotator cuff repair: a case control study. BMC Musculoskelet Disord. 2019;20(1):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Malavolta EA, Gracitelli MEC, Assunção JH, Ferreira Neto AA, Bordalo-Rodrigues M, de Camargo OP. Clinical and structural evaluations of rotator cuff repair with and without added platelet-rich plasma at 5-year follow-up: a prospective randomized study. Am J Sports Med. 2018;46(13):3134–3141. [DOI] [PubMed] [Google Scholar]
- 51. Mansat P, Cofield RH, Kersten TE, Rowland CM. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28(2):205–213. [DOI] [PubMed] [Google Scholar]
- 52. Martin Clark J, Millett PJ. Arthroscopic rotator cuff repair instruments and equipment: setting yourself up for success. In: Levine WN, Blaine TA, Ahmad CS, eds. Minimally Invasive Shoulder and Elbow Surgery. Informa Healthcare; 2007. [Google Scholar]
- 53. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861–1868. [DOI] [PubMed] [Google Scholar]
- 54. McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA III. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2):491–500. [DOI] [PubMed] [Google Scholar]
- 55. McIntyre LF, Bishai SK, Brown PB III, Bushnell BD, Trenhaile SW. Patient-reported outcomes after use of a bioabsorbable collagen implant to treat partial and full-thickness rotator cuff tears. Arthroscopy. 2019;35(8):2262–2271. [DOI] [PubMed] [Google Scholar]
- 56. Menge TJ, Tahal DS, Katthagen JC, Millett PJ. Arthroscopic acromioclavicular joint reconstruction using knotless coracoclavicular fixation and soft-tissue anatomic coracoclavicular ligament reconstruction. Arthrosc Tech. 2017;6(1):e37–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Millett PJ, Espinoza C, Horan MP, et al. Correction to: predictors of outcomes after arthroscopic transosseous equivalent rotator cuff repair in 155 cases. A propensity score weighted analysis of knotted and knotless self-reinforcing repair techniques at a minimum of 2 years. Arch Orthop Trauma Surg. 2017;137(12):1761. [DOI] [PubMed] [Google Scholar]
- 58. Millett PJ, Espinoza C, Horan MP, et al. Predictors of outcomes after arthroscopic transosseous equivalent rotator cuff repair in 155 cases: a propensity score weighted analysis of knotted and knotless self-reinforcing repair techniques at a minimum of 2 years. Arch Orthop Trauma Surg. 2017;137(10):1399–1408. [DOI] [PubMed] [Google Scholar]
- 59. Millett PJ, Horan MP, Maland KE, Hawkins RJ. Long-term survivorship and outcomes after surgical repair of full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2011;20(4):591–597. [DOI] [PubMed] [Google Scholar]
- 60. Millett PJ, Hussain ZB, Fritz EM, Warth RJ, Katthagen JC, Pogorzelski J. Rotator cuff tears at the musculotendinous junction: classification and surgical options for repair and reconstruction. Arthrosc Tech. 2017;6(4):e1075–e1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Millett PJ, Mazzocca A, Guanche CA. Mattress double anchor footprint repair: a novel, arthroscopic rotator cuff repair technique. Arthroscopy. 2004;20(8):875–879. [DOI] [PubMed] [Google Scholar]
- 62. Millett PJ, Warth RJ. Posterosuperior rotator cuff tears: classification, pattern recognition, and treatment. J Am Acad Orthop Surg. 2014;22(8):521–534. [DOI] [PubMed] [Google Scholar]
- 63. Millett PJ, Warth RJ, Dornan GJ, Lee JT, Spiegl UJ. Clinical and structural outcomes after arthroscopic single-row versus double-row rotator cuff repair: a systematic review and meta-analysis of level I randomized clinical trials. J Shoulder Elbow Surg. 2014;23(4):586–597. [DOI] [PubMed] [Google Scholar]
- 64. Mormino MA, Gross RM, McCarthy JA. Captured shoulder: a complication of rotator cuff surgery. Arthroscopy. 1996;12(4):457–461. [DOI] [PubMed] [Google Scholar]
- 65. Nho SJ, Slabaugh MA, Seroyer ST, et al. Does the literature support double-row suture anchor fixation for arthroscopic rotator cuff repair? A systematic review comparing double-row and single-row suture anchor configuration. Arthroscopy. 2009;25(11):1319–1328. [DOI] [PubMed] [Google Scholar]
- 66. Ntalos D, Sellenschloh K, Huber G, et al. Conventional rotator cuff versus all-suture anchors—a biomechanical study focusing on the insertion angle in an unlimited cyclic model. PLoS One. 2019;14(11):e0225648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Park MC. Biomechanical validation of rotator cuff repair techniques and considerations for a “technical efficiency ratio”. Arthroscopy. 2013;29(7):1230–1234. [DOI] [PubMed] [Google Scholar]
- 68. Park MC, ElAttrache NS, Ahmad CS, Tibone JE. “Transosseous-equivalent” rotator cuff repair technique. Arthroscopy. 2006;22(12):1360.e1-e5. [DOI] [PubMed] [Google Scholar]
- 69. Park MC, Peterson A, Patton J, McGarry MH, Park CJ, Lee TQ. Biomechanical effects of a 2 suture-pass medial inter-implant mattress on transosseous-equivalent rotator cuff repair and considerations for a “technical efficiency ratio.” J Shoulder Elbow Surg. 2014;23(3):361–368. [DOI] [PubMed] [Google Scholar]
- 70. Park MC, Peterson AB, McGarry MH, Park CJ, Lee TQ. Knotless transosseous-equivalent rotator cuff repair improves biomechanical self-reinforcement without diminishing footprint contact compared with medial knotted repair. Arthroscopy. 2017;33(8):1473–1481. [DOI] [PubMed] [Google Scholar]
- 71. Petri M, Warth RJ, Horan MP, Greenspoon JA, Millett PJ. Outcomes after open revision repair of massive rotator cuff tears with biologic patch augmentation. Arthroscopy. 2016;32(9):1752–1760. [DOI] [PubMed] [Google Scholar]
- 72. Plachel F, Traweger A, Vasvary I, Schanda JE, Resch H, Moroder P. Long-term results after arthroscopic transosseous rotator cuff repair. J Shoulder Elbow Surg. 2019;28(4):706–714. [DOI] [PubMed] [Google Scholar]
- 73. Pogorzelski J, Fritz EM, Horan MP, et al. Minimum five-year outcomes and clinical survivorship for arthroscopic transosseous-equivalent double-row rotator cuff repair. J Am Acad Orthop Surg. 2019;27(24):e1093–e1101. [DOI] [PubMed] [Google Scholar]
- 74. Randelli P, Cucchi D, Ragone V, de Girolamo L, Cabitza P, Randelli M. History of rotator cuff surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(2):344–362. [DOI] [PubMed] [Google Scholar]
- 75. Rokito AS, Cuomo F, Gallagher MA, Zuckerman JD. Long-term functional outcome of repair of large and massive chronic tears of the rotator cuff. J Bone Joint Surg Am. 1999;81(7):991–997. [DOI] [PubMed] [Google Scholar]
- 76. Rokito AS, Zuckerman JD, Gallagher MA, Cuomo F. Strength after surgical repair of the rotator cuff. J Shoulder Elbow Surg. 1996;5(1):12–17. [DOI] [PubMed] [Google Scholar]
- 77. Schlegel TF, Abrams JS, Bushnell BD, Brock JL, Ho CP. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg. 2018;27(2):242–251. [DOI] [PubMed] [Google Scholar]
- 78. Severud EL, Ruotolo C, Abbott DD, Nottage WM. All-arthroscopic versus mini-open rotator cuff repair: a long-term retrospective outcome comparison. Arthroscopy. 2003;19(3):234–238. [DOI] [PubMed] [Google Scholar]
- 79. Snow M, Hussain F, Pagkalos J, et al. The effect of delayed injection of leukocyte-rich platelet-rich plasma following rotator cuff repair on patient function: a randomized double-blind controlled trial. Arthroscopy. 2020;36(3):648–657. [DOI] [PubMed] [Google Scholar]
- 80. Snyder S. Arthroscopic fixation of rotator cuff tears: a preliminary report. Paper presented at: 12th Annual Meeting of the Arthroscopy Association of North America; April 4, 1993; Palm Desert, CA. [Google Scholar]
- 81. Swan KG, Jr, Baldini T, McCarty EC. Arthroscopic suture material and knot type: an updated biomechanical analysis. Am J Sports Med. 2009;37(8):1578–1585. [DOI] [PubMed] [Google Scholar]
- 82. Tauro JC. Arthroscopic “interval slide” in the repair of large rotator cuff tears. Arthroscopy. 1999;15(5):527–530. [DOI] [PubMed] [Google Scholar]
- 83. Tauro JC. Arthroscopic rotator cuff repair: analysis of technique and results at 2- and 3-year follow-up. Arthroscopy. 1998;14(1):45–51. [DOI] [PubMed] [Google Scholar]
- 84. Thal R. A technique for arthroscopic mattress suture placement. Arthroscopy. 1993;9(5):605–607. [DOI] [PubMed] [Google Scholar]
- 85. Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125(1):106–113. [DOI] [PubMed] [Google Scholar]
- 86. Tingart MJ, Apreleva M, Lehtinen J, Zurakowski D, Warner JJ. Anchor design and bone mineral density affect the pull-out strength of suture anchors in rotator cuff repair: which anchors are best to use in patients with low bone quality? Am J Sports Med. 2004;32(6):1466–1473. [DOI] [PubMed] [Google Scholar]
- 87. Vaishnav S, Millett PJ. Arthroscopic rotator cuff repair: scientific rationale, surgical technique, and early clinical and functional results of a knotless self-reinforcing double-row rotator cuff repair system. J Shoulder Elbow Surg. 2010;19(2 suppl):83–90. [DOI] [PubMed] [Google Scholar]
- 88. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983–991. [DOI] [PubMed] [Google Scholar]
- 89. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306–320. [DOI] [PubMed] [Google Scholar]
- 90. Warth RJ, Greenspoon JA, Bhatia S, Millett PJ. Arthroscopic double-row rotator cuff repair using a knotless, interconnected technique. Oper Tech Orthop. 2015;25(1):43–48. [Google Scholar]
- 91. Wilk KE, Crockett HC, Andrews JR. Rehabilitation after rotator cuff surgery. Tech Shoulder Elb Surg. 2000;1(2):128–144. [Google Scholar]
- 92. Wüst DM, Meyer DC, Favre P, Gerber C. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22(11):1146–1153. [DOI] [PubMed] [Google Scholar]
- 93. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81–92. [PubMed] [Google Scholar]
- 94. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Analysis of rotator cuff repair trends in a large private insurance population. Arthroscopy. 2013;29(4):623–629. [DOI] [PubMed] [Google Scholar]