Abstract
Background:
Depression and low mood are leading contributors to disability worldwide. Research indicates that clinical depression may be associated with low creatine concentrations in the brain and low prefrontal grey matter volume. Because subclinical depression also contributes to difficulties in day-to-day life, understanding the neural mechanisms of depressive symptoms in all individuals, even at a subclinical level, may aid public health.
Methods:
Eighty-four young adult participants completed the Depression, Anxiety and Stress Scale (DASS) to quantify severity of depression, anxiety and stress, and underwent 1H-Magnetic Resonance Spectroscopy of the medial prefrontal cortex and structural magnetic resonance imaging (MRI) to determine whole-brain grey matter volume.
Results/outcomes:
DASS depression scores were negatively associated (a) with concentrations of creatine (but not other metabolites) in the prefrontal cortex and (b) with grey matter volume in the right superior medial frontal gyrus. Medial prefrontal creatine concentrations and right superior medial frontal grey matter volume were positively correlated. DASS anxiety and DASS stress scores were not related to prefrontal metabolite concentrations or whole-brain grey matter volume.
Conclusions/interpretations:
This study provides preliminary evidence from a representative group of individuals who exhibit a range of depression levels that prefrontal creatine and grey matter volume are negatively associated with depression. While future research is needed to fully understand this relationship, these results provide support for previous findings, which indicate that increasing creatine concentrations in the prefrontal cortex may improve mood and well-being.
Keywords: Creatine, depression, grey matter volume, neuroimaging, prefrontal
Introduction
Depression and low mood are leading contributors to disability worldwide and can affect more than 300 million people at any one time (Britton, 2017). While most of the negative health and social effects of low mood are attributed to Major Depressive Disorder (MDD), individuals who experience subclinical depression (i.e. those who score below clinical thresholds on depression scales) also experience a significant impact on their daily functioning (Cuijpers et al., 2004). Importantly, the prevalence of subclinical depression/low mood may be increasing in society and may now affect a higher percentage of society (24%) than clinical depression (Van Zoonen et al., 2015). Because depression may be best understood as a continuum of symptoms, and because subclinical depression may be one of the best predictors of future MDD (Rodriguez et al., 2012; Van Zoonen et al., 2015), understanding the mechanisms of subclinical depressive symptoms in non-clinical groups may contribute to improvements in public mental health.
To aid development of novel therapies for depression, studies have attempted to determine the neurochemical mechanisms of depressive symptoms, yet many have ignored the potential importance of brain creatine. This is partly because when using the primary method for quantifying this endogenous compound in vivo (1H-Magnetic Resonance Spectroscopy (1H-MRS)), research groups have often referenced their metabolite of interest to creatine to ‘correct’ for concentrations of other metabolites (e.g. Kumar et al., 2002), on the basis that creatine was considered to be stable within regions of interest and across individuals (Li et al., 2003). However, prefrontal creatine is influenced by a range of factors, including cigarette smoking (Durazzo et al., 2016; Faulkner et al., 2021), cocaine use (Chang et al., 1997), cannabis use (Prescot et al., 2011), anxiety (Yue et al., 2012) and schizophrenia (Öngür et al., 2009), and can be influenced by other pathological conditions such as hepatic encephalopathy (Braissant et al., 2019), and has been shown to be almost absent in the brains of patients with cerebral creatine deficiency syndromes (e.g. Rackayova et al., 2017).
Currently, the potential relationship between brain creatine and depression is poorly understood. Specifically, no difference was found in anterior cingulate creatine metabolite concentrations between 19 depressed individuals diagnosed with MDD and 30 aged-matched controls (Auer et al., 2000), or in thalamic creatine concentrations between 18 young depressed individuals and 18 young non-depressed individuals (Mirza et al., 2006). Conversely, Kondo et al. (2016) reported a weak yet significant negative relationship between depression scores and concentrations of creatine in the frontal cortex of 22 adolescent participants diagnosed with MDD (p = 0.030; actual effect size not reported), indicating that high concentrations of creatine in the prefrontal cortex may be associated with lower levels of depression. Along these lines, Dechant et al. (1999) report that daily administration of 20 g creatine monohydrate for 4 weeks increased total brain creatine by up to 8.7% in a small sample of nine healthy individuals (see Allen, 2012, for a review), while Lyoo et al. (2012) report that daily supplementation of 5 g creatine monohydrate for 8 weeks augmented the antidepressant effects of escitalopram in 25 depressed females. Interestingly, Nemets and Levine (2013) report no significant effect of creatine supplementation when administered daily for only 4 weeks. However, this lack of an effect is likely due to, at least in part, the fact that the authors compared the effects of (a) 5 g creatine supplementation, (b) 10 g creatine supplementation and (c) placebo in small groups of only five, four and nine individuals, respectively. As such, creatine administration may positively influence depressive symptomatology, and determining whether there is a relationship between prefrontal creatine and depression may aid the treatment of this mood disorder.
Structural neuroimaging studies have also indicated that depression and low mood are related to lower grey matter volume in the prefrontal cortex. Three meta-analyses of 20+ studies that examined brain structure using voxel-based morphometry (VBM) revealed that compared to healthy controls, depressed patients exhibit lower grey matter volume bilaterally in the medial prefrontal cortex and anterior cingulate cortex (Bora et al., 2012; Lai, 2013; Wise et al., 2017). Furthermore, studies published more recently support these findings (e.g. Kandilarova et al., 2019). However, it is currently unknown whether this depression-related low prefrontal grey matter volume is related to alterations in prefrontal creatine concentrations.
In the current study, we examined the relationship between depression levels in a non-clinical sample and both prefrontal creatine metabolite concentrations (quantified using 1H-MRS) and grey matter volume (quantified using VBM). It was hypothesized that there would be a significant negative association between depression levels and both creatine concentrations and grey matter volume in the prefrontal cortex. For completeness, and on the basis of findings by Hasler et al. (2007) and Auer et al. (2000), who report that depressed individuals exhibit low prefrontal concentrations of glutamate and γ-aminobutyric acid (GABA) metabolites, we also performed exploratory analyses to determine the relationship between depression levels and concentrations of all metabolites quantified by the 1H-MRS sequence.
Methods
We report an analysis of data collected from two separate studies, both of which had the aim of collecting health-related magnetic resonance imaging (MRI) data in young adults. Both study protocols used the same MRI sequences for volumetric and 1H-MRS data (see below), and all data were acquired on the same 3T MRI scanner at the Combined Universities Brain Imaging Centre.
Participants
Across both studies, 84 participants were recruited via print and online advertisements. Thirty-eight of these subjects participated in Study 1, and 46 subjects participated in Study 2. All participants gave written informed consent after receiving a detailed explanation of their study procedures (approved by the University of Roehampton Research Ethics Committee) and were screened for eligibility. Exclusion criteria for both studies were as follows: self-report of psychiatric diagnoses, current drug use/abuse or dependence (other than tobacco and cannabis use), history of neurological injury or disease, pregnancy and contraindications for MRI (e.g. metal implants). Data from Study 1 were collected from November 2015 to March 2018, while data from Study 2 were collected from September 2017 to December 2019.
Questionnaire measures
Participants completed a demographics form (developed in-house) to determine age, gender, level of education, self-reported psychiatric comorbidity, neurological disorder and use of tobacco, cannabis and illicit drugs. Exposure to cigarettes was inferred from the average number of cigarettes smoked per day as in previous research (e.g. Durazzo et al., 2016; Faulkner et al., 2018, 2019, 2020). Participants also completed the Depression, Anxiety and Stress Scale (DASS), a 42-item questionnaire designed to quantify levels of depression, anxiety and stress; each of these emotional symptoms is assessed by summing the answers to fourteen 4-point Likert-type scale questions (Lovibond and Lovibond, 1995). On the Depression subscale, a score of 0 to 9 denotes no depression, 10 to 13 mild depression, 14 to 20 moderate depression, 21 to 27 severe depression, while 28+ denotes extremely severe depression. On the Anxiety subscale, a score of 0 to 7 denotes no anxiety, 8 to 9 mild anxiety, 10 to 14 moderate anxiety, 15 to 19 severe anxiety and 20+ denotes extremely severe anxiety. On the Stress subscale, a score of 0 to 14 denotes no stress, 15 to 18 mild stress, 19 to 25 moderate stress, 26 to 33 severe stress and 34+ denotes extremely severe stress.
To determine the influence of age, gender, cigarette smoking and cannabis use on depression, anxiety and stress, an analysis of variance (ANOVA) was constructed, in which scores from the DASS Depression, Anxiety or Stress subscales were added as the dependent variable (as relevant), and age, gender, the average number of cigarettes smoked per day and the average number of cannabis joints smoked per day were all added as separate factors.
1H-MRS data acquisition, preprocessing and analysis
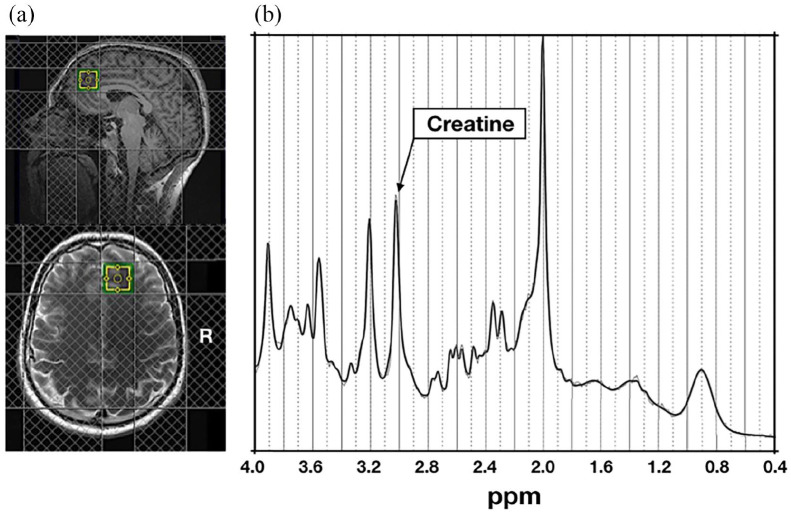
All 1H-MRS scans were acquired using the same 3T Siemens Magnetom TIM Trio MRI system using a 32-channel head coil. 1H-MRS in vivo spectra were acquired from the same 20 × 20 × 20 mm3 voxel located in the right medial prefrontal cortex (typical location shown in Figure 1(a)). The structure and function of the medial prefrontal cortex are related to the clinical features of depression (e.g. Farb et al., 2011); this voxel placement therefore allowed us to test our own hypotheses pertaining to relationships of depression and brain chemistry. A medial position was also chosen, as lateral voxels can be harder to place due to tissue boundaries. The voxel was placed manually by referring to the individual subject’s T1-weighted (magnetization-prepared rapid gradient echo (MPRAGE)) scan. Specifically, we ensured that the voxel was placed very close to the midline of the brain, and as anterior as possible while avoiding gyri and cerebrospinal fluid. As such, the voxel was placed both anterior and slightly dorsal to the corpus callosum. Spectra were acquired using a SPin ECho full Intensity-Acquired Localized (SPECIAL; Mlynarik et al., 2006) spectroscopy 1H-MRS sequence with water suppression (repetition time (TR) = 3000 ms, echo time (TE) = 8.5 ms, Phase cycle Auto, 192 averages from the right prefrontal cortex voxel; Godlewska et al., 2015). Water unsuppressed spectra (16 averages) were also acquired. Outer volume suppression slabs were applied 5 mm from the edge of each side of the voxel (six slabs in total), both to suppress signals originating outside of the right medial prefrontal voxel of interest, and to minimize motion artefact effects on spectra within the voxel.
Figure 1.
(a) Typical 1H-MRS voxel placement in the medial prefrontal cortex. (b) Example attained spectrum from the medial prefrontal voxel seen in (a).
Spectra were analysed using LCModel 6.3-1L, with a basis set consisting of 19 simulated spectra, as in Morgenroth et al. (2019) and Faulkner et al. (2021) (for the full basis set, see Supplementary Materials). This basis set was simulated using FID appliance (FID-A) (Simpson et al., 2017) for TE = 8.5 ms, magnetic field strength = ~3 T and assuming ideal radiofrequency pulses. Line widths and signal-to-noise ratios were estimated as less than 8 Hz and greater than 40 Hz, respectively (Godlewska et al., 2015). Cramér–Rao lower bounds (Kreis, 2016), line widths and signal-to-noise ratios did not differ as a function of DASS scores (i.e. levels of depression, anxiety or stress) or between Study 1 and Study 2 (see Supplementary Materials).
Water referencing and eddy current correction were used to quantify metabolite levels. When quantified in this way, such levels are influenced by cerebral spinal fluid, grey and white matter volumes of the region in which spectra are obtained (Srinivasan et al., 2006), as well as by individual differences in whole-cortical grey matter (Huster et al., 2007). We therefore corrected these metabolite levels for grey and white matter content within the right medial prefrontal voxel using the GABA Analysis Toolkit (Gannet 3.1, http://gabamrs.blogspot.co.uk/), adapted to work with Siemens SPECIAL data. Segmentation was performed using ‘new segment’ in Statistical Parametric Mapping 12 (SPM12) (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Cerebrospinal fluid and grey and white matter volumes were then accounted for in the expression of creatine using LCModel (Ernst et al., 1993; Gasporovic et al., 2006). For the specific calculation by which metabolites were corrected for using these volumes, see the Supplementary Materials.
To determine relationships between metabolite concentrations and DASS depression, anxiety or stress scores, an ANOVA was constructed in which DASS depression scores were added as the dependent variable, and age, gender, the average number of cigarettes smoked per day and daily cannabis use were all added as separate factors. For completeness, two exploratory ANOVAs were performed in which the dependent variables were (a) DASS anxiety scores and (b) DASS stress scores, to determine relationships between both anxiety and stress, respectively, and metabolite concentrations; these two ANOVAs both corrected for age, gender, daily cigarette smoking and daily cannabis use. Because the primary hypothesis pertained only to the relationship between depression and prefrontal creatine, and because all other analyses were considered secondary, corrections for multiple comparisons were not applied.
Structural MRI data acquisition, preprocessing and analysis
In both studies, high-resolution structural images were acquired using a T1-weighted MPRAGE sequence. Images were analysed using Computational Anatomy Toolbox 12 (CAT12; http://www.neuro.uni-jena.de/cat) implemented in SPM12 (Wellcome Trust Centre for Neuroimaging; fil.ion.ucl.ac.uk/spm/software/spm12/), as per the standard protocol (see http://www.neuro.uni-jena.de/cat12/CAT12-Manual.pdf); for a detailed description of the structural MRI (sMRI) data preprocessing steps, see the Supplementary Materials.
To determine the relationship between DASS depression scores and whole-brain grey matter volume, group-level analyses were performed by constructing a one-sample general linear model (GLM) in SPM12 that contained each participant’s modulated, normalized, segmented, registered and smoothed grey matter tissue segments, and one explanatory variable (DASS depression scores), along with separate variables for age, gender, daily cannabis use, smoking status (smokers vs non-smokers) and total intracranial volume to control for the influence of these variables. Contrasts were performed to identify regions in which whole-brain grey matter volume (a) positively and (b) negatively correlated with DASS depression scores. For completeness, two exploratory GLMs were performed; one that included DASS anxiety scores, and one that included DASS stress scores in place of depression scores. A threshold of p < 0.05 with Family-wise error (FWE) correction for multiple comparisons was applied to all contrasts.
To determine relationships between metabolite concentrations and grey matter volume that was associated with depression severity, bivariate correlations were performed; these models included only metabolite concentrations and values from significant clusters that were identified from the above volumetric contrasts.
All ANOVAs and correlational analyses were performed using both frequentist and Bayesian analyses. Frequentist analyses were performed using the Statistical Package for the Social Sciences (version 26, SPSS, Inc., Chicago, IL, USA). Bayesian analyses were performed using JASP (version 0.11.1; https://jasp-stats.org/download/).
For the frequentist analyses, a significance threshold of alpha = 0.05 (two-tailed) was adopted. For the Bayesian analyses, we adopted the thresholds set out by Jeffreys (1961); for a detailed description of these thresholds, see the Supplementary Materials.
Results
Participant characteristics
Of the 84 participants across both studies, 46 were male and 38 were female (mean age = 23.42 years, standard deviation (SD) = 4.50 years, range = 18–37 years). Forty-four participants self-reported tobacco use at least 1 day per week (mean cigarettes smoked per day by these 44 participants = 5.37, SD = 5.85). Forty-one participants self-reported cannabis use at least once per week (mean number of joints smoked per day by these 41 participants = 0.81, SD = 1.31). A full summary of participant characteristics can be seen in Table 1.
Table 1.
Participant characteristics.
| All participants | Depressed (DASS > 9) | Non-depressed (DASS < 10) | |
|---|---|---|---|
| n = 84 | n = 19 | n = 65 | |
| Gender (male/female) | 47/37 | 7/12 | 40/25 |
| Age (years) a | 23.41 (4.50) | 23.06 (3.15) | 23.45 (4.86) |
| Education (years) a | 15.77 (3.87) | 15.38 (3.96) | 15.88 (3.87) |
| DASS Depression a | 6.31 (6.25) | 16.17 (5.18)* | 3.44 (2.74)* |
| DASS Anxiety a | 5.52 (5.42) | 10.39 (7.29)* | 4.16 (3.84)* |
| DASS Stress a | 9.26 (7.79) | 16.61 (10.47)* | 7.19 (5.36)* |
| Cigarettes per day a | 5.37 (5.85) | 3.52 (3.04) | 5.75 (6.59) |
| Cannabis use b | 0.81 (1.31) | 0.59 (0.96) | 0.91 (1.40) |
| Cramér–Rao bound (creatine) | 2.26 (0.55) | 2.28 (0.47) | 2.24 (0.62) |
| FWHM | 0.04 (0.03) | 0.04 (0.01) | 0.03 (0.04) |
| GMV composition | 0.44 (0.06) | 0.47 (0.04) | 0.42 (0.06) |
| WMV composition | 0.52 (0.07) | 0.47 (0.05) | 0.53 (0.07) |
DASS: Depression, Anxiety and Stress Scale; FWHM: full width at half maximum; GMV: grey matter volume; WMV: white matter volume; SD: standard deviation.
Denotes mean (SD).
Denotes mean (SD) number of joints smoked per day.
Statistically significant (p < 0.05) difference between depressed and non-depressed individuals.
Depression scores
The mean self-reported score on the Depression subscale of the DASS was 6.31 (SD = 6.25, range = 0–24). Specifically, 65 participants scored between 0 and 9, and so were classified as ‘non-depressed’ (mean of these 65 participants = 3.28, SD = 2.72), while the remaining 19 participants scored 10+ and were thus classified as ‘depressed’ (mean of these 19 participants = 18.47, SD = 8.25); of these, 8 scored between 10 and 13 and were defined as ‘mildly depressed’ (mean = 10.33, SD = 0.71), 5 scored between 14 and 20 and were defined as ‘moderately depressed’ (mean = 17.00, SD = 2.22) and 6 scored between 21 and 27 and were defined as ‘severely depressed’ (mean = 22.00, SD = 1.20). The skewness and kurtosis values for depression scores were slightly outside the acceptable range, and so a square-root transformation was performed on this variable to render the data normally-distributed.
An ANOVA that contained DASS depression scores as the dependent variable and age, gender, smoking status and daily cannabis use as separate factors revealed that depression scores were not influenced by age, F(1, 76) = 1.334, p = 0.252, BF10 = 0.881. However, this ANOVA did reveal that female participants reported higher DASS depression scores than male participants, F(1, 76) = 6.446, p = 0.013, BF10 = 1.431 (see Supplemental Figure S1). Furthermore, this ANOVA also revealed that smokers and non-smokers did not differ in terms of DASS depression scores, F(1, 76) = 0.559, p = 0.457, BF10 = 0.198, and a bivariate correlation analysis revealed that depression scores were not associated with daily cannabis use (r = –0.231, p = 0.835, BF10 = 0.091).
Relationship between DASS depression scores and prefrontal creatine and other metabolites
An ANOVA that controlled for age, gender, smoking status and cannabis use revealed that there was an association between DASS depression scores and concentrations of creatine in the prefrontal cortex, F(1, 76) = 6.005, p = 0.017, BF10 = 3.834 (see Figure 2); specifically, participants who self-reported the highest depression scores exhibited the lowest creatine concentrations in the medial prefrontal cortex. There were no significant relationships between DASS depression scores and concentrations of any of the remaining metabolites (see Supplementary Materials). For the effects of age, gender, cannabis and tobacco use on metabolite concentrations, see the Supplementary Materials.
Figure 2.
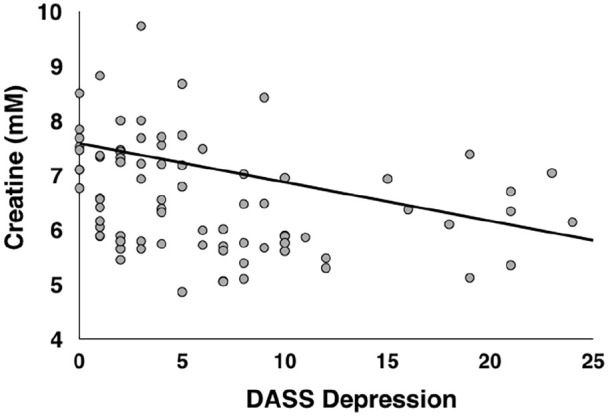
Relationship between DASS depression scores and creatine in the prefrontal voxel depicted in Figure 1(a). Creatine values are corrected for grey matter, white matter and cerebrospinal fluid, and are expressed in marcomolecules (mM).
Relationship between depression scores and grey matter volume
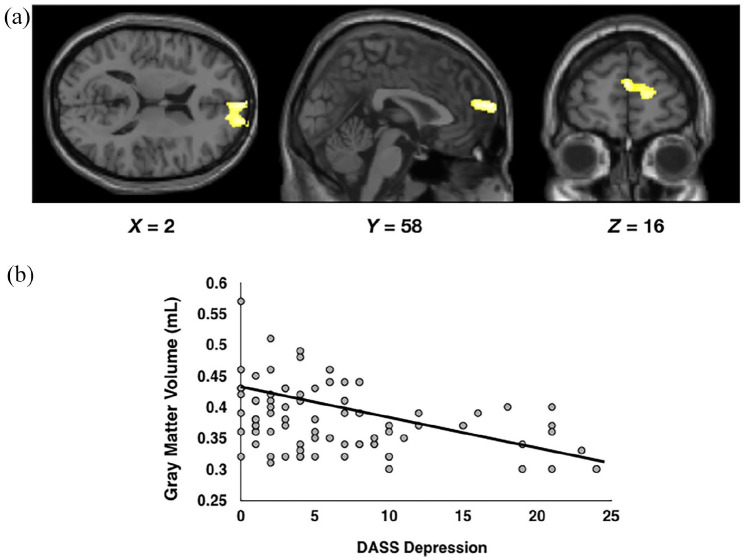
When controlling for age, gender, daily cigarette use, daily cannabis use and total intracranial volume, there was a significant negative association between DASS depression scores and grey matter volume in a cluster of 524 voxels in the right medial superior frontal gyrus; the local maxima for this cluster was x = 2, y = 58, z = 16 (see Figure 3).
Figure 3.
(a) Results of the whole-group F-test that examined brain regions in which whole-brain grey matter was associated with DASS depression scores. (b) Scatterplot depicting the relationship between DASS depression scores and grey matter volume within the cluster of voxels depicted in (a).
Relationship between prefrontal creatine and grey matter volume
A bivariate correlation revealed a significant positive correlation between prefrontal creatine concentrations and values extracted from the cluster of 524 voxels depicted in Figure 3(a) (values averaged across all voxels in the cluster; r = 0.225, p = 0.043, BF10 = 3.128). Finally, none of the remaining metabolite concentrations correlated with values extracted from the cluster of voxels depicted in Figure 3(a) (all ps > 0.187, all BFs < 0.919; see Figure 4).
Figure 4.
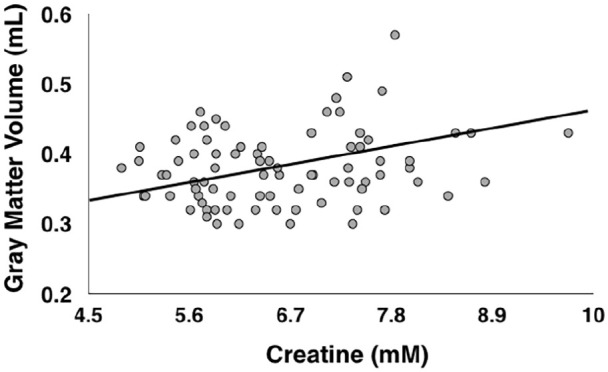
Correlation between concentrations of creatine in the voxel shown in Figure 1(a), and grey matter volume in the cluster of voxels shown in Figure 3(a) in all participants. Creatine values are corrected for grey matter, white matter and cerebrospinal fluid, and are expressed in marcomolecules (mM).
Anxiety and stress scores
ANOVAs that controlled for age, gender, daily cigarette use and daily cannabis use revealed that neither DASS anxiety scores nor DASS stress scores were associated with concentrations of creatine or any of the remaining metabolites (all ps > 0.163, all BF10 < 0.802; see Supplementary Materials). Finally, there were no significant associations between whole-brain grey matter volume and scores on the DASS Anxiety or Stress subscales.
Discussion
This is the first study to examine the relationship of both prefrontal creatine and grey matter volume with depression/low mood in a group of individuals who self-reported a wide range of depression severities. Our results suggest that individuals who experience subclinical depressive symptoms and low mood have lower concentrations of creatine and lower grey matter volume in the prefrontal cortex. These findings extend those of previous research, by indicating that depression, when considered as a continuum that ranges from no/subclinical depression to severe depression, is associated with low creatine and grey matter volume in the prefrontal cortex.
That greater depression severity was related to lower concentrations of prefrontal creatine was expected on the basis of findings from one study, which revealed a weak negative relationship between depression and frontal creatine (measured using 31-phosphorous MRS) in a small sample of adolescent depressed participants (Kondo et al., 2016). Interestingly, the relationship between brain creatine and magnitude of depression may be specific to the prefrontal lobes, as previous studies indicate there to be no relationship between depression and concentrations of creatine in the thalamus (Mirza et al., 2006) or anterior cingulate cortex (Auer et al., 2000).
The present findings could partly explain why increasing creatine levels via administration of creatine supplements has been shown to have potential in alleviating depressive symptoms in depressed patients (Kious et al., 2019; Kondo et al., 2011; Lyoo et al., 2012; Nemets and Levine, 2013; Roitman et al., 2007). However, while Kondo et al. (2016) examined the effects of daily administration of 2, 4 or 10 mg creatine on both depression and prefrontal creatine, they did so in only seven, eight and seven participants, respectively, meaning that the authors may have been underpowered to determine whether baseline prefrontal creatine concentrations can predict the observed treatment-induced improvements in mood. Future studies may wish to use magnetic resonance spectroscopy to determine whether pretreatment concentrations of creatine can predict such a response, and whether this effect is indeed specific to the prefrontal lobes, in a larger sample size than used by those authors. Furthermore, it should be noted that administration of creatine supplements do not currently lead to large increases in total brain creatine concentrations. For example, Dechant et al. (1999) report that daily administration of 20 mg creatine monohydrate for 4 weeks increased such levels by only 8.7%. Indeed, the blood–brain barrier has a relatively low permeability for creatine into the brain, due partly to the absence of the creatine transporter on the feet of astrocytes lining the microcapillary endothelial cells (see Braissant, 2012, for a review). As such, future studies may wish to determine ways to either optimize the efficiency of current creatine supplement protocols to increase levels of creatine in the brain, or ways to develop novel methods in which to increase such levels.
Currently, the mechanisms by which depression and low mood are associated with low brain creatine are poorly understood. Creatine has an important role in the regulation of many neurological functions, including sodium and calcium transport, and the synthesis, uptake and release of neurotransmitters (Allen, 2012), while impairments in all of these functions are considered to promote depression (Assis et al., 2009; Kious et al., 2019). Furthermore, many of the brain regions that express the creatine transporter, including the prefrontal cortex, are compromised in depression (e.g. Allen, 2012). Importantly, creatine is also involved in energy metabolism, in that the brain uses adenosine triphosphate (ATP) to convert creatine to phosphocreatine (Wallimann et al., 1992), and reduction in the release of ATP from astrocytes is considered one potential factor that may promote depression (Illes et al., 2020). Furthermore, because neuronal creatine is released from neurons in response to an action potential, and because creatine is subsequently transported back into the presynaptic neuron by the creatine transporter (see Jiddeke et al., 2012, for a review), creatine is considered by some to function as a neurotransmitter; it may thus be that alterations in its functioning as a neurotransmitter promote depression. In addition, administration of creatine can increase brain-derived neurotrophic factor (BDNF) in the hippocampus, which has itself been shown to have antidepressant effects (Pazini et al., 2016). However, these cellular mechanisms cannot be observed using 1H-MRS as this technique does not distinguish between intracellular and extracellular concentrations of creatine, nor does it allow for observation of creatine function at the level of the transporter. To truly determine the influence of creatine function (and indeed of creatine supplements) on depression, future studies could utilize other neuroimaging techniques, such as Positron Emission Tomography, alongside 1H-MRS and sMRI, to better examine prefrontal creatine function and affect at many stages throughout an individual’s lifespan, and to determine whether creatine supplements can alter mood via actions in the prefrontal cortex.
The present findings indicate that levels of depression, especially when quantified as in this study, may be related to concentrations of creatine in the prefrontal voxel observed in Figure 1(a), but not with other neurotransmitters in this specific brain region. Interestingly, while previous research (e.g. Auer et al., 2000) has used 1H-MRS to indicate that clinical depression is associated with low concentrations of glutamate in other brain regions such as the anterior cingulate cortex (which is considered to be adjacent to the prefrontal cortex), studies that have examined the relationship between depression and glutamate specifically in the prefrontal cortex have often corrected glutamate for concentrations of creatine, and have only examined the relationship of such concentrations with clinical depression (Hasler et al., 2007). As such, our results indicate that when examining the relationship between levels of depression that range from almost zero depression to high depression (which may be considered socially representative) and metabolite concentrations in the prefrontal cortex, the former are associated with concentrations of creatine, rather than glutamate. However, future work is needed to determine the replicability of this particular finding. Furthermore, our results also indicate that low prefrontal creatine and grey matter volume are specifically related to depression, as the DASS allows for identification of various aspects of emotional disturbance, and no meaningful or significant relationships between our brain measures and scores on the DASS Anxiety or Stress subscales were found.
The results of our VBM analyses replicate findings from previous studies (e.g. Bora et al., 2012; Lai, 2013), by indicating that higher levels of depression are associated with lower grey matter volume in the right superior medial frontal gyrus. As behavioural and cognitive data were not available from a sufficient sample of participants, our data cannot determine the functional relevance of reductions in grey matter in this brain region. However, low volume within the superior medial frontal gyrus may be associated with known depression-related cognitive deficits. For example, low grey matter volume and hypoactivation within this region are related to high levels of rumination (Johnson et al., 2009; Schiller et al., 2013) and an inability to inhibit a prepotent response (Li et al., 2010), both of which are associated with depressive symptoms (e.g. Kaiser et al., 2003; Nolen-Hoeksema et al., 1994). Future studies could therefore aim to determine whether therapies that act upon/prevent damage to this brain region can aid in the treatment of depression/low mood.
This study has several limitations. First, participants were typically young adults, and so our findings may not be generalizable to the entire public. Second, only 19 participants were classified as ‘depressed’ by the DASS because they scored greater than 9 on the Depression subscale, meaning that we may have lacked sufficient statistical power to detect small effects. Similarly, a larger sample size would have provided greater statistical power to detect small changes in brain metabolites such as Taurine that have less-dominant peaks and thus lower signal-to-noise. Third, a structured clinical interview (such as the Mini-International Psychiatric Interview – Sheehan et al., 1998 – for example) was not administered, meaning that detailed information regarding clinical symptoms of depression could not be determined. Furthermore, the fact that such an interview was not administered means that our sample potentially contains individuals who have psychiatric disorders, the symptoms of which could influence our findings. Fourth, our cross-sectional design did not allow us to make predictions regarding the causal effects of brain metabolites on depression; future studies may therefore wish to employ a longitudinal design. Fifth, we did not attempt to assess dietary intake of creatine, which may be associated with the experiencing of depressive symptomatology (Bakian et al., 2020). While it is unclear whether this association is causative (i.e. whether lower dietary creatine intake promotes depression in the general population), future studies may wish to assess participants’ dietary creatine intake when examining the relationship between depression and brain creatine in order to better understand this association. Furthermore, some participants self-reported light-to-moderate use of cannabis, and moderate use of tobacco cigarettes; while our analyses indicated cannabis use did not influence brain creatine or grey matter volume, there is a possibility that it had a small, yet undetectable effect. Along these lines, we did not specifically recruit participants who smoked cannabis, and instead collected data pertaining to daily cannabis use in these participants. While it could be considered that this approach yielded data that are representative of the average daily cannabis use in the larger population, it may have weakened our ability to determine whether there was an association in our sample between cannabis use and concentrations of brain creatine, in part because we could not construct a valid categorical variable for cannabis use (i.e. use vs no-use); indeed, Prescott et al. (2011) reported that cannabis users (defined as those who smoked cannabis more than 100 times in their life) exhibited lower brain creatine than non-cannabis users, and attempting to replicate this result could have yielded interesting information. In addition, because our 1H-MRS sequence only allowed for the quantification of metabolite concentrations in one voxel (see Figure 1), we were not able to determine whether level of depression is related to creatine (or indeed any other metabolite) in brain regions outside of the medial prefrontal cortex. Finally, cognitive measures were not obtained from a sufficient sample of participants, meaning that we could not determine whether low creatine is associated with depression-related cognitive deficits.
In summary, this study provides evidence in a representative group of individuals who exhibit a range of depression levels, that prefrontal creatine and grey matter volume are negatively related to low mood/depression. While future research is needed to achieve a more complete understanding of this relationship, these results support the findings of research which indicate that increasing concentrations of prefrontal creatine via creatine supplements may be able to improve an individual’s mood and/or alleviate depression.
Supplemental Material
Supplemental material, Supplementary_Materials for Relationship between depression, prefrontal creatine and grey matter volume by Paul Faulkner, Susanna Lucini Paioni, Petya Kozhuharova, Natasza Orlov, David J Lythgoe, Yusuf Daniju, Elenor Morgenroth, Holly Barker and Paul Allen in Journal of Psychopharmacology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was partly funded by a British Academy/Leverhulme Trust Research Grant, awarded to P.A.
Access to data: For access to data, please contact the corresponding author.
ORCID iD: Paul Faulkner  https://orcid.org/0000-0003-4475-3568
https://orcid.org/0000-0003-4475-3568
Supplemental material: Supplemental material for this article is available online.
References
- Allen PJ. (2012) Creatine metabolism and psychiatric disorders: Does creatine supplementation have therapeutic values? Neuroscience and Biobehavioural Reviews 36(5): 1442–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis LC, Rezin GT, Comim CM, et al. (2009) Effect of acute administration of ketamine and imipramine on creatine kinase activity in the brain of rats. Brazilian Journal of Psychiatry 31(3): 247–252. [DOI] [PubMed] [Google Scholar]
- Auer DP, Pütz B, Kraft E, et al. (2000) Reduced glutamate in the anterior cingulate cortex in depression: An in vivo proton magnetic resonance spectroscopy study. Biological Psychiatry 47(4): 305–313. [DOI] [PubMed] [Google Scholar]
- Bakian AV, Huber RS, Scholl L, et al. (2020) Dietary creatine intake and depression risk among US adults. Translational Psychiatry 10(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, et al. (2012) Gray matter abnormalities in major depressive disorder: A meta-analysis of voxel-based morphometry studies. Journal of Affective Disorders 138(1–2): 9–18. [DOI] [PubMed] [Google Scholar]
- Braissant O. (2012) Creatine and guanidinoacetate transport at blood-brain and blood-cerebrospinal fluid barriers. Journal of Inherited Metabolic Disease 35: 655–664. [DOI] [PubMed] [Google Scholar]
- Braissant O, Rackayova V, Pierzchala K, et al. (2019) Longitudinal neurometabolic changes in the hippocampus of a rat model of chronic hepatic encephalopathy. Journal of Hepatology 71(3): 505–515. [DOI] [PubMed] [Google Scholar]
- Britton J. (2017) Death, disease, and tobacco. Lancet 389(10082): 1861–1862. [DOI] [PubMed] [Google Scholar]
- Chang L, Mehringer M, Ernst T, et al. (1997) Neurochemical alterations in asymptomatic abstinent cocaine users: A proton magnetic resonance spectroscopy study. Biological Psychiatry 42(12): 1104–1114. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, de Graaf R, van Dorselaer S. (2004) Minor depression: Risk profiles, functional disability, health care use and risk of developing major depression. Journal of Affective Disorders 79(1–3): 71–79. [DOI] [PubMed] [Google Scholar]
- Dechant P, Pouwels PJW, Wilken B, et al. (1999) Increase in total creatine in human brain after oral supplementation of creatine-monohydrate. Journal of Physiology 277(3): 698–704. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Mon A, et al. (2016) Chronic cigarette smoking in healthy middle-aged individuals is associated with decreased regional brain N-aceytlaspartate and glutamate levels. Biological Psychiatry 79(6): 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Kries R, Ross BD. (1993) Absolute quantitation of water and metabolites in the human brain: Compartments and water. Journal of Magnetic Resonance 102(1): 1–8. [Google Scholar]
- Farb NAS, Anderson AK, Bloch RT, et al. (2011) Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological Psychiatry 70(4): 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P, Dean AC, Ghahremani DG, et al. (2020) Neural basis of smoking-related difficulties in emotion regulation. International Journal of Neuropsychopharmacology 23: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P, Ghahremani DG, Tyndale RF, et al. (2018) Functional connectivity of the raphe nuclei: Link to tobacco withdrawal in smokers. International Journal of Neuropsychopharmacology 21(9): 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P, Ghahremani DG, Tyndale RF, et al. (2019) Neural basis of smoking-induced relief of craving and negative affect: Contribution of nicotine. Addiction Biology 24: 1087–1095. [DOI] [PubMed] [Google Scholar]
- Faulkner P, Lucini Paioni S, Kozhuharova P, et al. (2021) Daily and intermittent smoking are associated with low prefrontal volume and low concentrations of glutamate, creatine, myo-inositol, and N-acetylaspartate. Addiction Biology 26: e12986. [DOI] [PubMed] [Google Scholar]
- Gasporovic C, Song T, Devier D, et al. (2006) Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine 55(6): 1219–1226. [DOI] [PubMed] [Google Scholar]
- Godlewska BR, Near J, Cowen PJ. (2015) Neurochemistry of major depression: A study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 232(3): 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, et al. (2007) Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. JAMA Psychiatry 64(2): 193–200. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, et al. (2007) Morphologic asymmetry of the human anterior cingulate cortex. NeuroImage 34(3): 888–895. [DOI] [PubMed] [Google Scholar]
- Illes P, Rubini P, Yin H, et al. (2020) Impaired ATP release from brain astrocytes may be a cause of major depression. Neuroscience Bulletin 36: 1281–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys H. (1961) Theory of Probability (3rd edn). Oxford: Oxford University Press and Clarendon Press. [Google Scholar]
- Jiddeke M van de Kamp, Jakobs C, Gibson KM, et al. (2012). New insights into creatine transporter deficiency: The importance of recycling creatine in the brain. Journal of Inherited Metabolic Disease volume 36: 155–156. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchel KJ, et al. (2009) Medial cortex activity, self-reflection and depression. Social, Cognitive and Affective Neuroscience 4(4): 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Unger J, Kiefer M, et al. (2003) Executive control deficit in depression: Event-related potentials in a Go/Nogo task. Psychiatry Research: Neuroimaging 122(3): 169–184. [DOI] [PubMed] [Google Scholar]
- Kandilarova S, Stoyanov D, Sirakov N, et al. (2019) Reduced gray matter in frontal and temporal areas in depression: Contributions from voxel-based morphometry study. Acta Neuropsychiatrica 31(5): 252–257. [DOI] [PubMed] [Google Scholar]
- Kious BM, Kondo DG, Renshaw PF. (2019) Creatine for the treatment of depression. Biomolecules 9(406): 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo DG, Forrest LN, Shi X, et al. (2016) Creatine target engagement with brain bioenergetic: A dose-ranging phosphorous-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids 48: 1941–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo DG, Sung Y-H, Hellem TL, et al. (2011) Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorous magnetic resonance spectroscopy study. Journal of Affective Disorders 135(0): 354–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis R. (2016) The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magnetic Resonance in Medicine 75: 15–18. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thomas A, Lavretsky H, et al. (2002) Frontal white matter biochemical abnormalities in late-life major depression detected with proton magnetic resonance spectroscopy. The American Journal of Psychiatry 159(4): 630–636. [DOI] [PubMed] [Google Scholar]
- Lai CH. (2013) Gray matter volume in major depressive disorder: A meta-analysis of voxel-based morphometry studies. Psychiatry Research: Neuroimaging 211(1): 37–46. [DOI] [PubMed] [Google Scholar]
- Li BSY, Wang H, Gonen O. (2003) Metabolite ratios to assumed stable creatine may confound the quantification of proton brain MR spectroscopy. Magnetic Resonance Imaging 21(8): 923–928. [DOI] [PubMed] [Google Scholar]
- Li CT, Lin CP, Chou KH, et al. (2010) Structural and cognitive deficits in remitting and non-remitting recurrent depression: A voxel-based morphometric study. NeuroImage 50: 347–356. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. (1995) Manual for the Depression Anxiety Stress Scales. Sydney, NSW, Australia: Psychology Foundation of Australia. [Google Scholar]
- Lyoo IK, Yoon S, Kim -T-K, Hwang J, et al. (2012) A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. The American Journal of Psychiatry 169(9): 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza Y, O’Neill J, Smith EA, et al. (2006) Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus depression and healthy controls. Journal of Child Neurology 21(2): 102–111. [DOI] [PubMed] [Google Scholar]
- Mlynarik V, Gambarota G, Frenkel H, et al. (2006) Localized short-echo time proton spectroscopy with full signal-intensity acquisition. Magnetic Resonance in Medicine 56(5): 965–970. [DOI] [PubMed] [Google Scholar]
- Morgenroth E, Orlov N, Lythgoe DJ, et al. (2019) Altered relationship between prefrontal glutamate and activation during cognitive control in people with high trait anxiety. Cortex 117: 53–63. [DOI] [PubMed] [Google Scholar]
- Nemets B, Levine J. (2013) A pilot dose-finding clinical trial of creatine monohydrate augmentation to SSRIs/SNRIs/NASA antidepressant treatment in major depression. International Clinical Psychopharmacology 28(3): 127–133. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Parker LE, Larson J. (1994) Ruminative coping with depressed mood following loss. Journal of Personality and Social Psychology 67(1): 92–104. [DOI] [PubMed] [Google Scholar]
- Öngür D, Prescot AP, Jensen JE, et al. (2009) Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Research: Neuroimaging 172(1): 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazini FL, Cunha MP, Rosa JM, et al. (2016) Creatine, similar to ketamine, counteracts depressive-like behaviour induced by corticosterone via P13K/Akt/mTOR pathway. Molecular Neurobiology 53: 6818–6834. [DOI] [PubMed] [Google Scholar]
- Prescot AP, Locatelli AE, Renshaw PF, et al. (2011) Neurochemical alterations in adolescent chronic marijuana smokers: A proton MRS study. NeuroImage 57(1): 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackayova V, Cudalbu C, Pouwels PJ, et al. (2017) Creatine in the central nervous system: From magnetic resonance spectroscopy to creatine deficiencies. Analytical Biochemistry 529: 144–157. [DOI] [PubMed] [Google Scholar]
- Rodriguez MR, Nuevo R, Chatterji S, et al. (2012) Definitions and factors associated with subthreshold conditions: A systematic review. BMC Psychiatry 12(181): 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman S, Green T, Osher Y, et al. (2007) Creatine monohydrate in resistant depression: A preliminary study. Bipolar Disorders 9(7): 754–758. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Minkel J, Smoski MJ, et al. (2013) Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. Journal of Affective Disorders 151(2): 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59(20): 34–57. [PubMed] [Google Scholar]
- Simpson R, Devenyi GA, Jezzard P, et al. (2017) Advanced processing and simulation of MRS data using the FID appliance (FID-A) – An open source, MATLAB-based toolkit. Magnetic Resonance in Medicine 77(1): 23–33. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Cunningham C, Chen A, et al. (2006) TE-averaged two-dimensional proton spectroscopic imaging of glutamate at 3T. NeuroImage 30(4): 1171–1178. [DOI] [PubMed] [Google Scholar]
- Van Zoonen K, Kleiboer A, Beekman ATF, et al. (2015) Reasons and determinants of help-seeking in people with a sub-clinical depression. Journal of Affective Disorders 173: 105–112. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, et al. (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. The Biochemical Journal 281(1): 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T, Radua J, Cardoner N, et al. (2017) Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: Evidence from voxel-based meta-analysis. Molecular Psychiatry 22: 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Liu M, Nie X, et al. (2012) Quantitative 3.0T MR spectroscopy reveals decreased creatine concentration in the dorsolateral prefrontal cortex of patients with social anxiety disorder. PLoS ONE 7(10): e48105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials for Relationship between depression, prefrontal creatine and grey matter volume by Paul Faulkner, Susanna Lucini Paioni, Petya Kozhuharova, Natasza Orlov, David J Lythgoe, Yusuf Daniju, Elenor Morgenroth, Holly Barker and Paul Allen in Journal of Psychopharmacology