Abstract
Malignant mesothelioma is a neoplasm of serosal surfaces, most commonly affecting the pleura. The peritoneum, pericardium, and tunica vaginalis are less frequently involved. Malignant mesothelioma with EWSR1-ATF1 fusion in young adults was recently reported in the literature. Here, we present two pediatric cases of EWSR1-ATF1 translocation-associated malignant mesothelioma in the peritoneum and pericardium respectively. Both cases lacked a known exposure history. Microscopy in both cases showed predominantly epithelioid morphology with ample eosinophilic cytoplasm, and immunohistochemistry was positive for pan-keratin, calretinin, and WT1. Both cases showed EWSR1-ATF1 gene rearrangement by RNA sequencing, which was instrumental in confirming the diagnosis of malignant mesothelioma and to exclude more common pediatric sarcomas, especially in the context of limited sampling.
Keywords: peritoneum, pericardium, mesothelioma, RNA sequencing, immunohistochemistry, EWSR1, gene rearrangement
Introduction
Malignant mesothelioma is a neoplasm of the serosal surfaces of body cavities. The most common site of involvement is pleura, then peritoneum. The pericardium or tunica vaginalis is the primary site in less than 5% of cases. 1 Pediatric mesotheliomas comprise less than 5% of total mesotheliomas, with similar anatomical sites and frequencies of involvement to adults. 2 Asbestos and radiation exposure are strong risk factors for adult pleural mesothelioma, but the association is weaker with peritoneal mesothelioma, and is only rarely found in pericardial mesothelioma and in pediatric mesothelioma cases.3,4 Proposed risk factors in children include fetal isoniazid exposure, or ataxia-telangiectasia, but the pathophysiology of pediatric cases largely remains unknown.5,6
Genomic profiling studies of peritoneal mesothelioma have demonstrated overlapping genetic alterations with pleural mesothelioma, such as BAP1 inactivation/loss, and deletions of CDKN2A and NF2.7–9 The roles of these genes in pericardial mesothelioma have not been widely reported. Recently, malignant mesothelioma with genetic alterations in ALK, EWSR1, FUS1 or YY1 have been reported.10–13
In this report, two adolescent patients with mesothelioma harboring EWSR1-ATF1 fusion are described, both of whom were 15 years of age at diagnosis. Patient #1 had peritoneal mesothelioma and is the youngest patient to date reported to have peritoneal mesothelioma with EWSR1-ATF1 rearrangement. Patient #2 had pericardial mesothelioma, the first reported pericardial mesothelioma with EWSR1-ATF1 fusion.
Case Reports
Patient #1
This previously healthy 15-year old male presented with a two-month history of weight loss and constitutional symptoms. Ultrasound and CT scan showed a mesenteric mass. Abdominal fluid drainage and incisional biopsy were performed. After the procedure, he developed bowel obstruction and underwent a wider resection of the mass and adjacent small bowel for symptom management.
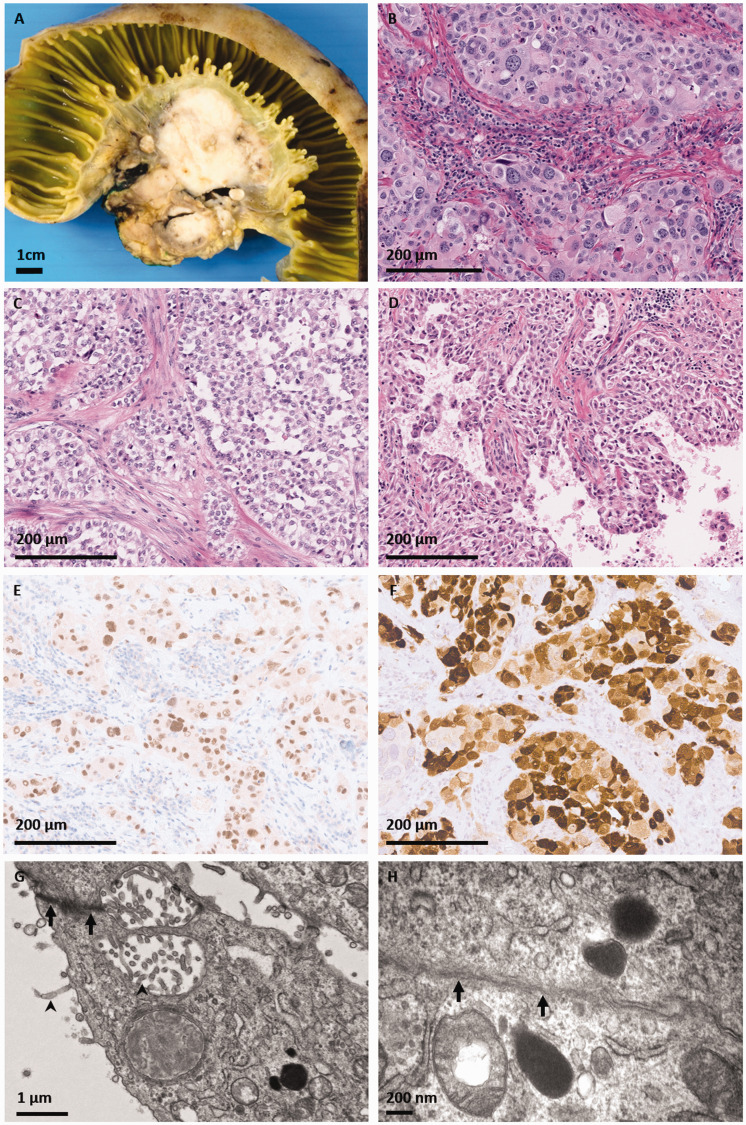
Grossly, the resected tumor was solid, tan-white, and centered in the small bowel mesentery. It abutted the muscularis propria but did not invade into the superficial bowel tissue layers (Figure 1(A)). Microscopically, the tumor was predominantly composed of nests or sheets of large epithelioid cells, with round nuclei of variable diameters and eosinophilic (Figure 1(B)) to clear (Figure 1(C)) cytoplasm. Papillary structures were seen in a minority of sections (Figure 1(D)). Up to 3 mitoses per 10 high power fields, including atypical mitoses, were counted.
Figure 1.
Patient #1, presenting with a peritoneal mass. A, Grossly, the resected tumor was a solid tanwhite mass centered in the small bowel mesentery, abutting the muscularis propria but not invading into the superficial bowel tissue layers. On hematoxylin and eosin stained microscopic sections, the tumor was predominantly composed of nests or sheets of epithelioid cells with round vesicular nuclei of varying diameters and (B) eosinophilic or (C) clear cytoplasm. D, Papillary structures were seen in a minority of sections. Immunohistochemistry for (E) WT-1 and (F) calretinin was positive in tumor cells. Electron microscopy revealed a poorly differentiated tumor with (G) microvilli (arrowhead) and desmosomes (arrow). H, There was evidence of basement membrane formation (arrowhead).
Immunohistochemistry (IHC) was positive for the epithelial markers AE1/AE3, CAM5.1, and EMA. Vimentin was strongly positive. Desmin showed patchy positivity. Multiple mesothelial lineage markers such as WT-1 (Figure 1(E)), calretinin (Figure 1(F)), and CK5/6 were positive. Markers for melanocytic differentiation (S100, Melan A, HMB45, and SOX10) were negative. INI-1 (SMARCB1) protein expression was retained. No cytoplasmic inclusions were identified on PAS-D. In light of the positive mesothelial markers, BAP1 immunohistochemistry was performed, and tumor cells showed retention of this protein.
Breakapart FISH was positive for EWSR1 gene rearrangement. RNA sequencing using Illumina TruSight RNA Fusion Panel identified EWSR1-ATF1 fusion with the breakpoints located in exon 13 of EWSR1 and exon 5 of ATF1. Ultrastructural analysis revealed a poorly differentiated tumor with epithelioid features including microvilli and desmosomes (Figure 1(G)), as well as basement membrane formation (Figure 1(H)). Based on these data, a final diagnosis of malignant peritoneal mesothelioma was made.
Patient #2
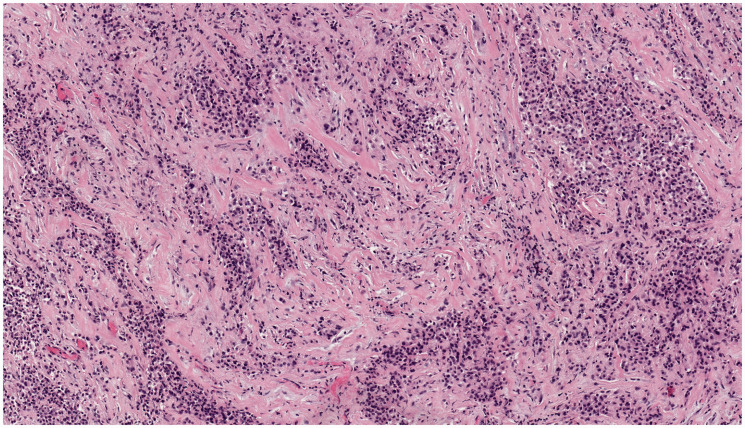
This 15-year-old male patient presented with a pericardial mass. Core needle biopsies were performed. Microscopically, the tumor showed infiltrating cords and nests of monomorphic epithelioid cells within a sclerotic stroma (Figure 2). The tumor cells showed ample eosinophilic cytoplasm with hyperchromatic eccentric nuclei. IHC was positive for pan-keratin, calretinin and WT-1, supporting a mesothelial lineage. RNA sequencing using Illumina TruSight RNA Fusion Panel also identified EWSR1-ATF1 fusion with breakpoints in exon 8 of EWSR1 and exon 4 of ATF1. These results supported a final diagnosis of primary pericardial mesothelioma.
Figure 2.
Patient #2, presenting with a pericardial mass. On hematoxylin and eosin stained microscopic sections, the tumor showed infiltrating cords and nests of epithelioid cells within a sclerotic stroma. The cells were monomorphic with hyperchromatic eccentric nuclei and eosinophilic cytoplasm. Photomicrograph taken at 100× original magnification.
Discussion
This report describes two cases of pediatric mesothelioma with EWSR1-ATF1 fusion. Both cases showed the typical histology and immunohistochemistry profile of malignant mesothelioma. Because mesothelioma is rare in children, and because some more common pediatric malignancies may show at least focally similar histology to mesothelioma, or have similar immunohistochemistry markers to mesothelioma, there is a differential diagnosis that should be considered. In particular, Patient #1’s tumor, which was extensively sampled and showed variable morphology, had areas with focal cytoplasmic clearing and nested growth pattern that was reminiscent of clear cell sarcoma (CCS). However, the tumor showed no immunohistochemistry or ultrastructural evidence of melanocytic differentiation. Another sarcoma with a nested growth pattern is alveolar soft part sarcoma, but no characteristic PAS-D cytoplasmic inclusions were identified in this patient’s tumor. The positive epithelial and mesenchymal IHC markers were similar to those of desmoplastic small round cell tumor (DSRCT), but the histomorphology of the tumor was not that of DSRCT. Finally, poorly differentiated carcinoma was a consideration, but would be highly unusual in this age group. RNA sequencing was crucial for confirming the final diagnosis of malignant mesothelioma with EWSR1 gene rearrangement. This malignancy was characterized in a case series by Desmeules et al., which included four patients with EWSR1-ATF1 or FUS-ATF1 fusion-positive malignant mesotheliomas, three of whom had peritoneal-based disease. The age of presentation ranged from 21-34 years, and the three patients with available clinical information had no asbestos exposure history. Three cases in which BAP1 IHC was performed showed retained expression. 11
EWSR1 is a multifunctional RNA-binding protein belonging to the FET (FUS/TLS, EWS, and TAF15) protein family, which also includes FUS and TAF15. Both the EWSR1 and FUS genes are involved in tumorigenesis by fusing with various transcription factor coding genes, leading to fusion oncogene formation. EWSR1 or FUS fusing with various CREB-transcription factor family members (ATF1, CREB1, and CREM) leads to a wide variety of neoplasms, including CCS, clear cell-like tumor of the gastrointestinal tract (CCLTGT), angiomatoid fibrous histiocytoma (AFH), primary pulmonary myxoid sarcoma (PPMS), soft tissue myoepithelial tumor (SMET), hyalinizing clear cell carcinoma (HCCC), angiosarcoma of the parotid, and a group of malignant epithelioid neoplasms with predilection for mesothelial-lined cavities.14–17 Though the fusion proteins driving these neoplasms may be the same, these tumors can be separated by careful consideration of clinical presentation, histomorphology, and immunohistochemistry. For example, AFH and angiosarcoma of the parotid both show minimal morphologic resemblance to mesothelioma and are negative for mesothelial markers. CCS and CCLTGT can be ruled out by negative melanocytic markers. HCCC is a low grade neoplasm of the salivary gland that has not been described in the peritoneum or pericardium. PPMS and SMET can be separated from mesothelioma based on their site and lack of mesothelial immunohistochemistry profile. Recently, Argani et al. 16 published a case series of malignant epithelioid neoplasms with predilection for mesothelial-lined cavities. In these tumors, EWSR1 or FUS was fused to CREB transcription factors, and showed histologic features intermediate between AFH and epithelioid mesothelioma. The epithelioid component of the tumors described in Argani et al. showed morphologic resemblance to the tumors that we describe in this report, but calretinin was negative. In contrast, both of our cases were strongly positive for calretinin, supporting their mesothelial lineage. Furthermore, ultrastructural analysis of one of our cases clearly demonstrated epithelial or mesothelial elements such as desmosomes, basement membranes, and microvilli, while the tumors described in Argani et al. did not show ultrastructural evidence of mesothelial differentiation.
Mesothelioma with EWSR1/FUS-ATF1 fusion is likely a distinct subgroup of mesothelioma with an alternative pathogenesis that does not involve common pathways such as BAP1, CDKN2A and NF2. Morphologically, translocation-associated mesotheliomas show histologic overlap with non-translocated mesotheliomas. Both can have epithelioid morphology with variable papillary, nested, and solid growth patterns. While non-translocated mesothelioma can have sarcomatoid morphology, to date only epithelioid histology has been described in mesotheliomas with EWSR1-ATF1 or FUS-ATF1. Both of the cases in our report showed epithelioid morphology.
To conclude, the two patients with EWSR1-ATF1 associated mesothelioma described in this report are the youngest patients reported with this malignancy. This report highlights the importance of including malignant mesothelioma in the differential diagnosis of pediatric tumors with EWSR1-ATF1 gene rearrangements. In the pediatric population, sarcomas with EWSR1 gene rearrangement occur more frequently than mesothelioma. Correlating the correct tumor morphology with the underlying genetic alteration is essential, due to the existence of tumors with similar morphology or immunohistochemistry profiles. Furthermore, the clinical context, such as age, location, and past medical history, can considerably narrow down a broad differential diagnosis. Finally, RNA sequencing is a complementary and powerful tool that can identify the fusion gene driving the malignancy and help to establish a definitive diagnosis.
Ethics and Consent
Per UBC Guidance and Sick Kids Standard Operating Procedure, this case series does not require REB review as it comprises no more than two separate cases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Hezhen Ren https://orcid.org/0000-0002-2779-7297
Anna F Lee https://orcid.org/0000-0002-6703-0656
References
- 1.Hillerdal G. Malignant mesothelioma 1982: review of 4710 published cases. Br J Dis Chest. 1983;77(4):321–343. [PubMed] [Google Scholar]
- 2.Fraire AE, Cooper S, Greenberg SD, Buffler P, Langston C. Mesothelioma of childhood. Cancer. 1988;62(4):838–847. [DOI] [PubMed] [Google Scholar]
- 3.Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol. 2007;18(6):985–990. [DOI] [PubMed] [Google Scholar]
- 4.Webb J, Yiu YW, Giastefani S, Carr-White G. Pericardial mesothelioma. QJM. 2016;109(9):631–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosas-Salazar C, Gunawardena SW, Spahr JE. Malignant pleural mesothelioma in a child with ataxia-telangiectasia. Pediatr Pulmonol. 2013;48(1):94–97. [DOI] [PubMed] [Google Scholar]
- 6.Tuman K. Mesothelioma in child with prenatal exposure to isoniazid. Lancet. 1980;316(8190):362. [DOI] [PubMed] [Google Scholar]
- 7.Thurneysen C, Opitz I, Kurtz S, Weder W, Stahel RA, Felley-Bosco E. Functional inactivation of NF2/merlin in human mesothelioma. Lung Cancer. 2009;64(2):140–147. [DOI] [PubMed] [Google Scholar]
- 8.Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9(6):2108–2113. [PubMed] [Google Scholar]
- 9.Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43(7):668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung YP, Dong F, Watkins JC, et al. Identification of ALK rearrangements in malignant peritoneal mesothelioma. JAMA Oncol. 2018;4(2):235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmeules P, Joubert P, Zhang L, et al. A subset of malignant mesotheliomas in young adults are associated with recurrent EWSR1/FUS-ATF1 fusions. Am J Surg Pathol. 2017;41(7):980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panagopoulos I, Thorsen J, Gorunova L, et al. RNA sequencing identifies fusion of the EWSR1 and YY1 genes in mesothelioma with t(14;22)(q32;q12). Genes Chromosomes Cancer. 2013;52(8):733–740. [DOI] [PubMed] [Google Scholar]
- 13.Argani P, Lian DWQ, Agaimy A, et al. Pediatric mesothelioma with ALK fusions: a molecular and pathologic study of 5 cases. Am J Surg Pathol. 2021;45(5):653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol. 2012;36(7):e1–e11. [DOI] [PubMed] [Google Scholar]
- 15.Flucke U, Mentzel T, Verdijk MA, et al. EWSR1-ATF1 chimeric transcript in a myoepithelial tumor of soft tissue: a case report. Hum Pathol. 2012;43(5):764–768. [DOI] [PubMed] [Google Scholar]
- 16.Argani P, Harvey I, Nielsen GP, et al. EWSR1/FUS–CREB fusions define a distinctive malignant epithelioid neoplasm with predilection for mesothelial-lined cavities. Mod Pathol. 2020;33(11):2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gru AA, Becker N, Pfeifer JD. Angiosarcoma of the parotid gland with a t(12;22) translocation creating a EWSR1-ATF1 fusion: a diagnostic dilemma. J Clin Pathol. 2013;66(5):452–454. [DOI] [PubMed] [Google Scholar]