Abstract
Acute myocardial infarction (AMI) has been a preclinical and clinical concern due to high hospitalization rate and mortality. This study was aimed at evaluating the effectiveness and safety of Shexiang Baoxin Pill (SBP) for AMI and exploring the possible mechanism of oxidative stress. Six databases were searched on March 26, 2021. Twenty-four studies were included and accessed by the RoB 2.0 or SYRCLE tool. Compared with routine treatment (RT), SBP showed the effectiveness in the clinical efficacy (RR = 1.15, 95% CI [1.06, 1.25]), left ventricular ejection fraction (LVEF) (SMD = 0.73, 95% CI [0.62, 0.95]), glutathione (GSH) (SMD = 2.07, 95% CI [1.51, 2.64]), superoxide dismutase (SOD) (SMD = 0.92, 95% CI [0.58, 1.26]), malondialdehyde (MDA) (SMD = −4.23, 95% CI [-5.80, -2.66]), creatine kinase-myocardial band (CK-MB) (SMD = −4.98, 95% CI [-5.64, -4.33]), cardiac troponin I (cTnI) (SMD = −2.17, 95% CI [-2.57, -1.76]), high-sensitivity C-reactive protein (Hs-CRP) (SMD = −1.34, 95% CI [-1.56, -1.12]), interleukin-6 (IL-6) (SMD = −0.99, 95% CI [-1.26, -0.71]), triglycerides (TG) (SMD = −0.52, 95% CI [-0.83, -0.22]), flow-mediated dilation (FMD) (SMD = 1.39, 95% CI [1.06, 1.72]), von Willebrand Factor (vWF) (SMD = −1.77, 95% CI [-2.39, -1.15]), nitric oxide (NO) (SMD = 0.89, 95% CI [0.65, 1.13]), and recurrent rate (RR = 0.30, 95% CI [0.15, 0.59]). But SBP adjunctive to RT plus PCI had no improvements in almost pooled outcomes except for the Hs-CRP (SMD = −1.19, 95% CI [-1.44, -0.94]) and TG (SMD = −0.25, 95% CI [-0.48, -0.02]). Laboratory findings showed that SBP enhanced the endothelial nitric oxide synthase (eNOS) activity and regulated laboratory indexes especially for homocysteine. In conclusion, SBP has adjunctive effects on AMI via the mechanism of antioxidative stress. The current evidence supports the use of SBP for mild and moderate AMI patients.
1. Introduction
Acute myocardial infarction (AMI) is the sudden damage to the myocardium due to insufficient blood flow to the heart. It is characterized by chest pain, chest discomfort, and acute shortness of breath [1]. Globally, AMI has become the leading cause of hospitalization and death [2]. Early revascularization and primary percutaneous coronary intervention (PCI) restore blood flow to the culprit coronary artery and reduce AMI mortality rate [3, 4]. However, immediate multivessel PCI might cause additional risks, e.g., induction of further ischemia, volume overload, and renal impairment due to the use of an increased dose of contrast material [5, 6]. Concurrently, the abrupt restoration therapy of coronary flow may induce reversible impairment of myocardial contractility, ventricular arrhythmias, and microvascular dysfunction. The myocardial ischemia/reperfusion (I/R) injury leads to myocyte necrosis, slows cardiomyocyte healing, and results in heart failure [7, 8]. Thus, prevention of I/R injury in AMI could reduce the injury.
Shexiang Baoxin Pill (SBP) is a classical Chinese medicine (CM) formula for cardiovascular diseases including AMI and stable angina pectoris [9–11], which has been approved by the Chinese Food and Drug Administration [12]. A pharmacological study indicates that SBP reduces cardiac infarct volume, suppresses inflammation, and promotes angiogenesis in the heart [12]. SBP is composed of 7 Chinese medicines or extracts including Moschus, Radix Ginseng, Calculus Bovis, Cortex Cinnamomi, Styrax, Venenum Bufonis, and Borneolum Syntheticum. Ginsenosides and cinnamaldehyde, active components of SBP, regulate energy metabolism in cardiomyocytes [13] and inhibit reactive oxygen species (ROS) production and autophagy [14]. However, the efficacy and mechanisms of SBP for AMI have not been systematically evaluated. This review was aimed at evaluating the efficacy and mechanisms of SBP through clinical studies and experimental studies with AMI animal models.
2. Materials and Methods
2.1. Search Strategy
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplementary Table 1) [15], this registered study (PROSPERO, no. CRD42021245957) searched six electronic databases: PubMed, Embase, Cochrane, Chinese National Knowledge Infrastructure (CNKI), China Science and Technology Journal (VIP), and Wanfang, from the date of database establishment to March 26, 2021. The combination of MeSH terms and keywords were used as follows: “Shexiang Baoxin” AND “acute myocardial infarction”. The PubMed database retrieval strategy is shown in Supplementary Table 2.
2.2. Eligibility Criteria
Inclusion criteria were as follows: (1) preclinical experiment (PE) or randomized controlled trial (RCT) studying SPB; (2) animal models of AMI were induced by operation ligation [16, 17], or patients met diagnostic criteria for AMI [18–20]; (3) SBP was an explored mechanism in preclinical experiments and was used as intervention or adjunctive to routine treatment (RT) in the clinical observation group; and (4) the clinical observation group and control group received RT or PCI. Exclusion criteria were as follows: (1) repetitive studies, comment, clinical experience, case report, review, data mining research, and protocol; (2) non-RCT and PE not studying oxidative stress; (3) PE or RCT contained the intervention of moxibustion, acupuncture, or other CM except for SBP; and (4) the study lacked essential data even though the principal authors were contacted.
2.3. Data Extraction
(1) Basic information of the included experiments (the first author, publication year, animal species, sex, number of animals, weight, intervention, and experiment duration) and trials (the first author, publication year, sample size, age information of the patients, intervention, and trial duration) were extracted; (2) all outcome indicators of experiments were extracted; (3) the primary outcome indicator (clinical efficacy rate) and second outcome indicators (cardiac function, oxidative stress, myocardial enzyme, inflammatory cytokines, blood lipid level, vascular endothelial function, and complication rate) of trials were extracted; and (4) endpoint data and baseline data were extracted for each outcome.
2.4. Quality Assessment
Six aspects of the version 2 of the Cochrane Risk of Bias Tool (RoB 2.0) [21] were assessed for the included RCTs: randomization process, deviations from the intended interventions, missing outcome data, outcome measurements, selection of the reported results, and overall bias according to the three criteria of “low risk,” “high risk,” or “some concerns.” Two researchers (JG and ZQ) assessed the included studies individually, and the third researcher (HC) resolved the discrepancies. GRADE (Grading of Recommendations, Assessment, Development and Evaluations) was used to evaluate evidence certainty of meta-analysis results. The Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) [22] risk of bias tool was used for Pes, including sequence generation, baseline characteristics, allocation concealment, random housing, blinding of performance, random outcome assessment, blinding of detection, incomplete outcome data, selective outcome reporting, and other sources of bias.
2.5. Statistical Analysis
The Stata 17.0 software (Stata Corp., College Station, TX, USA) was applied to statistical analysis: (1) a random effects model was adopted for pooling studies with high heterogeneity while a fixed effects model was applied for studies with low heterogeneity; (2) Cohen's d and 95% CI were used for continuous variables; (3) RR (relative risk) and 95% CI were used for categorical variables; (4) weight (%) was used to indicate a percentage of each study contributing to the pooled intervention effects; (5) a p value < 0.05 was considered statistically different; (6) heterogeneity was evaluated by Q statistics and I2, and the p value in Q statistics was <0.05 or I2 > 50% presented high heterogeneity or otherwise low heterogeneity; and (7) sensitivity analysis and subgroup analysis were carried out when studies had significant heterogeneity.
3. Results
3.1. Eligible Studies
A total of 862 studies were retrieved through the initial search, and 541 studies were obtained after screening out duplicate studies. Subsequently, 510 studies were excluded by reviewing titles or abstracts. In the remaining 31 studies, 7 studies were excluded following the full-text screen. The flow chart of screening is shown in Figure 1.
Figure 1.
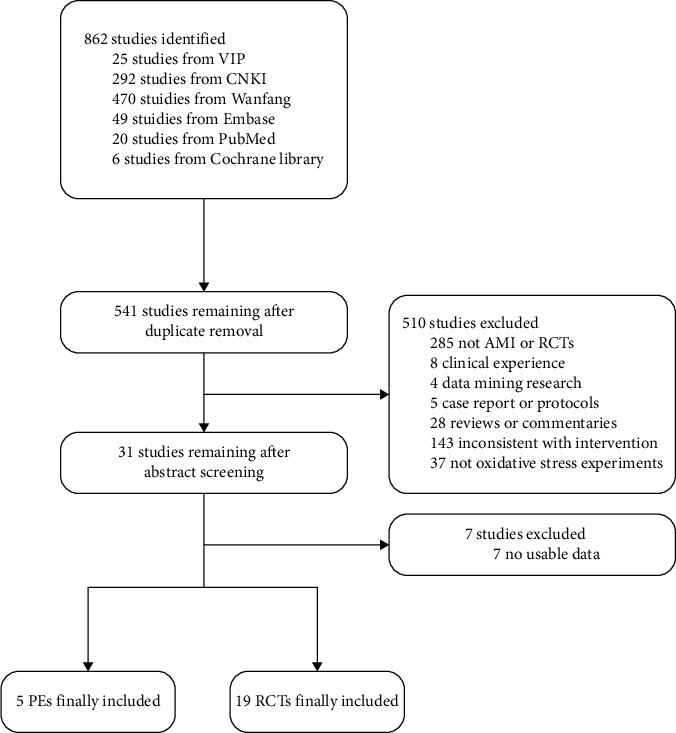
Flow chart.
3.2. Study Characteristics
Nineteen RCTs [23–41] and five PEs [42–46] were included in this study, involving 1849 patients (observation group: 926, control group: 923) and 209 animals, respectively. The years of publication range from 1999 to 2021. The shortest experimental duration is 5 days, and the longest is 15 days. As for RCTs, the shortest duration of treatment is 2 weeks, and the longest is 24 weeks. The number of animals ranges from 6 to 81, and the sample size of patients is from 59 to 200 in each study. In the included PEs, three experiments [43–45] were carried out in metabolomics, one experiment [42] involved pharmacodynamics, and one experiment [46] was carried out in quantitative proteomics. In the included RCTs, 5 trials [37–41] used PCI both in the observation group and in the control group. The basic information of included studies is shown in Tables 1 and 2.
Table 1.
Basic information of the included preclinical experiments.
| ID | Animal species | Gender | Number of animals | Age | Weight | Intervention | Experimental duration | Experimental type |
|---|---|---|---|---|---|---|---|---|
| Luo X P 1999 | SD rats (NR) | Male | 49 | NR | 250-300 g | SBP | 14 days | Pharmacodynamics |
| Xiang L 2013 | SD rats (NR) | Male | 56 | NR | 200 ± 20 g | SBP, SFSBP, seven components∗ | 15 days | Metabonomics |
| Liu Q 2017 | SD rats (SPF) | Male | 81 | NR | 200 ± 20 g | Ginsenosides in SBP | 5 days | Metabonomics |
| Jiang P 2011 | SD rats (NR) | Male | 17 | NR | 200 ± 15 g | SBP | 4 days | Metabonomics |
| Yu F 2021 | SD rats (SPF) | Male | 6 | 7 weeks | 200-230 g | SBP | 15 days | Quantitative proteomics |
Note: SD: Sprague-Dawley; SPF: specific pathogen-free; SBP: Shexiang Baoxin Pill; SFSBP: simplified formula of SBP; NR: not reported. ∗Including muskone, ginsenoside, ginsenoside, cinnamic acid, cholic acid, bufalin, and borneol.
Table 2.
Basic information of the included randomized controlled trials.
| ID | Sample size (T/C) | Mean age (years) | Diagnostic criteria | Intervention | Comparison | Duration of treatment | Outcomes |
|---|---|---|---|---|---|---|---|
| Chen ZH 2013 | 105 (50/55) | T: 58.9 ± 9.3 C: 61.4 ± 10.4 |
I | SBP plus RT | RT | 8 weeks | ②④⑤⑥ |
| Luo Y C 2015 | 80 (40/40) | T: 55.8 ± 19.6 C: 55.8 ± 19.6 |
II | SBP plus RT | RT | 4 weeks | ⑧ |
| Ge R L 2015 | 70 (35/35) | T: 59.9 ± 4.2 C: 60.4 ± 4.1 |
I | SBP plus RT | RT | 8 weeks | ②⑤⑥ |
| Yang G L 2013 | 120 (60/60) | T: 58.2 ± 7.1 C: 56.9 ± 6.8 |
II | SBP plus RT | RT | 24 weeks | ②④⑧ |
| Ma C 2020 | 90 (45/45) | T: 50.5 ± 4.8 C: 50.4 ± 4.3 |
Compliant with II | SBP plus RT | RT | 2 weeks | ①②⑤⑦⑧ |
| Tian F Q 2016 | 64 (32/32) | T: 57.4 ± 5.2 C: 56.5 ± 5.3 |
II | SBP plus RT | RT | 2 weeks | ①③④⑧ |
| Wang S S 2016 | 106 (53/53) | T: 60.5 ± 8.7 C: 61.1 ± 9.1 |
Compliant with II | SBP plus RT | RT | 2 weeks | ①②⑤⑦ |
| Bai X 2020 | 82 (41/41) | T: 56.4 ± 10.5 C: 57.3 ± 10.6 |
Compliant with II | SBP plus RT | RT | 2 weeks | ②⑤⑧ |
| Wei L N 2018 | 96 (48/48) | T: 57.2 ± 8.3 C: 56.9 ± 8.1 |
Compliant with II | SBP plus RT | RT | 2 weeks | ①②⑤⑦⑧ |
| Jiang F J 2020 | 60 (30/30) | T: 40.1 ± 3.4 C: 39.7 ± 4.3 |
Compliant with II | SBP plus RT | RT | 2 weeks | ①⑧ |
| Zhang X T 2017 | 140 (70/70) | T: 63.9 ± 8.6 C: 64.5 ± 8.7 |
Compliant with II | SBP plus RT | RT | NR | ①⑧ |
| Xu J 2017 | 90 (45/45) | T: 56.2 ± 6.8 C: 55.9 ± 8.6 |
III | SBP plus RT | RT | 12 weeks | ②⑥ |
| Yang F 2015 | 120 (60/60) | T: 64.1 ± 7.9 C: 63.9 ± 8.1 |
Compliant with II | SBP plus RT | RT | 10 weeks | ①③④⑧ |
| Feng B 2020 | 83 (43/40) | T: 57.5 ± 3.3 C: 58.6 ± 3.6 |
II | SBP plus RT | RT | 2 weeks | ③④ |
| Huang P D 2016 | 88 (44/44) | T: 72.2 ± 6.5 C: 72.7 ± 6.1 |
II | SBP plus RT plus PCI | RT plus PCI | 8 weeks | ①②③④⑤⑥ |
| Jiang D J 2020 | 80 (40/40) | T: 55.0 ± 2.4 C: 55.0 ± 2.3 |
Compliant with II | SBP plus RT plus PCI | RT plus PCI | 2 weeks | ②⑦ |
| Xu F L 2018 | 200 (100/100) | T: 59.4 ± 10.1 C: 59.8 ± 10.4 |
Compliant with II | SBP plus RT plus PCI | RT plus PCI | 12 weeks | ⑤⑥⑧ |
| Lin G Q 2010 | 59 (30/29) | T: 60.7 ± 8.2 C: 61.0 ± 7.4 |
Compliant with II | SBP plus RT plus PCI | RT plus PCI | 24 weeks | ①②④⑤ |
| Ma R J 2019 | 116 (60/56) | T: 62.2 ± 8.3 C: 62.0 ± 7.5 |
Compliant with II | SBP plus RT plus PCI | RT plus PCI | 4 weeks | ②④⑧ |
Note: T: treatment group; C: control group; SBP: Shexiang Baoxin Pill; RT: routine treatment (including oxygen inhalation, vascular dilation, anticoagulation, and thrombolysis); PCI: percutaneous coronary intervention; CPGs: clinical practice guidelines; NR: not reported; I: diagnostic criteria of AMI developed by the World Health Organization; II: diagnostic criteria of AMI developed by the Chinese Cardiovascular Society; III: diagnostic criteria of AMI developed by the American Heart Association and American College of Cardiology; ①: clinical efficacy rate; ②: cardiac function; ③: oxidative stress; ④: AMI evaluation index; ⑤: inflammatory factors; ⑥: blood lipid level; ⑦: vascular endothelial function; ⑧: complication rate.
3.3. Risk of Bias
Five PEs [42–46] were assessed by the SYRCLE risk of bias tool (Supplementary Table 3). Due to lack of reporting, random housing and blinding of performance were judged as “some concerns” in all PEs. Three PEs [42, 44, 45] without reporting randomization were judged as “some concerns,” and three PEs neglected specific baseline report and thus increased the risk of bias, which were judged as “some concerns.” Nineteen RCTs [23–41] were assessed by using the RoB 2.0 tool (Supplementary Table 4). Among the assessments, four RCTs [27–29, 37] with a good design and report were assessed as “low” risks, but almost RCTs presented the risk of “some concerns” due to missing outcome data or selection of the reported result; even two RCTs [34, 36] with incomplete data or results led to the “high” risks.
3.4. Preclinical Experiments
Five experiments [42–46] were included that studied SBP for AMI in AMI animal models. The histology analysis in an experiment [42] showed that the endothelial nitric oxide synthase (eNOS) was mainly distributed in the myocardial interstitium, and SBP increased the expression of eNOS in the left ventricle. The metabonomics analysis showed that SBP downregulated hippuric acid, homocysteine, 5-methylcytosine, PGPC, and allantoin, which were involved in oxidative injury [43]. Another study analyzed the effects of SBP ginsenosides and found that ginsenoside Rg1 and Rb3 downregulated indoleacrylic acid, Rc upregulated 6-hydroxymelatonin and downregulated thymidine, and Re downregulated thymidine, indicating antioxidative effects of SBP [44]. SBP downregulated homocysteine to protect against ROS-induced endothelial cell injury [43, 45]. The proteomics analysis showed that peroxiredoxin-3 in SBP protects against oxidative stress in a rat model of myocardial infarction [46].
3.5. Clinical Trials
3.5.1. Clinical Efficacy
Nine RCTs [27–29, 31–33, 35, 37, 40] reporting a clinical efficacy rate were pooled. Among the subgroup analysis (Figure 2), SBP plus RT showed a better clinical efficacy rate than RT alone (RR = 1.15, 95% CI [1.06, 1.25], p < 0.05) with low heterogeneity (Q (6) = 8.17, p = 0.23, I2 = 35.00%). But SBP adjunctive to RT plus PCI did not significantly improve the clinical efficacy rate (RR = 1.09, 95% CI [0.89, 1.35], p > 0.05) and showed high heterogeneity (Q (1) = 4.54, p = 0.03, I2 = 77.96%).
Figure 2.
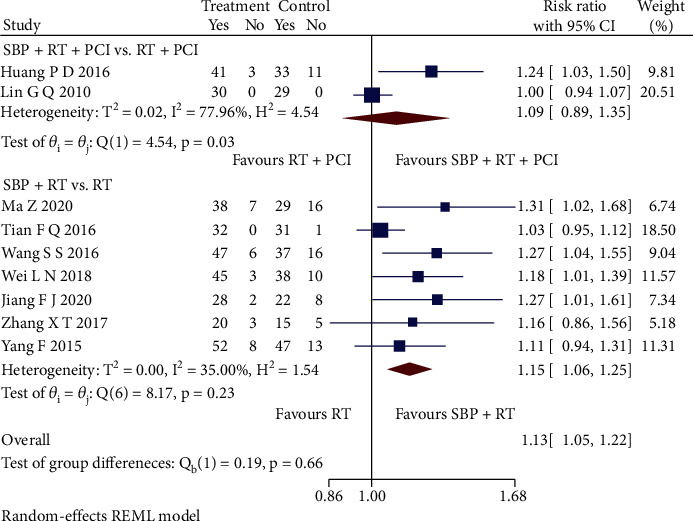
Forest plot of subgroup analysis on the clinical efficacy rate.
3.5.2. Cardiac Function
Twelve RCTs [23, 25–27, 29–31, 34, 37, 38, 40, 41] reporting cardiac function were pooled and conducted subgroup analysis, as shown in Figures 3(a)–3(c). The subgroup analysis revealed that SBP plus RT significantly presented a higher left ventricular ejection fraction (LVEF) level than RT (SMD = 0.73, 95% CI [0.62, 0.95], p < 0.05; Q (7) = 9.27, p = 0.23, I2 = 20.75%, low heterogeneity), but SBP adjunctive to RT plus PCI had no significant difference (SMD = 0.64, 95% CI [-0.16, 1.45], p > 0.05; Q (3) = 33.46, p < 0.05, I2 = 92.31%, high heterogeneity) compared with RT plus PCI. Sensitivity analysis indicated that differences in PCI operations could cause the heterogeneity. Further, compared with RT, SBP plus RT had a significant improvement in left ventricular end-diastolic diameter (LVEDD) (SMD = −0.79, 95% CI [-1.09, -0.49], p < 0.05; Q (1) = 0.01, p = 0.93, I2 = 0, low heterogeneity), left ventricular end-diastolic volume (LVEDV) (SMD = −1.21, 95% CI [-1.47, -0.95], p < 0.05; Q (2) = 1.16, p = 0.56, I2 = 0, low heterogeneity), left ventricular end-systolic diameter (LVESD) (SMD = −1.15, 95% CI [-1.46, -0.83], p < 0.05; Q (1) = 0.28, p = 0.60, I2 = 0, low heterogeneity), and left ventricular end-systolic volume (LVESV) (SMD = −1.06, 95% CI [-1.30, -0.82], p < 0.05; Q (4) = 6.03, p = 0.20, I2 = 33.96%, low heterogeneity). Besides, SBP adjunctive to RT plus PCI had a significant improvement in LVEDD (SMD = −0.99, 95% CI [-1.86, -0.12], p < 0.05) with the high heterogeneity (Q (1) = 8.34, p < 0.05, I2 = 88.00%). Sensitivity analysis indicated that the heterogeneity could be caused by measuring methods.
Figure 3.
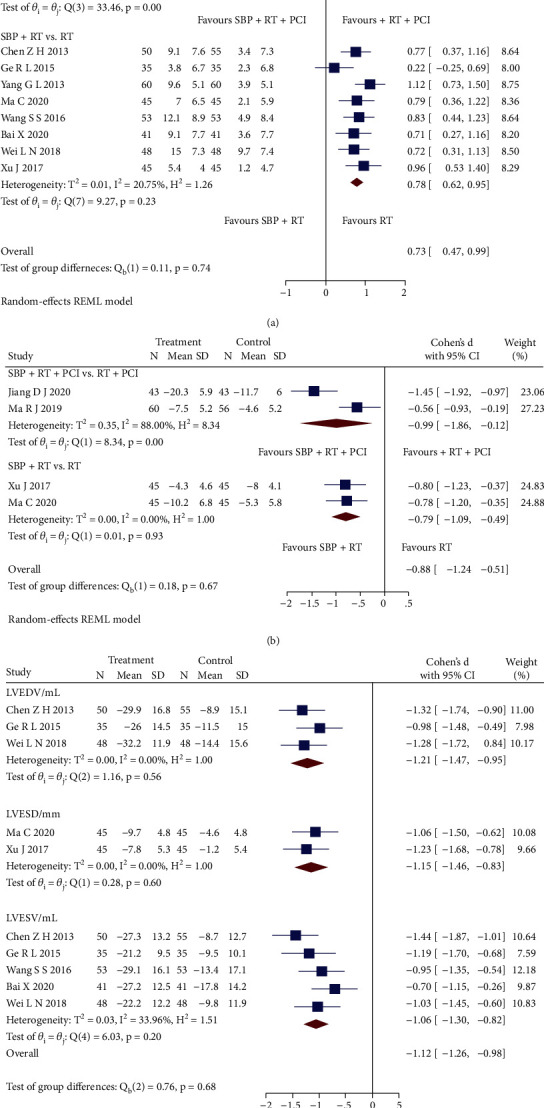
(a) Forest plot of subgroup analysis on the LVEF. (b) Forest plot of subgroup analysis on the LVEDD. (c) Forest plot of subgroup analysis on the LVEDV, LVESD, and LVESV.
3.5.3. Oxidative Stress
Based on the reports of three oxidative stress indicators, two RCTs [28, 36] were pooled, as shown in Figure 4. Compared with RT, SBP adjunctive to RT had significant effectiveness of increasing both glutathione (GSH) level (SMD = 2.07, 95% CI [1.51, 2.64], p < 0.05; Q (1) = 1.94, p = 0.16, I2 = 48.44%, moderate heterogeneity) and superoxide dismutase (SOD) level (SMD = 0.92, 95% CI [0.58, 1.26], p < 0.05; Q (1) = 0.28, p = 0.59, I2 = 0, low heterogeneity) and decreasing malondialdehyde (MDA) level (SMD = −4.23, 95% CI [-5.80, -2.66], p < 0.05; Q (1) = 7.21, p = 0.01, I2 = 86.12%, high heterogeneity). Sensitivity analysis indicated that differences in measuring time could cause the heterogeneity.
Figure 4.
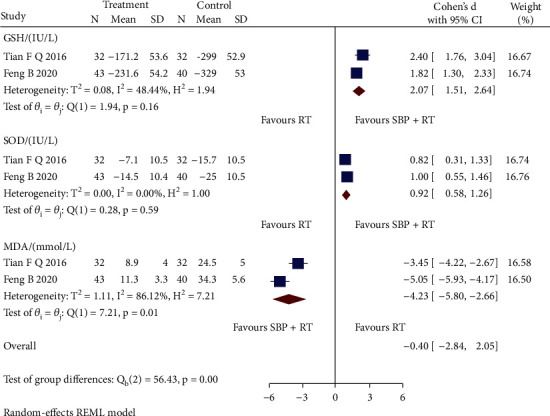
Forest plot of subgroup analysis on the GSH, SOD, and MDA.
3.5.4. Laboratory Indexes
(1) Myocardial Enzymes. Two RCTs [28, 36] reported creatine kinase-myocardial band (CK-MB) and cardiac troponin I (cTnI). The subgroup analysis showed that SBP plus RT had significant effectiveness in CK-MB (SMD = −4.98, 95% CI [-5.64, -4.33], p < 0.05; Q (1) < 0.05, p = 1.00, I2 = 0, low heterogeneity) and cTnI (SMD = −2.17, 95% CI [-2.57, -1.76], p < 0.05; Q (1) = 0.01, p = 0.94, I2 = 0, low heterogeneity) (Figure 5(a)).
Figure 5.
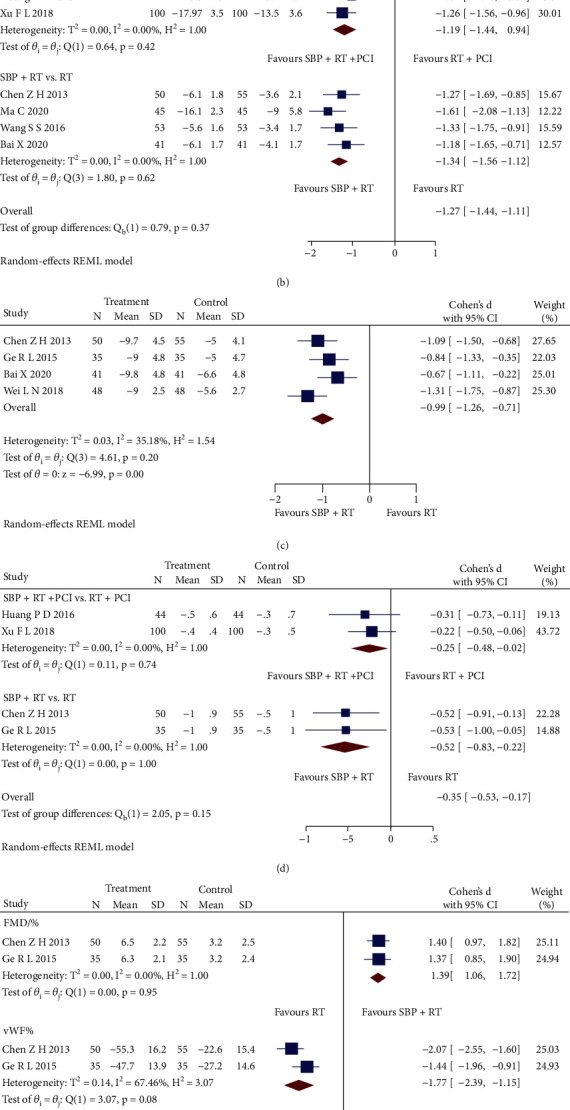
(a) Forest plot of subgroup analysis on the CK-MB and cTnI. (b) Forest plot of subgroup analysis on the Hs-CRP. (c) Forest plot of pooled analysis on the IL-6. (d) Forest plot of subgroup analysis on the TG. (e) Forest plot of subgroup analysis on the FMD and vMF. (f) Forest plot of pooled analysis on the NO.
(2) Inflammatory Factors. SBP plus RT significantly declined the levels of high-sensitivity C-reactive protein (Hs-CRP) (SMD = −1.34, 95% CI [-1.56, -1.12], p < 0.05; Q (3) = 1.80, p = 0.62, I2 = 0, low heterogeneity) and interleukin-6 (IL-6) (SMD = −0.99, 95% CI [-1.26, -0.71], p < 0.05; Q (3) = 4.61, p = 0.20, I2 = 35.18%, low heterogeneity), as shown in the pooled analysis of six RCTs [23, 25, 27, 29–31] (Figures 5(b)and 5(c), respectively). The pooled analysis of other two RCTs [37, 39] showed that SBP adjunctive to RT plus PCI had a significant improvement in Hs-CRP (SMD = −1.19, 95% CI [-1.44, -0.94], p < 0.05; Q (1) = 0.64, p = 0.42, I2 = 0, low heterogeneity) (Figure 5(b)).
(3) Blood Lipid Level. Four RCTs reporting triglyceride (TG) level were pooled by subgroup analysis of interventions. As shown in Figure 5(d), both SBP plus RT (SMD = −0.52, 95% CI [-0.83, -0.22], p < 0.05; Q (1) < 0.05, p = 1.00, I2 = 0, low heterogeneity) and SBP adjunctive to RT plus PCI (SMD = −0.25, 95% CI [-0.48, -0.02], p < 0.05; Q (1) = 0.11, p = 0.74, I2 = 0, low heterogeneity) significantly lowered TG level when compared with RT and RT plus PCI, respectively.
(4) Vascular Endothelial Function. Compared with RT, SBP plus RT had significant improvements in the flow-mediated dilation (FMD) level (SMD = 1.39, 95% CI [1.06, 1.72], p < 0.05; Q (1) < 0.05, p = 0.95, I2 = 0, low heterogeneity) and von Willebrand Factor (vWF) level (SMD = −1.77, 95% CI [-2.39, -1.15], p < 0.05; Q (1) = 3.07, p = 0.08, I2 = 67.46%, high heterogeneity) in the subgroup analysis of two RCTs [23, 25] (Figure 5(e)). Sensitivity analysis indicated that differences in the measuring method could contribute to the heterogeneity. Three RCTs [27, 29, 31] reporting the nitric oxide (NO) level were pooled. The analysis showed that SBP plus RT had a higher level of NO compared with RT alone (SMD = 0.89, 95% CI [0.65, 1.13], p < 0.05; Q (2) = 0.15, p = 0.93, I2 = 0, low heterogeneity) (Figure 5(f)).
3.5.5. Complication Rate
Seven RCTs [26, 30, 32, 33, 35, 39, 41] reported recurrent AMI, and nine RCTs [24, 26–28, 30–33, 35] reported complication cases. SBP adjunctive to RT significantly reduced the AMI recurrent rate than RT alone (RR = 0.30, 95% CI [0.15, 0.59], p < 0.05; Q (4) = 1.45, p = 0.84, I2 = 0, low heterogeneity), but SBP adjunctive to RT plus PCI did not present significant difference (RR = 0.64, 95% CI [0.20, 2.09], p > 0.05; Q (1) = 0.76, p = 0.38, I2 = 0, low heterogeneity) (Figure 6(a)). As for the overall complication cases, SBP plus RT significantly reduced the overall complication rate than RT alone (RR = 0.47, 95% CI [0.39, 0.57], p < 0.05; Q (8) = 3.95, p = 0.86, I2 = 0, low heterogeneity), as shown in Figure 6(b).
Figure 6.
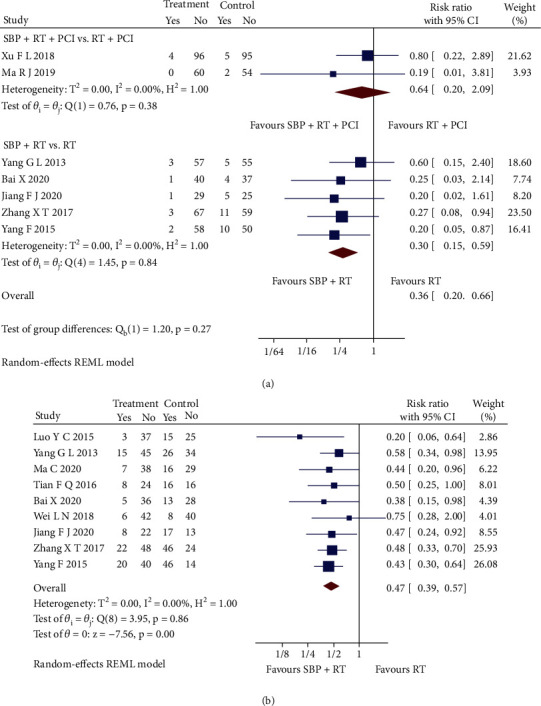
(a) Forest plot of subgroup analysis on the recurrent AMI rate. (b) Forest plot of pooled analysis on the overall complication rate.
4. Discussion
This review explored the clinical evidence of SBP for AMI in RCTs and antioxidant effects of SBP for AMI in PEs, respectively. In the PEs, we found that SBP enhanced the eNOS activity during oxidative stress and regulated several laboratory indexes involving oxidative damage, including hippuric acid, homocysteine, 5-methylcytosine, PGPC, allantoin indoleacrylic acid, 6-hydroxymelatonin, and thymidine. From the pooled analysis of RCTs, SBP plus RT showed significantly improved clinical efficacy rate, cardiac function, and vascular endothelial function and reduced myocardial enzyme, inflammatory cytokines, blood lipid level, and complication rate. Hence, SBP not only showed the benefits for AMI in RCT but also had antioxidative effects in AMI animal models. Notably, the adjunctive effects of SBP adjunctive to RT plus PCI were diminished in most outcomes except for Hs-CRP and TG levels. It may be attributed to the ceiling effect of PCI for AMI treatment.
Oxidative stress plays a major role in cardiovascular diseases [47–49]. Combined with our preclinical review, SBP removes superoxide anion (O2•−) through several antioxidative stress mechanisms (Figure 7). SBP protects against endothelial cell injury by activating eNOS activity and promoting nitric oxide (NO) production [50, 51]. Excessive O2•− reacts with NO to form peroxynitrite (ONOO−) [52–54]. ONOO− in turn oxidizes eNOS to produce O2•−. Superoxide dismutases (SODs) upregulated by SBP are capable of preserving NO bioactivity [55] and clearing away O2•− [56]. Hence, SODs suppress the reaction of O2•− and NO from producing ONOO− as well as catalyzing O2•− into hydrogen peroxide (H2O2). H2O2 finally is detoxified into water and oxygen by glutathione peroxidases (GPx) or peroxiredoxins (Prx) [57]. The study indicates that SBP enhances the activity of peroxiredoxin-3 (Prx-3) to remove H2O2. Another study reported that reactive oxygen radicals from the autooxidation of homocysteine in plasma probably lead to oxidative damage of endothelial cells [58]. Several active components of SBP have been identified. Cinnamaldehyde decreases the O2•− generation through the toll-like receptor 4-NADPH oxidase 4 (TLR4-NOX4) pathway in the lipopolysaccharide-induced cardiac dysfunction [14]. Ginsenoside Rc, one component of SBP, activates a histone deacetylase, sirtuin type 1 (SIRT1), and suppresses ROS [13, 59]. The collecting evidence indicates that SBP has antioxidant effects on AMI. As SBP consists of multiple Chinese medicines, the antioxidant effects could involve multiple active components and multiple mechanisms. A few questions have not been fully addressed; e.g., how many active components are in SBP? What are the mechanisms of these active components suppressing ROS?
Figure 7.
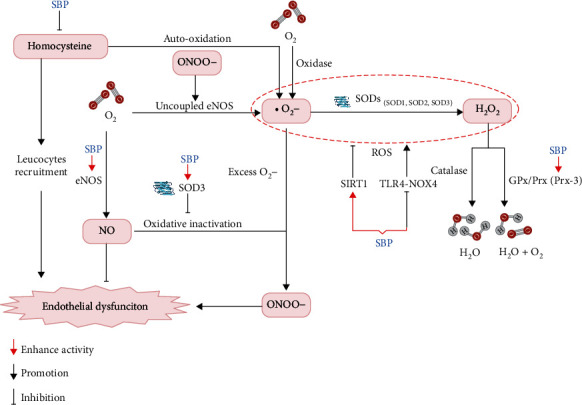
Reactions of the superoxide involving the possible effectiveness of SBP.
This systematic review and meta-analysis included RCTs to evaluate the clinical effects of SBP following the PRISMA guideline. Nevertheless, the overall quality of included RCTs is poor according to the risk of bias assessment, and half of the pooled analysis involving PCI showed high heterogeneity. Compared with RT plus PCI, we found that the SBP adjunctive to RT plus PCI does not significantly affect the AMI recurrence rate and even decreases the LVEF level in one study [40]. Given the inadequate inclusion of RCTs involving PCI, the study cannot confirm the effectiveness of SBP adjunctive to RT plus PCI even though it significantly improves the Hs-CRP and TG levels. Hence, our findings suggest that SBP can be recommended for patients with mild or moderate AMI rather than severe AMI patients requiring PCI treatment. Whether SBP benefits patients with severe AMI needs to be evaluated by rigorous RCTs further.
5. Conclusions
SBP protects against oxidative stress in AMI via multiple mechanisms. Clinical evidence indicates that SBP adjunctive to RT improves the clinical efficacy rate, cardiac function, and other clinical indexes of AMI. The current evidence supports the use of SBP for mild and moderate AMI patients.
Acknowledgments
This study is supported by the Shenzhen Fundamental Research Program (JCYJ20180306173745092), Chinese Medicine Development Fund (19SB2/012A), HMRF (17181831), and Shanghai Hutchison Pharmaceuticals Ltd. (2018009).
Contributor Information
Zhang-Jin Zhang, Email: zhangzj@hku.hk.
Haiyong Chen, Email: haiyong@hku.hk.
Disclosure
The funders were not involved in the collection, management, analysis, and interpretation of data.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
HC and ZZ conceived and designed the study. HC revised the manuscript. JG, ZQ, and QH performed the data analysis and wrote the manuscript. LF, CL, WC, HZ, PM, XX, and ML retrieved studies and extracted data. All authors revised and approved the final manuscript. Jianbo Guo, Zongshi Qin, and Qingyong He contributed equally to this work.
Supplementary Materials
Supplementary Table 1: PRISMA 2009 checklist.
Supplementary Table 2: the search strategy of the PubMed database.
Supplementary Table 3: risk of bias judgments for preclinical experiments (SYRCLE).
Supplementary Table 4: risk of bias judgments for randomized controlled trials (RoB 2.0).
References
- 1.Anderson J. L., Morrow D. A. Acute myocardial infarction. New England Journal of Medicine . 2017;376(21):2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E. J., Go A. S., et al. Executive summary: Heart Disease and Stroke Statistics—2016 Update. Circulation . 2016;133(4):447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 3.Hall M., Dondo T. B., Yan A. T., et al. Association of clinical factors and therapeutic strategies with improvements in survival following non-ST-elevation myocardial infarction, 2003-2013. JAMA . 2016;316(10):1073–1082. doi: 10.1001/jama.2016.10766. [DOI] [PubMed] [Google Scholar]
- 4.Roger V. L., Weston S. A., Gerber Y., et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation . 2010;121(7):863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Waha S., Jobs A., Eitel I., et al. Multivessel versus culprit lesion only percutaneous coronary intervention in cardiogenic shock complicating acute myocardial infarction: a systematic review and meta-analysis. European Heart Journal Acute Cardiovascular Care . 2018;7(1):28–37. doi: 10.1177/2048872617719640. [DOI] [PubMed] [Google Scholar]
- 6.Thiele H., Akin I., Sandri M., et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. New England Journal of Medicine . 2017;377(25):2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 7.Seewald M., Coles J. A., Jr., Sigg D. C., Iaizzo P. A. Featured article: pharmacological postconditioning with delta opioid attenuates myocardial reperfusion injury in isolated porcine hearts. Experimental Biology and Medicine . 2017;242 doi: 10.1177/1535370216684041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schönberger T., Jürgens T., Müller J., et al. Pivotal role of phospholipase D1 in tumor necrosis factor-α-mediated inflammation and scar formation after myocardial ischemia and reperfusion in mice. The American Journal of Pathology . 2014;184(9):2450–2464. doi: 10.1016/j.ajpath.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Wen J., Ma X., Zhang L., et al. Therapeutic efficacy and safety of Shexiang Baoxin pill combined with trimetazidine in elderly patients with heart failure secondary to ischaemic cardiomyopathy. Medicine . 2018;97(51) doi: 10.1097/MD.0000000000013580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong T., Wang J., Ma X., et al. Shexiang Baoxin pills as an adjuvant treatment for chronic heart failure: a system review and meta-analysis. Evidence-based Complementary and Alternative Medicine . 2018;2018:13. doi: 10.1155/2018/6949348.6949348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K. J., Zhu J. Z., Bao X. Y., Zheng Q., Zheng G. Q., Wang Y. Shexiang Baoxin pills for coronary heart disease in animal models: preclinical evidence and promoting angiogenesis mechanism. Frontiers in Pharmacology . 2017;8 doi: 10.3389/fphar.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu L., Sun X., Chen C., Qin Y., Guo X. Shexiang Baoxin pill, derived from the traditional Chinese medicine, provides protective roles against cardiovascular diseases. Frontiers in Pharmacology . 2018;9 doi: 10.3389/fphar.2018.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Q., Su H., Qi B., et al. A SIRT1 activator, ginsenoside Rc, promotes energy metabolism in cardiomyocytes and neurons. Journal of the American Chemical Society . 2021;143(3):1416–1427. doi: 10.1021/jacs.0c10836. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H., Zhang M., Zhou F., et al. Cinnamaldehyde ameliorates LPS-induced cardiac dysfunction via TLR4-NOX4 pathway: the regulation of autophagy and ROS production. Journal of Molecular and Cellular Cardiology . 2016;101:11–24. doi: 10.1016/j.yjmcc.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine . 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangogiannis N. G. Pathophysiology of myocardial infarction. Comprehensive Physiology . 2009;5 doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Liu X., Ren B., Rupp H., Takeda N., Dhalla N. S. Modification of myosin gene expression by imidapril in failing heart due to myocardial infarction. Journal of Molecular and Cellular Cardiology . 2002;34(7):847–857. doi: 10.1006/jmcc.2002.2023. [DOI] [PubMed] [Google Scholar]
- 18.Ibanez B., James S., Agewall S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) European Heart Journal . 2002;39 [Google Scholar]
- 19.Roffi M., Patrono C., Collet J. P., et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment Elevation. European Heart Journal . 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K., Alpert J. S., Jaffe A. S., et al. Third universal definition of myocardial infarction. Journal of the American College of Cardiology . 2016;60 doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Sterne J. A., Savović J., Page M. J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ . 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Hooijmans C. R., Rovers M. M., de Vries R. B., Leenaars M., Ritskes-Hoitinga M., Langendam M. W. SYRCLE's risk of bias tool for animal studies. BMC Medical Research Methodology . 2014;14(1) doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z. H., Ma L. Effects of Shexiangbaoxin pill on inflammation indicators, cardiac and vascular endothelial function in patients with acute myocardial infarction. Journal of Chongqing Medical University . 2014;38 [Google Scholar]
- 24.Luo Y. C. Influence of Shexiangbaoxin pills on QT interval dispersion in acute myocardial infarction patients. Chinese Journal of Modern Drug Application . 2015;9 [Google Scholar]
- 25.Ge R. L. Thirty-five cases with acute myocardial infarction treated with Shexiang Baoxin pill. Henan Traditional Chinese Medicine . 2015;35 [Google Scholar]
- 26.Yang G. L., Song H. X., Liu J. H., Li H. Y., Liu P., Cao X. M. Clinical study on the treatment of acute non-ST segment elevation myocardial infarction with Shexiang Baoxin pill. Henan Traditional Chinese Medicine . 2013;33 [Google Scholar]
- 27.Ma C., Xu Z. W., Zhao S., et al. Effect of Shexiang Baoxin pill and low molecular weight heparin calcium in the treatment of acute ST segment elevation myocardial infarction. China Medical Herald . 2020;17 [Google Scholar]
- 28.Tian F. Q. Study on the anti-peroxidation effect of shexiang baoxin pill combined with urokinase in the treatment of acute myocardial infarction. Modern Journal of Integrated Traditional Chinese and Western Medicine . 2016;25 [Google Scholar]
- 29.Wang S. S. Clinical effect of Shexiangbaoxin pill combined with fasudil on acute myocardial infarction and the impact on micro-inflammatory state and vascular endothelial function. Practical journal of cardio-cerebrovascular disease . 2016;24 [Google Scholar]
- 30.Bai X. Shexiang Baoxin pill combined with atorvastatin in the treatment of acute myocardial infarction. Systems Medicine . 2020;5 [Google Scholar]
- 31.Wei L. N., Pan D. B., Huang J. S. Effects of Shexiang Baoxin pill and atorvastatin on cardiac function and the levels of serum ET, NO, CRP, IL-6 in patients with acute myocardial infarction. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovvascular Disease . 2018;16 [Google Scholar]
- 32.Jiang F. J. Observation on the curative effect of Shexiang Baoxin pill combined with atorvastatin on acute myocardial infarction. Chinese Journal of Clinical Rational Drug Use . 2020;13 [Google Scholar]
- 33.Zhang X. T. Efficacy evaluation of Shexiang Baoxin pill combined with atorvastatin in the treatment of acute myocardial infarction. The World's Latest Medical Information Digest . 2017;17 [Google Scholar]
- 34.Xu J., Li H. N. Clinical observation on the treatment of acute myocardial infarction by Shexiang Baoxin pill combined with atorvastatin. Journal of Clinical Medical . 2017;4 [Google Scholar]
- 35.Yang F., Jiang X. J., Shen Y. B. Clinical study on Shexiang Baoxin pills combined with atorvastatin in treatment of acute myocardial infarction. Drugs & Clinic . 2015;30 [Google Scholar]
- 36.Feng B., Hong S. Y. Effects of Shexiang Baoxin pill combined with atenolol for patients with acute myocardial infarction. Journal of Clinical Medicine in Practice . 2020;24 [Google Scholar]
- 37.Huang P. D., Wu Y. H., Gu W., Dong L. P. Clinical efficacy of heart musk of pill combined with western medicine for acute myocardial infarction in elderly patients. Chinese Journal of Evidence-Based Cardiovascular Medicine . 2016;8 [Google Scholar]
- 38.Jiang D. J., Yan X. B., Yu Z. Z. Effects of Shexiang Baoxin pill combined with tirofiban on vascular endothelial function and cardiac function after PCI in patients with acute myocardial infarction. Shanghai Medical and Pharmaceutical Journal . 2020;41 [Google Scholar]
- 39.Xu F. L., Jiang W., Huang L., Yin J., Zai W. X., Gao T. Y. Effect of Shexiang Baoxin pill on prognosis of patients with acute coronary syndrome after PCI. Acta Chinese Medicine . 2018;33 [Google Scholar]
- 40.Lin G. Q. Evaluate the effect of SXBXW for patients with acute myocardial infarction after PCI . Fujian University of Traditional Chinese Medicine; 2010. [Google Scholar]
- 41.Ma R. J., Cai S. L. Clinical study on Shexiang Baoxin pills combined with benazepril for patients with acute ST-segment elevation myocardial infarction after PCI. Journal of New Chinese Medicine . 2017;51 [Google Scholar]
- 42.Luo X. P., Zeng Z. Y., Shi H. M., Fan W. H., Zai R. H. Effects of heart-protecting musk pill on the expression of myocardial endothelial nitric oxide synthase and left ventricular function after myocardial infarction in rats. Chinese Herbal Medicine . 1999;2 [Google Scholar]
- 43.Xiang L., Jiang P., Wang S., et al. Metabolomic strategy for studying the intervention and the synergistic effects of the shexiang baoxin pill for treating myocardial infarction in rats. Evidence-based Complementary and Alternative Medicine . 2013;2013:11. doi: 10.1155/2013/823121.823121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q. Pharmacokinetics and pharmacodynamics study of Ginsenosides in Shexiang Baoxin pill based on metabonomics approach . Second Military Medical University; 2017. [Google Scholar]
- 45.Jiang P., Dai W., Yan S., et al. Biomarkers in the early period of acute myocardial infarction in rat serum and protective effects of Shexiang Baoxin Pill using a metabolomic method. Journal of Ethnopharmacology . 2011;138(2):530–536. doi: 10.1016/j.jep.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 46.Yu F., Yu Y., Tian S., et al. Quantitative proteomics reveals Shexiang Baoxin pill exerts cardioprotective effects by preserving energy metabolism in a rat model of myocardial infarction. Journal of Ethnopharmacology . 2021;266 doi: 10.1016/j.jep.2020.113460. [DOI] [PubMed] [Google Scholar]
- 47.Griendling K. K., Alexander R. W. Oxidative stress and cardiovascular disease. Circulation . 2021;96 [PubMed] [Google Scholar]
- 48.Lüscher T. F. Ageing, inflammation, and oxidative stress: final common pathways of cardiovascular disease. European Heart Journal . 2015;36(48):3381–3383. doi: 10.1093/eurheartj/ehv679. [DOI] [PubMed] [Google Scholar]
- 49.Chang X., Zhang T., Zhang W., Zhao Z., Sun J. Natural drugs as a treatment strategy for cardiovascular disease through the regulation of oxidative stress. Oxidative Medicine and Cellular Longevity . 2020;2020:20. doi: 10.1155/2020/5430407.5430407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ungvari Z., Tarantini S., Kiss T., et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nature Reviews. Cardiology . 2018;15(9):555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo X. P., Li Y., Fan W. H., Shi H. M., Wang S. Y., Dai R. H. Effect of heart-protecting musk pill on nitric oxide metabolism of arterial wall in cholesteriol-fed rabbits. Chinese Journal of Critical Care Medicine . 1999;19 [Google Scholar]
- 52.Pacher P., Beckman J. S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews . 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown G. C., Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovascular Research . 2007;75(2):283–290. doi: 10.1016/j.cardiores.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Szabó C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nature Reviews. Drug Discovery . 2007;6(8):662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 55.Kim H. W., Lin A., Guldberg R. E., Ushio-Fukai M., Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circulation Research . 2007;101(4):409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 56.Ning R. B., Xu C. S., Chai D. J., Lin J. X. Effect of Shexiang Baoxin pills proliferation and oxidative stress induced by H(2)O(2) in human umbilical vein endothelial cells. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovvascular Disease . 2007;9 [Google Scholar]
- 57.Stöcker S., Van Laer K., Mijuskovic A., Dick T. P. The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs. Antioxidants & Redox Signaling . 2018;28(7):558–573. doi: 10.1089/ars.2017.7162. [DOI] [PubMed] [Google Scholar]
- 58.Welch G. N., Loscalzo J. Homocysteine and atherothrombosis. The New England Journal of Medicine . 1998;338(15):1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Liang X., Chen Y., Zhao X. Screening SIRT1 activators from medicinal plants as bioactive compounds against oxidative damage in mitochondrial function. Oxidative Medicine and Cellular Longevity . 2016;2016:9. doi: 10.1155/2016/4206392.4206392, [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: PRISMA 2009 checklist.
Supplementary Table 2: the search strategy of the PubMed database.
Supplementary Table 3: risk of bias judgments for preclinical experiments (SYRCLE).
Supplementary Table 4: risk of bias judgments for randomized controlled trials (RoB 2.0).


