Abstract
Background
Lipedema is a loose connective tissue disease predominantly in women identified by increased nodular and fibrotic adipose tissue on the buttocks, hips and limbs that develops at times of hormone, weight and shape change including puberty, pregnancy, and menopause. Lipedema tissue may be very painful and can severely impair mobility. Non-lipedema obesity, lymphedema, venous disease, and hypermobile joints are comorbidities. Lipedema tissue is difficult to reduce by diet, exercise, or bariatric surgery.
Methods
This paper is a consensus guideline on lipedema written by a US committee following the Delphi Method. Consensus statements are rated for strength using the GRADE system.
Results
Eighty-five consensus statements outline lipedema pathophysiology, and medical, surgical, vascular, and other therapeutic recommendations. Future research topics are suggested.
Conclusion
These guidelines improve the understanding of the loose connective tissue disease, lipedema, to advance our understanding towards early diagnosis, treatments, and ultimately a cure for affected individuals.
Keywords: Lipedema, lymphedema, hypermobility, chronic venous disease, standard of care
Introduction
Lipedema is a disease of fibrotic loose connective (adipose) tissue (LCT) on the lower abdomen, hips, buttocks, and limbs of females, sparing the trunk, hands, and feet. Lipedema is rare in men. A trigger for the development of lipedema tissue may be an increase in fluid and connective tissue remodeling that occurs alongside body changes during puberty, childbirth, menopause, stress associated with lifestyle change, or by altering tissue structure after surgery or trauma. 1 A hallmark of lipedema tissue is inflammation2,3 resulting in tissue fibrosis and pain, and in some cases, the tissue may become numb. 4
First described in 1940 by Allen and Hines at Mayo Clinic in the US 5 and by Moncorps from Germany, 6 lipedema remains under-recognized in part, because it is assumed to be a usual hereditary component of female fat. 7 Lipedema is confused with non-lipedema obesity or lymphedema due to increased leg size. 8 Under-recognition or misdiagnosis can delay identification of lipedema for decades. 7 Therefore, patient access to appropriate and timely treatment is often diminished 9 and patients frequently find themselves blamed for their condition, including self-blame. 10 However, lipedema can be treated to reduce pain and edema, maintain mobility, and improve quality of life while slowing disease progression, therefore timely diagnosis is paramount.
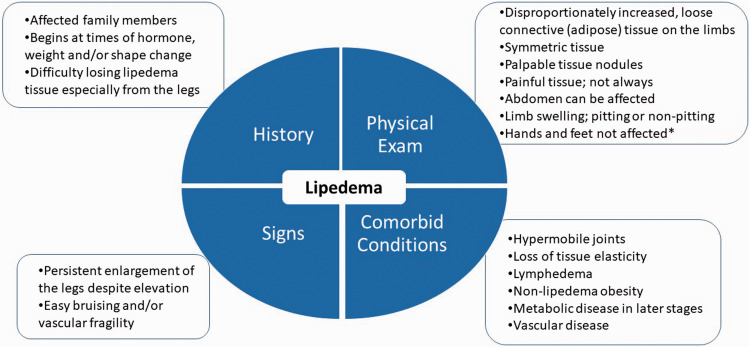
Lipedema is identified by clinical exam 11 with diagnostic criteria to help guide the clinical diagnosis (Figure 1). Skin and lipedema LCT are graded by stage and location (Figure 2). Lipedema tissue, body mass index (BMI), metabolic disease and lymphedema increase with stage.4,12
Figure 1.
Diagnostic considerations for lipedema supported by expert opinion of the United States standard of care committee. *∼30% of women with lipedema can have fat tissue on the hands likely due to loss of elasticity in the tissue. 4
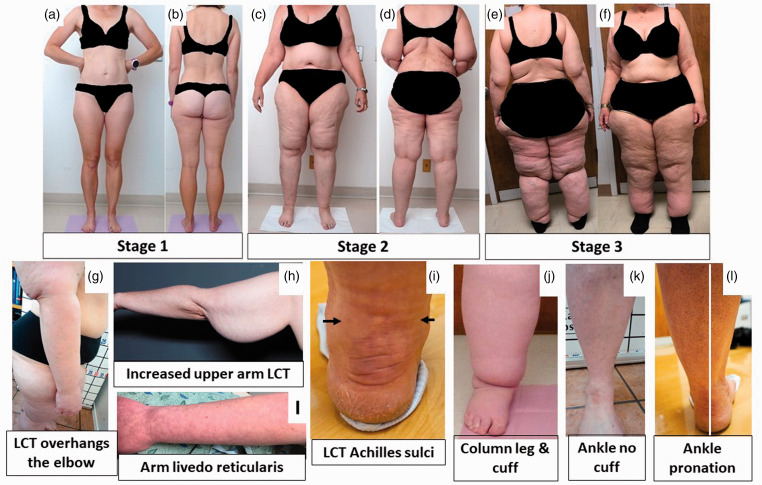
Figure 2.
Stages and features of lipedema. (a) to (f): Front and back pictures of women with lipedema Stages 1 to 3. Staging references the legs, however women pictured also have arm involvement. Stage 1 skin has a smooth texture with subdermal pebble-like feel due to underlying loose connective tissue fibrosis. Lipedema Stage 2 women have more lipedema tissue than women with Stage 1 and skin dimpling due to progressed fibrotic changes and excess tissue. Palpable nodules may be more numerous and larger. Note the full Achilles sulci in pictures (d) to (f). In Lipedema Stage 2 arms, the tissue begins to hang off the arm and full arm involvement shows a more pronounced wrist cuff. Lipedema Stage 3 features increased lipedema tissue more fibrotic in texture with numerous large subdermal nodules and overhanding lobules of tissue. Patient (e) and (f) has lipedema, non-lipedema obesity and lipolymphedema. Types I to V describe the locations of lipedema tissue. Type I, lipedema tissue is present under the umbilicus and over hips and buttocks, Type II, under the umbilicus to knees (a, b), Type III, under the umbilicus to ankles (c to f), Type IV, arms (a to f) and Type V, lower legs (not shown). A tissue cuff at the ankle or wrist may be present in all stages. (g): Lipedema tissue overhangs the elbow. (h): Lipedema tissue often hangs well below the arm due to loss of elasticity and heaviness of the tissue. (i): Livedo reticularis is often a feature of lipedema. (j): Close view of tissue filling the Achilles sulci. (k): Close view of a column type lipedema leg with an obvious ankle cuff. (l): An ankle of a woman with lipedema without an ankle cuff (compare to (k)). (m): Pronation of the ankle commonly found in women with lipedema. Consent was obtained for use of all photos. LCT: loose connective tissue.
Although guidelines are available from other countries,13–17 a published guideline remains an unmet medical need to improve and expand care for people with lipedema in the US.
Methods
In 2019, 21 lipedema expert panelists and a parliamentarian gathered at the Fat Disorders Resource Society Annual Meeting in 2019 to review the literature and develop consensus SOC guidelines for lipedema in the US. A structured questionnaire of 96 consensus statements in REDCap 18 was completed by all panelists prior to the meeting, then panelist average responses were summarized, presented and discussed at the meeting (Round 1). After presentations on SOC guidelines from other countries including pathophysiology, diagnostic criteria,13–17 imaging, and medical, manual, and surgical treatments for patients with lipedema, consensus, defined as 75% agreement amongst panelists, was reached on 90 statements (Round 2). Panelist responses to the summary were collected, summarized again by representative panelists, and presented to panelists two more times to reach a final consensus on 85 statements following the Delphi survey technique. 19
Consensus statements were scored by panelists and averaged using the GRADE system 20 which classifies recommendations as strong (Grade 1 or ⊕) or weak (Grade 2 or ⊕⊕), according to the balance between benefits, risks, burden, and cost, and the degree of confidence in estimates of benefits, risks, and burden, and quality of evidence as high (Grade A), moderate (Grade B), or low (Grade C) according to factors including the risk of bias, precision of estimates, the consistency of the results, and the directness of the evidence as suggested by UpToDate. 21 References evaluated to score consensus statements in this document follow the statement directly and/or in succeeding paragraph(s) or sections.
US SOC meeting goals
Agree on a description of lipedema and a consensus SOC for the US.
Develop and publish clinical practice guidelines for use by providers, patients, and families.
This consensus standard of care guideline accomplishes Goal 1. Additional content is available online. 22 Consensus statements are graded to reflect the strength or weakness based on the current published evidence.
1.0 Lipedema overview
1.1 Lipedema should be regarded as a LCT disease versus a disease of just adipocytes (fat).11,23 (⊕A)
Fat is a loose connective tissue. In addition to adipocytes, immune cells and fibroblasts, LCT has an extracellular matrix of fibers (e.g. collagen and elastin) that supports, protects, and connects tissues. Blood vessels and cells contribute fluid to the extracellular matrix. Fluid exits through lymphatic vessels 24 or remains in the tissue bound to glycosaminoglycans and proteoglycans. Glycosaminoglycans bind sodium and water due to their strong negative charge. Glycosaminoglycans increase when extracellular matrix water and/or salt increases.
When excess fluid is present, LCT becomes compliant, 25 allowing more fluid to collect, stimulating proteoglycan synthesis. Excess fluid limits cell access to oxygen resulting in hypoxia, inflammation and fibrosis. 26 Extracellular matrix fluid, free and bound to proteoglycans, also increases in lymphedema.27,28 When excess fluid collects in the extracellular matrix, it is called edema. 29
1.2 Extracellular matrix edema in lipedema tissue is bound to proteoglycans. (⊕C)
Despite a lack of visible fluid in lipedema tissue on ultrasound, 30 extracellular fluid is higher in the tissue of women with lipedema compared to matched controls. 31 Sodium is also higher in the skin and LCT of women with lipedema. 32 Lipedema tissue has an enlarged extracellular matrix where proteoglycans reside.2,33 In support, multiple proteoglycans are upregulated in excess adipose tissue in individuals with obesity. 34 These data suggest an increase in proteoglycan-bound fluid in lipedema tissue.
1.3 Lipedema has a distinct distribution of pathologic tissue that differs from non-lipedema obesity (Figure 2). 8 (⊕A)
In women with lipedema, but without non-lipedema obesity, gynoid (not truncal) loose connective tissue is disproportionately increased and fibrotic (Figures 2 and 3), with greater numbers of M2 macrophages, unlike the prevalence of M1 macrophages in non-lipedema obesity.2,35 Furthermore, an inflammatory angiogenesis 3 is present in lipedema LCT but not in the tissue of people with non-lipedema obesity. 36
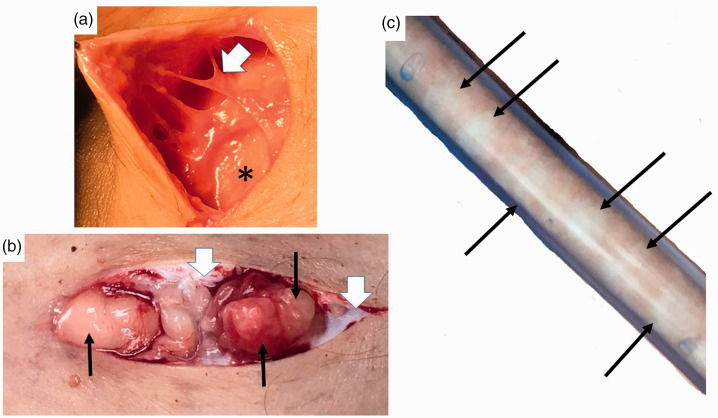
Figure 3.
Nodules and thickened extracellular matrix fibers in lipedema calf loose connective tissue. (a) Example of thick fibrotic fibers (white arrowhead) connecting skin to superficial fascia (*). The abnormal fibers when palpated through the wound are firm and thick and less mobile due to fibrosis in comparison to adjacent fibers. (b) Three nodules under the skin (black arrows) that can be palpated through the skin as firm and that when removed feel firm. Notice extensive scar under the skin (white arrowheads). (c) Lipedema nodules (black arrows) intermingled amongst yellow fat obtained during modified suction lipectomy.
Source: Photos courtesy of Jaime Schwartz.
1.4 Lipedema LCT can affect the abdomen. 12 (⊕B)
Lipedema tissue is on the abdomen, 4 often with metabolic disease (Figures 1 and 2). 12
1.5 Disproportionate distribution of lipedema tissue along with joint hypermobility and muscle weakness 37 impact postural stability and balance often resulting in a hyperlordotic curve in the lumbar spine, valgus knee, ankle pronation and plantar arch flattening. 38 (⊕B)
1.6 Lipedema tissue is resistant to reduction by diet, exercise, or bariatric surgery.39–42 (⊕B)
When weight loss occurs, a greater degree of tissue is lost from the trunk exaggerating the disproportion. Fibrosis of LCT, as in lipedema, inhibits weight loss by usual measures. 43
1.7 Rice-grain, pearl-sized or larger nodules in LCT should be part of the diagnostic criteria for lipedema (Figure 1).4,42 (⊕B)
Fibrosis of lipedema tissue is present in the extracellular matrix space 2 and within fibers forming fibrotic nodules palpable through the skin (Figure 3).
1.8 A Microangiopathy of blood and lymphatic vessels underlies lipedema pathology. (⊕B)
Lipedema LCT can have capillary fragility 44 and livedo reticularis (Figure 2) and are prone to easy bruising. Increased numbers of dilated micro-blood vessels in lipedema 3 contribute excess fluid to the extracellular matrix. Elevated M2 macrophages,2,3 lymphocyte subtypes 2 and platelet factor 4, an inflammatory marker elevated in conditions of lymphatic disease including all stages of lipedema, 45 suggest inflammation drives the microangiopathy in lipedema. Impairment in lymphatic outflow in lipedema contributes to excess fluid in the extracellular matrix. 46
1.9 Comorbidities of lipedema include lymphedema, 4 non-lipedema obesity, 12 venous disease (5.0 Arterial and venous disorders in lipedema section) and joint disease (Figure 1; Table 1). (⊕A)
Table 1.
Multidisciplinary team to assess people with lipedema at any time including prior to lipedema reduction surgery.
| Team | Domain |
|---|---|
| Medical | Lipedema, lymphedema, bariatric, dermatological, endocrine, gastrointestinal, neurological, orthopedic, pain, sleep, vascular |
| Nutrition | Healthy and sustainable eating plan |
| Behavioral/Psychiatric | Depression, anxiety, eating disorders, body dysmorphic disorder 14 ; especially prior to any life-changing surgery or significant dietary change |
| Compression specialist | Compression garment selection and fitting |
| Certified lymphatic therapist | Tissue structure and mobilization, lymphatic function, nutrition, posture, gait, exercise, home self-care |
Hypermobile joints were present in ∼50% of women with lipedema consistent with a connective tissue disease, such as hypermobile Ehlers Danlos Syndrome. 12 Reduced elasticity of the skin 25 and aorta 47 in women with lipedema confirm lipedema as a connective tissue disease. 48 Comorbidities should individually be evaluated and treated based on current guidelines for each disease.
1.10 Assessment for hypermobility by the Beighton criteria 49 or questionnaire 50 should be considered when lipedema is diagnosed. (⊕C)
1.11 When there is a concern that lymphedema is present concurrently with lipedema, a nuclear medicine lymphangioscintigraphy exam of the legs, arms or both, should be conducted to assess the integrity and function of the lymphatic system. 46 (⊕A) This exam may also guide treatment when lymphedema is present. Lymphangioscintigraphy findings in lipedema include convoluted lymphatic vessels in the legs that slow transit of radionuclide. 46 (⊕A)
1.12 Women with lipedema who develop lymphedema have lipolymphedema. (⊕A) Lipolymphedema is lipedema that has progressed to clinically identifiable lymphedema, a risk that increases concomitant with stage. 4
1.13 Lipedema tissue is frequently painful especially when touched. (⊕B)
On a numerical pain scale from 0 (none) to 10 (unbearable), 80% of women with lipedema scored ≥5, and 11% rated their pain as unbearable. 51 The etiology of pain in lipedema is unclear, 52 though histology findings of inflammation and hypoxia may be contributing elements.2,3 Painful lipedema tissue may be misdiagnosed as fibromyalgia.
Painful lipedema tissue is not an absolute requirement for the diagnosis of lipedema (Figure 1). 52 In a seminal paper on lipedema, only 40%–50% of women had pain or tenderness in the tissue. 53 Conservative therapies can reduce lipedema tissue pain (3.0 Conservative and other therapies section), yet people still retain a diagnosis of lipedema. In a family with lipedema and no pain, a gene mutation in AKR1C1, reducing aldo-keto reductase activity, should increase levels of the potent analgesic, allopregnanolone, 54 while at the same time decreasing prostaglandin F2α levels and raising progesterone levels, both of which stimulate adipogenesis. 55
1.14 Lipedema is a common disease. (⊕C)
Prevalence estimates for lipedema range from 6.5% in children in the US, 56 6%–8% in women in Germany, 16 and 15%–19%57,58 in vascular clinics. If these numbers are valid and applied to the US population, then millions of women in the US have lipedema.
1.15 Lipedema can be inherited. (⊕B)
Genes for lipedema are thought to pass from parent to offspring in an autosomal dominant manner with sex limitation.59,60 One gene for lipedema has been identified, AKR1C1, a gene encoding for aldo-keto reductase that catalyzes the reduction of progesterone to its inactive form. 55 Elevation of progesterone due to a mutation in AKR1C1 should increase adipogenesis, as in lipedema. 61 Genes associated with lipedema as part of a syndrome have been reviewed. 62
1.16 Lipohypertrophy is a condition in women that is very similar to lipedema but without edema and pain. 42 Women with lipohypertrophy have tissue that looks like lipedema, have difficulty losing weight, but do not have pain or edema. Some authors state lipohypertrophy is a pre-lipedema condition 63 while others consider it a synonym for lipedema. 7 Lipohypertrophy is also used to describe obesity affecting the limbs and trunk. 64 More research is needed to determine if lipohypertrophy is different from lipedema. (⊕C)
1.17 Lipedema and its concomitant pain and inability to lose tissue mass by usual measures can increase the incidence of depression, anxiety, or eating disorders. 65 (⊕B)
Eighty-five percent of women state lipedema affects their mental health, coping abilities and self-esteem. 66 Depression was observed in 18%–35% of people with lipedema, exceeding average population prevalence levels. 67 On a standardized measure of health-related quality of life, anxiety or depression was found in 42% of people with lipedema. 68 In other studies, self-reported anxiety affected 18%–30% of people with lipedema.69,70 Psychological pain scores were also high in women with lipedema. 51 Early diagnosis and treatment may mitigate the impact of lipedema on mental health.
In a study of 100 people with lipedema, 74% had a history of eating disorders, 12% with periodic binge eating attacks, 8% with bulimia, and 16% with anorexia nervosa. 71
1.18 A Mental health consultation should be offered to people with lipedema when there are signs and symptoms of depression, anxiety or eating disorders. (⊕B)
Improved mental health increases self-care by women with lipedema. 72
2.0 Medical treatment
2.1 Signs and symptoms of lipedema can be treated to maintain and improve quality of life including pain, edema, and mobility; earlier treatment provides better results.17,42,64,66,73 (⊕B)
2.2 A Complete patient evaluation and assessment identifies impairments that can be addressed with medications, therapy, or referrals to other providers (Table 1).11,17 (⊕C)
2.3 Barriers to treatment of lipedema include difficulty of self-care, mobility limitations, social stigma attached to increased body size and physical limitations, anxiety, depression, 65 lack of social support, 12 availability of knowledgeable healthcare providers and affordability of services and limitations of some non-surgical treatments to reduce lipedema tissue.37,66,74 (⊕B)
There are no known medications that specifically treat lipedema.
2.4 Use of medications and supplements for lipedema should focus on reducing tissue inflammation, fibrosis, swelling, and pain. 11 (⊕⊕C)
Medications for metabolic complications that arise from obesity in people with lipedema should follow standard guidelines. 75 (⊕A)
2.5 Medications that increase edema should be avoided in people with lipedema. 11 (⊕A)
2.6 Medications that promote weight gain should be avoided and replaced with medications that are weight neutral or that promote weight loss when possible. 76 (⊕A)
2.7 Thiazolidnediones increase subcutaneous adipose tissue and should be avoided in people with lipedema. 77 (⊕C)
2.8 Long-term use of diuretics should be avoided in people with lipedema. 78 (⊕B)
Diuretics do not treat the main cause of edema in lipedema which is inflammation.2,3
2.9. Sympathomimetic amines that constrict arterioles and lower intracapillary pressure can be considered for edema treatment. 78 (⊕⊕C)
People with lipedema treated with sympathomimetic amines had reduced weight, body size, edema and pain and improved quality of life. 69
2.10. Metformin should be considered for people with lipedema and metabolic complications. (⊕A)
Metformin inhibits hypoxia-induced fibrosis in adipose tissue, 79 and can reverse fibrosis after injury. 80
2.11. Thyroid function should be assessed in people with lipedema. (⊕A)
Hypothyroidism was found in 27%–36% of women with lipedema.4,67,70
2.12. Diosmin can be considered for treatment of lipedema tissue. (⊕⊕C)
Diosmin, a biologically active polyphenol often in combination with its precursor, hesperidin, reduces oxidative stress markers in people with chronic venous disease, 81 improves venous elasticity, 82 functions as a lymphagogue reducing edema, 81 reduces microvascular permeability, 83 and improves vascular, 84 neuropathic 85 and radicular pain.
2.13. Eating plans for people with lipedema should minimize postprandial insulin and glucose fluctuations (⊕C) and be sustainable long-term. (⊕C)
Healthy eating patterns for lipedema can be whole food, enzyme rich, plant-based86,87 or ketogenic. 88 Research favors vegetable-based low-carbohydrate diets which correlate with decreased all-cause mortality over animal-based diets. 89
2.14. Vitamin D levels should be monitored and normalized for people with lipedema. (⊕C)
Vitamin D levels decrease with increasing BMI. 90
2.15. Lipedema tissue does not reduce significantly after diet, exercise, or bariatric surgery39–42 likely due to the fibrotic component of loose connective tissue. (⊕C)
Weight reduction of non-lipedema obesity is beneficial to reduce metabolic complications following published guidelines. 75 A BMI greater than 50 kg/m2 can induce metabolic complications, lymphedema and exacerbate lipedema. 91
2.16. Women with lipedema may have sleep issues including sleep apnea; sleep assessment should be considered especially in later stages. 4 (⊕C)
2.17. While sex hormones can affect fluid retention, a causative role for sex hormones in the expression of lipedema remains speculative. When necessary, lower doses of sex hormones for birth control or hormone replacement should be considered. 11 (⊕⊕C)
3.0. Conservative and other therapies
3.1. People with lipedema should be assessed for lipedema, lymphedema, posture, balance, muscle strength, gait and joint hypermobility by a therapist with certified lymphedema therapist (CLT) training. 92 (⊕C)
People with lipedema may benefit from postural and core exercises, 12 muscle strengthening exercises, gait training, neuromuscular re-education, and deep abdominal breathing to increase lymphatic flow 93 and stimulate the parasympathetic system. Education and training should be performed by a qualified practitioner.
3.2. Standard conservative therapy for lipedema includes nutritional guidance (2.0 Medical treatment section), manual therapy, compression garments, recommendations for a pneumatic compression device (external pump)94,95 and a home exercise plan. 37 (⊕C)
3.3. Manual therapies, sequential pneumatic compression pumps96,97 and exercise 98 should improve lipedema tissue by decreasing pain and increasing lymphatic flux, which in turn increases movement of glycosaminoglycans from the extracellular matrix into lymphatic vessels. 99 (⊕C)
3.4. Standard manual therapy for lipedema includes soft tissue mobilization to reduce pain, inflammation100,101 and musculoskeletal restrictions, and manual lymphatic drainage as part of an individualized comprehensive therapy program to stimulate lymphatic flow and reduce edema. 102 (⊕C)
3.5. Lipedema tissue should be mobilized deeper with myofascial release, other manual techniques or instrument assisted soft tissue therapy to reduce fibrotic restrictions and improve the interstitial space while considering patient tolerance and tissue integrity.100,101 These therapies do not harm the lymphatic system. (⊕C)
3.6. Compression needs vary depending on patient presentation, pain, and physical ability to don/doff garments or compression bandages (Table 2).16,103 (⊕A)
Table 2.
Compression class level (CCL) recommendations for lipedema.a
| Stage | Recommendation |
|---|---|
| Stage 1 | Micro-massage garment (10–20 mm Hg) as needed. |
| Stage 2 | Micro-massage, CCL I or II as tolerated when pain, swelling or heaviness are present. |
| Stage 3 | Micro-massage; CCL I or CCL II as tolerated when pain, swelling or heaviness are present. May have to layer different garments. |
| Lipedema with lipolymphedema | CCL should be determined individually based on patient presentation, physical ability and tolerance, and caregiver support. May have to layer different garments. |
aCCL I = ∼20–30 mmHg, CCL II = ∼30–40 mmHg.
3.7. Compression garments for lipedema provide comfort and reduce pain by supporting the tissues especially if there is interference by lipedema tissue pads, 104 and manage edema.7,105 (⊕B)
3.8. Selection of compression styles, fabric and strength should be individualized. Compression garment styles can be combined to cover the arms, hands, legs, feet, trunk, or pelvis. 103 (⊕C)
Fabrics range from lightweight and micro-massage, to circular knit to flat knit, the latter providing the strongest containment. 106 Certified lymphedema therapists may suggest modifications for compression garments, inelastic compression garments, “donning aides”, or adaptive equipment. Multilayer, short-stretch compression wraps, or inelastic Velcro may be required to contain fluid. The strength of garments or the compression class level is made independent of fabric type and according to lipedema stage (Table 2). If pain increases with compression, the compression class level may be decreased, or garments layered. A higher compression class level does not equate to better results. 103
3.9. Pneumatic compression devices stimulate lymphatic flow 96 and are an option for at-home lipedema and lymphedema management when there are no contraindications.96,107 (⊕A)
Pneumatic compression devices provide pain reduction and may provide better control of swelling than self-manual lymphatic drainage. 108 Use of pneumatic compression devices and early mobilization can reduce the risk of deep venous thromboembolism following lipedema reduction surgery. 109 Pressure levels can be altered and cotton padding added between the skin and device if discomfort is experienced with pneumatic compression device use. 64
3.10. Exercise programs for people with lipedema should be individually prescribed, started slowly, and progressed as tolerated.37,66 (⊕B)
3.11. Mobility can be improved by therapeutic interventions for flexibility, posture, joint protection, strengthening (including pelvic floor) and conditioning.37,66 (⊕C)
3.12. Beneficial home exercise plans for people with lipedema include swimming/aquatics, elliptical machines, yoga, stationary bikes, whole body vibration and walking. Impact levels may vary but should remain tolerable and sustainable for long-term adherence.37,66 (⊕C)
3.13. People with lipedema undertaking exercise programs ideally would be followed long-term with regular assessment. 37 (⊕C)
3.14. Home care for lipedema (self-management or with caregiver assistance) is essential to mitigate progression and optimize quality of life. 10 (⊕C)
Daily self-care includes skin care (to prevent breakdown under fat lobules, and to prevent infection when lymphedema is present), compression garments, pneumatic compression pumps, self-massage, a healthy eating plan, home exercise plan, adequate sleep and psychosocial support including social networks.
4.0. Surgical treatment
4.1. Lipedema reduction surgery is currently the only available technique for removing abnormal lipedema tissue such as adipocytes, nodules, fibrotic extracellular matrix, and other non-adipocyte components. It is also the only treatment that slows progression of lipedema and ideally would be performed before complications and disabilities from lipedema develop.110,111 (⊕C)
4.2. Lipedema reduction surgery utilizes suction lipectomy (liposuction), excision and manual extraction that spares blood and lymphatic vessels. 17 (⊕⊕C)
Lipedema reduction surgery significantly improves symptoms,110–112 mobility, stance, gait, 38 valgus rotation/deformity of the knee and ankle, quality of life, and redistributes and restores the plantar arch. 113 It also improves lymphatic symptoms, reducing the need for compression and manual therapy110–112,114 and improves lymphatic function as shown by radionucleotide lymphangioscintigraphy. 115
The types of suction lipectomy recommended for people with lipedema are based around tumescent liposuction which uses a solution injected into the tissue to decrease pain and bleeding. 116 Other mechanical methods can also be used such as Water Assisted Liposuction (WAL)114,117 and Power Assisted Liposuction (PAL). 118
To date, all studies showing clinical improvements for women with lipedema used tumescence or WAL techniques.110–112,119 There is little published information on the safety of laser or ultrasound technology for removing lipedema tissue.
4.3. Candidates for lipedema reduction surgery should generally be in good health
People with lipedema are different from the general population in that BMI is not a reliable indicator of overall health. 17 (⊕C)
4.4. There is no age limit for which people will benefit from lipedema reduction surgery. 17 (⊕C)
4.5. Indications for lipedema reduction surgery include a diagnosis of lipedema with demonstrated compliance and adherence to or failure of conservative therapies (3.0 Conservative and other therapies section ).13–17,66 (⊕C)
4.6. Lipedema reduction surgery does not fit traditional volume limits for liposuction
Debulking lipedema tissue may require larger than traditional suction aspirate volumes 120 and multiple surgeries with proper intervals in-between. This is not cosmetic liposuction as there are mobility, pain and health benefits when removing lipedema tissue.13–17 (⊕⊕B)
4.7. Women with lipedema should be treated with conservative therapy prior to lipedema reduction therapy (4.0 Surgical treatment section). People may travel to receive surgery and rely on a therapy team in their hometown for pre- and post-operative care. In the weeks before surgery, a certified lymphedema therapist can perform a pre-surgical screening to guide “prehab” exercise, perform manual therapies and recommend compression garments for the patient.13–17 (⊕⊕B)
4.8. If the patient has lipolymphedema, complete decongestive therapy performed prior to surgery should include an intensive volume reduction phase, ideally 3–4 treatments per week. 107 (⊕C)
4.9. Before surgery, two sets of off the shelf, made to measure or inelastic garments or a combination of micro-massage garment and short stretch bandages should be prescribed. 107 Compression garments should be replaced 3 or 4 times during the first year. Garments must be worn regularly as non-compliance risks a rebound of edema. 107 (⊕C)
4.10. People with lipedema, especially higher stages, are at increased risk for venous thromboembolism and pulmonary embolus after surgery. We recommend venous thromboembolism risk stratification and treatment when indicated (5.0 Arterial and venous disorders in lipedema section). (⊕A)
4.11. A Pre-surgical venous duplex ultrasound and/or treatment of chronic venous disease should be considered especially in patients with lipolymphedema prior to lipedema reduction surgery. 121 (⊕⊕A)
Varicose veins from chronic venous disease increase the risk of venous thromboembolism in the legs; 122 treatment of chronic venous disease decreases this risk. 123 Varicose veins may increase the risk of intra-operative blood loss during surgical treatment of lipedema. 124
4.12. Lipedema reduction surgery can be safely accomplished in an outpatient setting
Consider overnight observation after surgery for significant comorbid medical illness or high-volume aspirate. 17 (⊕⊕B)
4.13. Lipedema reduction surgery can be safely performed under local or general anesthesia. 17 (⊕B)
4.14. Lipedema reduction surgery is not without risk and may cause long-term complications including lymphatic injury. 125 (⊕C)
4.15. Lipedema reduction surgery should be performed by surgeons experienced in the care of people with lipedema, with expert knowledge of the anatomy and function of lymphatic collection systems, using meticulous care to avoid lymphatic injury.66,116 (⊕B)
4.16. Lipedema reduction surgery may be less effective in advanced stages of lipedema 66 and in women with lipedema and severe obesity110–112,119 although recent data demonstrate a greater reduction of symptoms in more advanced cases. 73 Surgery may involve multiple procedures, however, the optimal time between procedures is unknown. (⊕⊕B)
4.17. Blunt cannulas no larger than 2–4 mm should be used during lipedema reduction surgery
Larger cannulas increase the risk for lymphatic injury, and the risk of rare, but deadly fat embolism. Cannulas greater than 4 mm should only be used in people with advanced stage lipedema and only for deep plane liposuction. 126 (⊕C)
4.18. Longitudinal technique should be used during lipedema reduction surgery to avoid damaging lymphatic vessels. 127 (⊕C)
4.19. Anemia is a risk with large volume liposuction in people with lipedema
Hemoglobin levels should be followed pre- and post-operatively in higher risk individuals. 128 (⊕C)
4.20. Large tissue sacks may remain after successful surgery and weight loss, for which subsequent plastic surgery in the form of dermo-lipectomy may be required. (⊕C)
These surgical recommendations align with published standard of care guidelines and long-term studies.16,110–112,119 UK guidelines suggest lipedema reduction surgery after 6–12 months of adherence to conservative therapy. 13 Dutch guidelines suggest lipedema reduction surgery for people no longer responding to conservative therapy. 66 We recommend women with lipedema discuss lipedema reduction surgery with healthcare providers for a pre-surgical assessment, get a referral to a trained therapist (3.0 Conservative and other therapies section), and be assessed for significant and treatable vascular disease (5.0 Arterial and venous disorders in lipedema section) prior to undergoing lipedema reduction surgery.
4.21. People with early stage lipedema should wear a postoperative compression garment for at least 2–3 months to manage post-operative edema. 107 People with advanced lipedema and/or lipolymphedema may need to continue compression garments for life.15,120 If people find it difficult to don and doff compression garments, two garments with a lesser level compression can be layered to achieve adequate compression. (⊕C)
4.22. Post-surgical care should be performed by a certified lymphedema therapist 2–3 times a week as soon after surgery as possible until swelling subsides.120,129 Certified lymphedema therapists or a qualified fitter can monitor compression needs. (⊕⊕B)
4.23. Complete decongestive therapy is either no longer needed or the need reduced in people after recovery from lipedema reduction surgery. 112 (⊕⊕A)
5.0. Arterial and venous disorders in lipedema
5.1. The arterial and venous vascular status of people with lipedema should be evaluated.47,121 (⊕⊕C)
Most people with lipedema have leg pain, all have leg swelling, either pitting or non-pitting, and many have underlying chronic venous disease. 121
The physical examination should include inspection and palpation of pulses in the limbs. Pulse palpation in people with lipedema may be difficult and painful due to limb size.
5.2. It is important to differentiate leg pain in lipedema from peripheral arterial disease. (⊕⊕A)
Peripheral arterial disease is common especially when major risk factors are present. 130 Compression garments are a standard treatment for people with lipedema with signs of lymphatic impairment; however, compression garments are contraindicated for people with severe peripheral arterial disease.
5.3. If peripheral arterial disease is clinically suspected, ankle brachial index is recommended with whole leg or single segment (foot and ankle) assessment. (⊕A)
Arm or leg enlargement in lipedema may affect accuracy of the blood pressure measurements in this test and cause pain. 131 If performing an ankle brachial index is not possible, measurement of a toe brachial index may be helpful. Forearm or wrist blood pressure measurement may be an alternative in this population. Other options include using a 4 MHz Doppler ultrasound probe (over the standard 8 MHz probe) and/or a larger blood pressure cuff. 132
The arterial duplex ultrasound can eliminate the need for invasive procedures such as arteriography or computed tomography angiography.
5.4. Common venous conditions seen in people with lipedema include increased risk for venous thromboembolism and conditions associated with chronic venous disease: varicose veins, chronic venous insufficiency, and telangiectasias (spider veins). 121 (⊕C)
5.5. Lipedema, especially later stages, is associated with multiple comorbid conditions that increase the risk of venous thromboembolism, which includes superficial thrombophlebitis, deep vein thrombosis and pulmonary embolism. 133 (⊕C)
5.6. Providers should perform a venous thromboembolism risk assessment score 134 for people with lipedema and follow venous thromboembolism prophylaxis treatment guidelines. (⊕C)
Independent risk factors for venous thromboembolism based on Caprini risk stratification, 134 especially for women with Stage 3 lipedema include:
BMI >40 kg/m2 (1 point)
Varicose veins (1 point)
Swollen legs, including loss of definition of bony prominences (1 point)
Decreased mobility 113 (1 point) (⊕A)
5.7. Chronic venous disease can present with leg swelling and pain and should be considered in the differential diagnosis of lipedema. (⊕B)
Chronic venous disease is the most common vascular disorder in all populations. Chronic venous disease is the presence of morphological (i.e., venous dilation) or functional (e.g., venous reflux) abnormalities manifested by symptoms and/or signs indicating the need for further investigation or treatment. There is little data on lipedema and chronic venous disease. Two studies state ∼25% of women with lipedema have venous disease and one study showed 50% of women with lipedema and lipolymphedema had chronic venous insufficiency.53,121,135 Lipedema and chronic venous disease often co-exist, share similar leg symptoms, and could exacerbate each other. Advancing age, female gender, and BMI compound an underlying relationship between lipedema and chronic venous disease. 136
Symptoms of chronic venous disease include: leg pain, fatigue, heaviness, swelling, pruritus, restless legs and night cramps. 137 The leg pain of chronic venous disease is generally worse with dependency and relieved by elevation. Symptoms of chronic venous disease are relieved by compression garments and walking. In people with lipedema, leg elevation does not improve swelling, and compression garments often cause pain.
The physical exam for chronic venous disease includes inspection of the arms and legs comparing each to the contralateral limb. Physical signs of chronic venous disease include telangiectasia, varicose veins, hyperpigmentation, erythema, inflammation, dryness, corona phlebectatica, lipodermatosclerosis, atrophie blanche and leg ulceration. Edema, pitting or non-pitting, should be noted.
Secondary lymphedema in people with lipedema can be difficult to evaluate. Not only does secondary lymphedema often occur in the absence of a positive Stemmer's sign, 138 it requires palpation of tissue density and heaviness. As much as 0.5 L of fluid can be present in the calf/ankle before it is noticed. Women with any stage of lipedema may manifest lymphedema although it is more likely in more advanced stages.4,46
5.8. The Clinical-Etiological-Anatomical-Pathophysiological (CEAP) classification for venous disease should be determined for people with lipedema. 139 (⊕A)
5.9. The venous evaluation of people with lipedema includes a bilateral, lower extremity duplex ultrasound evaluation of the deep and superficial venous systems assessing for valvular insufficiency (reflux), 121 acute or chronic thrombosis 133 and patterns of obstructive flow. (⊕A)
The scan should evaluate reflux in the superficial truncal veins (great saphenous, small saphenous and accessory saphenous), measure truncal vein diameters, and map large refluxing tributaries. These scans can be difficult to perform and assess in people with severe obesity, extensive lipedema, and lower extremity edema.
Duplex ultrasound may be helpful when the clinical examination for lipedema is unclear. For example, dermal thickness was normal in people with lipedema, while dermal thickness was increased and fluid was present in the loose connective tissue in cases of lymphedema. 140
Knowing when to treat chronic venous disease in people with lipedema is challenging without published data. Generally, it is accepted to consider treatment of chronic venous disease when superficial truncal reflux is present, the symptoms interfere with activities of daily living, and people do not respond to conservative therapy (compression garments, manual therapy, 3.0 Conservative and other therapies section). (⊕B)
5.10. Providers should determine whether symptoms are from lipedema, chronic venous disease or both as they share many symptoms. 121 (⊕B)
One goal for people with lipedema is to improve discomfort. It is important to give reasonable expectations for chronic venous disease treatment outcomes including that overall leg shape, edema, and underlying symptoms from lipedema most likely will not improve. However, by removing the chronic venous disease component, people can expect an overall net improvement of end of day symptoms. 139
5.11. Consider thermal and non-thermal treatment modalities of chronic venous disease in people with lipedema. 141 (⊕C)
There are two modalities for treating superficial truncal reflux in chronic venous disease: non-thermal and thermal. Non-thermal methods cause less inflammation, injury to adjacent structures, damage to adjacent lymphatics or nerves, risk of anesthetic complications, noxious needle punctures, and pre- and post-treatment discomfort. Thermal modalities are theoretically more likely to injure adjacent lymphatics; however, thermal ablation is more widely available and more effective on larger diameter veins. 142
5.12. Due to alterations in lymphatic vessels in people with lipedema, 46 when thermal ablation is used to treat chronic venous disease in the proximal saphenous vein segments, generous peri-vascular tumescent anesthesia should be infiltrated, especially at the sapheno-femoral and sapheno-popliteal junctions, to increase protection of surrounding lymphatics. 143 (⊕C)
5.13. The decision of whether to treat distal saphenous segments and/or large tributaries of chronic venous disease in people with lipedema must be individualized. 144 (⊕⊕C)
Monitoring treatment
Though there is no lipedema specific health-related quality of life evaluation tool, several outcome instruments have been used to differentiate lipedema from lymphedema including the SF-36 145 and the Patient Benefit Index. 146
Research
How or why lipedema occurs is poorly understood, and for affected individuals, the signs and symptoms of progression remain unexplored. Key areas of research include:
Defining the penetrance of pain and its mechanisms,
Pathomechanism of muscle strength loss,
Connective tissue aspect of lipedema including hypermobile joints,
Difference and differential diagnosis between lipohypertrophy and lipedema,
Overall prevalence and incidence of lipedema as well as its demographic distribution.
Further research should focus on how to optimize treatment for people with lipedema, with a particular focus on patient quality of life, nutritional guidance, management of comorbid diseases, deeper tissue techniques to reduce inflammation and fibrosis, earlier diagnosis to allow for intervention and education, psychosocial support, as well as pre- and post-surgical protocols to improve care and assess medium- to long-term outcomes.
Conclusion
These findings are the consensus statements of US-based expert panelists put forth as a standard of care guideline for people with lipedema in the US. It is our goal and aspiration that that this guideline will improve the understanding of the loose connective tissue disease, lipedema, and that increased research and awareness of lipedema will advance our understanding towards increased diagnosis, improvements of treatments, and ultimately a cure for the community of affected individuals.
Acknowledgements
The authors thank the Lipedema Foundation, the Fat Disorders Resource Society, Jaime Schwartz, and Ethan Larson for material support.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KLH received research funding from Raziel Therapeutics, is on the Speaker’s Board for Tactile Medical, and has received honorarium for speaking engagements from Sigvaris and Lymphapress. MS received consulting money from Microaire. LBM received honorarium for speaking engagements for Lymphapress. LAK received honorarium for speaking engagements from Compression Guru. SMD is on the Speaker’s Bureau and Scientific Advisory Board for Tactile Medical. TFW received research funding from Raziel Therapeutics and has received honorarium for speaking engagements from Sigvaris and Tactile Medical. PCD is a consultant for PureTech Health and received grant funding from LymphaTouch. KL is on the Advisory Board for Aria Health. EI received honorarium for speaking engagements for Sigvaris. NJP receives consulting fees from the Obesity Medicine Association, has an independent contractor relationship with Medifast, and has received speaking fees from Integrity Continuing Education, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this research was funded by the National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) grant 1 R13 HL147503-01 (Karen L. Herbst).
Ethical approval: All patients provided consent for use of their photos.
Guarantor: KLH
Contributorship: All authors attended the original consensus meeting and contributed to the original ideas for this paper. KLH obtained grant funding, organized the original meeting for the standard of care committee, provided questions and summaries for consensus statements and collated written portions of the submitted paper from authors. All authors researched the literature, assisted in drafting of the different sections of the paper, edited multiple versions of the manuscript and approved the final version of the manuscript.
ORCID iDs: Karen L Herbst https://orcid.org/0000-0002-9079-9754
Thomas Wright https://orcid.org/0000-0003-4900-2800
References
- 1.Buck DW, 2nd, Herbst KL. Lipedema: a relatively common disease with extremely common misconceptions. Plast Reconstr Surg Glob Open 2016; 4: e1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felmerer G, Stylianaki A, Hägerling R, et al. Adipose tissue hypertrophy, an aberrant biochemical profile and distinct gene expression in lipedema. J Surg Res 2020; 253: 294–303. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ghadban S, Cromer W, Allen M, et al. Dilated blood and lymphatic microvessels, angiogenesis, increased macrophages, and adipocyte hypertrophy in lipedema thigh skin and fat tissue. J Obes 2019; 2019: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst K, Mirkovskaya L, Bharhagava A, et al. Lipedema fat and signs and symptoms of illness, increase with advancing stage. Arch Med 2015; 7: 1–8. [Google Scholar]
- 5.Allen EV, Hines EAJ. Lipedema of the legs: a syndrome characterised by fat legs and orthostatic edema. Proc Staff Meet Mayo Clin 1940; 15: 184–187. [Google Scholar]
- 6.Moncorps CB, Brinkhaus G, Herteld F, et al. Experimentelle untersuchungen zur frage akrozyanotischer zustandsbilder. Arch Derm Syph 1940; 186: 209–215. [Google Scholar]
- 7.Todd M. Lipoedema: presentation and management. Br J Community Nurs 2010; 15: S10–S16. [DOI] [PubMed] [Google Scholar]
- 8.Buso G, Depairon M, Tomson D, et al. Lipedema: a call to action!. Obesity (Silver Spring) 2019; 27: 1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shavit E, Wollina U, Alavi A. Lipoedema is not lymphoedema: a review of current literature. Int Wound J 2018; 15: 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fetzer A, Wise C. Living with lipoedema: reviewing different self-management techniques. Br J Community Nurs 2015; 20: S14–S19. [DOI] [PubMed] [Google Scholar]
- 11.Herbst KL. Subcutaneous adipose tissue diseases: Dercum disease, lipedema, familial multiple lipomatosis and Madelung disease. In: Purnell J, Perreault L. (eds) Endotext. Massachusetts: MDText.com, 2019. [PubMed] [Google Scholar]
- 12.Torre YS, Wadeea R, Rosas V, et al. Lipedema: friend and foe. Horm Mol Biol Clin Investig 2018; 33: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppel T, Cunneen J, Fetzer S, et al. Best practice guidelines: the management of lipoedema. Wounds UK, 2017: 13: 1–36.
- 14.Alcolea JM, Alonso AB, Arroyo BA, et al. Documento de consenso lipedema 2018. In: 33rd National congress of the Spanish society of aesthetic medicine (SEME) Malaga, Barcelona, Spain, 22-24 February 2018. Barcelona: LITOGAMA S.L.
- 15.Peprah K, MacDougall D. Liposuction for the treatment of lipedema: a review of clinical effectiveness and guidelines. Ottawa: Canadian Agency for Drugs and Technologies in Health, 2019. [PubMed] [Google Scholar]
- 16.Reich-Schupke S, Schmeller W, Brauer WJ, et al. S1 guidelines: lipedema. J Dtsch Dermatol Ges 2017; 15: 758–767. [DOI] [PubMed] [Google Scholar]
- 17.Sandhofer M, Hanke CW, Habbema L, et al. Prevention of progression of lipedema with liposuction using tumescent local anesthesia: results of an international consensus conference. Dermatol Surg 2020; 46: 220–228. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–1015. [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Kunz R, et al. GRADE: going from evidence to recommendations. Br Med J 2008; 336: 1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Policy U. Grading guide. In: Post TW. (ed.) UpToDate. Waltham, MA: UpToDate, 2020. [Google Scholar]
- 22.Herbst KL. Lipedema is not just fat. Lipedemacom, 2020.
- 23.McKee TJ, Perlman G, Morris M, et al. Extracellular matrix composition of connective tissues: a systematic review and Meta-analysis. Sci Rep 2019; 9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levick JR, Michel CC. Microvascular fluid exchange and the revised starling principle. Cardiovasc Res 2010; 87: 198–210. [DOI] [PubMed] [Google Scholar]
- 25.Guyton AC. Pressure-volume relationships in the interstitial spaces. Invest Ophthalmol 1965; 4: 1075–1084. [PubMed] [Google Scholar]
- 26.Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol 2015; 208: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts MA, Mendez U, Gilbert RJ, et al. Increased hyaluronan expression at distinct time points in acute lymphedema. Lymphat Res Biol 2012; 10: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates DO, Levick JR, Mortimer PS. Change in macromolecular composition of interstitial fluid from swollen arms after breast cancer treatment, and its implications. Clin Sci (Lond) 1993; 85: 737–746. [DOI] [PubMed] [Google Scholar]
- 29.Mortimer PS, Levick JR. Chronic peripheral oedema: the critical role of the lymphatic system. Clin Med (Lond) 2004; 4: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iker E, Mayfield CK, Gould DJ, et al. Characterizing lower extremity lymphedema and lipedema with cutaneous ultrasonography and an objective computer-assisted measurement of dermal echogenicity. Lymphat Res Biol 2019; 17: 525–530. [DOI] [PubMed] [Google Scholar]
- 31.Crescenzi R, Donahue PMC, Weakley S, et al. Lipedema and Dercum's disease: a new application of bioimpedance. Lymphat Res Biol 2019; 17: 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crescenzi R, Marton A, Donahue PMC, et al. Tissue sodium content is elevated in the skin and subcutaneous adipose tissue in women with lipedema. Obesity (Silver Spring) 2018; 26: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suga H, Araki J, Aoi N, et al. Adipose tissue remodeling in lipedema: adipocyte death and concurrent regeneration. J Cutan Pathol 2009; 36: 1293–1298. [DOI] [PubMed] [Google Scholar]
- 34.Pessentheiner AR, Ducasa GM, Gordts PLSM. Proteoglycans in obesity-associated metabolic dysfunction and meta-inflammation. Front Immunol 2020; 11: 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009; 58: 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Esch-Smeenge J, Damstra RJ, Hendrickx AA. Muscle strength and functional exercise capacity in patients with lipoedema and obesity: a comparative study. J Lymphoedema 2017; 12: 27–31. [Google Scholar]
- 38.Stutz JJ. Liposuktion beim lipödem zur verhinderung von gelenkspätkomplikationen (Liposuction of lipedema to prevent later joint complications). Vasomed 2011; 23: 1–6. [Google Scholar]
- 39.Bast JH, Ahmed L, Engdahl R. Lipedema in patients after bariatric surgery. Surg Obes Relat Dis 2016; 12: 1131–1132. [DOI] [PubMed] [Google Scholar]
- 40.Pouwels S, Huisman S, Smelt HJM, et al. Lipoedema in patients after bariatric surgery: report of two cases and review of literature. Clin Obes 2018; 8: 147–150. [DOI] [PubMed] [Google Scholar]
- 41.Pouwels S, Smelt HJ, Said M, et al. Mobility problems and weight regain by misdiagnosed lipoedema after bariatric surgery: illustrating the medical and legal aspects. Cureus 2019; 11: e5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiedner M, Aghajanzadeh D, Richter DF. Differential diagnoses and treatment of lipedema. Plast Aesthet Res 2020; 7: 10. [Google Scholar]
- 43.Bel Lassen P, Charlotte F, Liu Y, et al. The FAT score, a fibrosis score of adipose tissue: predicting weight-loss outcome after gastric bypass. J Clin Endocrinol Metab 2017; 102: 2443–2453. [DOI] [PubMed] [Google Scholar]
- 44.Szolnoky G, Nagy N, Kovacs RK, et al. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology 2008; 41: 161–166. [PubMed] [Google Scholar]
- 45.Ma W, Gil HJ, Escobedo N, et al. Platelet factor 4 is a biomarker for lymphatic-promoted disorders. JCI Insight 2020; 5: e135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forner-Cordero I, Olivan-Sasot P, Ruiz-Llorca C, et al. Lymphoscintigraphic findings in patients with lipedema. Rev Esp Med Nucl Imagen Mol 2018; 37: 341–348. [DOI] [PubMed] [Google Scholar]
- 47.Szolnoky G, Nemes A, Gavaller H, et al. Lipedema is associated with increased aortic stiffness. Lymphology 2012; 45: 71–79. [PubMed] [Google Scholar]
- 48.Jagtman BA, Kuiper JP, Brakkee AJ. [Measurements of skin elasticity in patients with lipedema of the Moncorps rusticanus type]. Phlebologie 1984; 37: 315–319. [PubMed] [Google Scholar]
- 49.Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis 1973; 32: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hakim AJ, Grahame R. A simple questionnaire to detect hypermobility: an adjunct to the assessment of patients with diffuse musculoskeletal pain. Int J Clin Pract 2003; 57: 163–166. [PubMed] [Google Scholar]
- 51.Stutz JJ. Alles über das Lipödem. Lymphe & Gesundheit 2015; 1–9 (human med AG Offprint).
- 52.Gensior MHL, Cornely M. Der Lipödemschmerz, seine Folgen auf die Lebensqualität betroffener Patientinnen – Ergebnisse einer Patientenbefragung mittels Schmerzfragebogen [Pain in lipoedema, fat in lipoedema and its consequences: results of a patient survey based on a pain questionnaire]. Handchir Mikrochir Plast Chir 2019; 51: 249–254. [DOI] [PubMed] [Google Scholar]
- 53.Wold LE Hines EA JrandAllen EV.. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann Intern Med 1951; 34: 1243–1250. [DOI] [PubMed] [Google Scholar]
- 54.Nair AS, Diwan S. Allopregnanolone: a neurosteroid for managing acute and chronic pain conditions. Saudi J Anaesth 2019; 13: 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michelini S, Chiurazzi P, Marino V, et al. Aldo-keto reductase 1C1 (AKR1C1) as the first mutated gene in a family with nonsyndromic primary lipedema. Int J Mol Sci 2020; 21: 6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schook CC, Mulliken JB, Fishman SJ, et al. Differential diagnosis of lower extremity enlargement in pediatric patients referred with a diagnosis of lymphedema. Plast Reconstr Surg 2011; 127: 1571–1581. [DOI] [PubMed] [Google Scholar]
- 57.Herpertz U. Krankheitsspektrum des Lipödems an einer Lymphologischen Fachklinik - Erscheinungsformen. Mischbilder und Behandlungsmöglichkeiten 1997; 5: 301–307. [Google Scholar]
- 58.Forner-Cordero I, Szolnoky G, Forner-Cordero A, et al. Lipedema: an overview of its clinical manifestations, diagnosis and treatment of the disproportional fatty deposition syndrome - systematic review. Clin Obes 2012; 2: 86–95. [DOI] [PubMed] [Google Scholar]
- 59.Földi E, Földi M. Das lipödem. In Földi M, Földi E, Kubik S. (eds) Lehrbuch der Lymphologie für Mediziner, Masseure und Physiotherapeuten. Munich: Elsevier, Urban & Fischer, 2005, pp.443–453. [Google Scholar]
- 60.Child AH, Gordon KD, Sharpe P, et al. Lipedema: an inherited condition. Am J Med Genet A 2010; 152A: 970–976. [DOI] [PubMed] [Google Scholar]
- 61.Mendes AM, Madon RJ, Flint DJ. Effects of cortisol and progesterone on insulin binding and lipogenesis in adipocytes from normal and diabetic rats. J Endocrinol 1985; 106: 225–231. [DOI] [PubMed] [Google Scholar]
- 62.Paolacci S, Precone V, Acquaviva F, et al. Genetics of lipedema: new perspectives on genetic research and molecular diagnoses. Eur Rev Med Pharmacol Sci 2019; 23: 5581–5594. [DOI] [PubMed] [Google Scholar]
- 63.Herpertz U. Das lipödem. Lymphologie 1995; 19: 1–11. [PubMed] [Google Scholar]
- 64.Reich-Schupke S, Altmeyer P, Stücker M. Thick legs not always lipedema. J Dtsch Dermatol Ges 2013; 11: 225–233. [DOI] [PubMed] [Google Scholar]
- 65.Dudek JE, Białaszek W, Gabriel M. Quality of life, its factors, and sociodemographic characteristics of Polish women with lipedema. BMC Women’s Health 2021; 21: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halk AB, Damstra RJ. First Dutch guidelines on lipedema using the international classification of functioning, disability and health. Phlebology 2017; 32: 152–159. [DOI] [PubMed] [Google Scholar]
- 67.Bauer AT, von Lukowicz D, Lossagk K, et al. New insights on lipedema: the enigmatic disease of the peripheral fat. Plast Reconstr Surg 2019; 144: 1475–1484. [DOI] [PubMed] [Google Scholar]
- 68.Romeijn JRM, de Rooij MJM, Janssen L, et al. Exploration of patient characteristics and quality of life in patients with lipoedema using a survey. Dermatol Ther (Heidelb) 2018; 8: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herbst KL, Abu-Zaid L, Fazel MT. Question-based self-reported experience of patients with subcutaneous adipose tissue (SAT) disease prescribed sympathomimetic amines. Med Res Archiv 2019; 7: 1–17. [Google Scholar]
- 70.Beltran K, Herbst KL. Differentiating lipedema and Dercum's disease. Int J Obes (Lond.) 2017; 41: 240–245. [DOI] [PubMed] [Google Scholar]
- 71.Kraus RH. and 2015. 2015:1–9. LG. All about lipedema. Lymphe Gesundheit 2015. 2015. [Google Scholar]
- 72.Dudek JE, Bialaszek W, Ostaszewski P. Quality of life in women with lipoedema: a contextual behavioral approach. Qual Life Res 2016; 25: 401–408. [DOI] [PubMed] [Google Scholar]
- 73.Baumgartner A, Hueppe M, Meier-Vollrath I, et al. Improvements in patients with lipedema 4, 8 and 12 years after liposuction. Phlebology 2021; 36: 152–159. [DOI] [PubMed] [Google Scholar]
- 74.Wollina U. [Lipedema: up-to-date of a long forgotten disease.]. Wien Med Wochenschr 2017; 167: 343–348. [DOI] [PubMed] [Google Scholar]
- 75.Bays HE, Mw Christensen S, Tondt J, et al. Obesity algorithm slides. Scarborough: Obesity Medicine Association, 2020. [Google Scholar]
- 76.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2015; 100: 342–362. [DOI] [PubMed] [Google Scholar]
- 77.Kajita K, Mori I, Hanamoto T, et al. Pioglitazone enhances small-sized adipocyte proliferation in subcutaneous adipose tissue. Endocr J 2012; 59: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 78.Kenny J-ES. The revised starling principle: implications for rational fluid therapy. PulmCCM. Epub ahead of print 7 July 2016. Available at: http://pulmccm.org/main/2016/ ards-review/revised-starling-principle-implications-rational-fluid-therapy/
- 79.Li X, Li J, Wang L, et al. The role of metformin and resveratrol in the prevention of HIF1-alpha accumulation and fibrosis in hypoxic adipose tissue. Br J Pharmacol 2016; 5: 13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kheirollahi V, Wasnick RM, Biasin V, et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun 2019; 10: 2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feldo M, Wozniak M, Wojciak-Kosior M, et al. Influence of Diosmin treatment on the level of oxidative stress markers in patients with chronic venous insufficiency. Oxid Med Cell Longev 2018; 2018: 2561705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ibegbuna V, Nicolaides AN, Sowade O, et al. Venous elasticity after treatment with Daflon 500 mg. Angiology 1997; 48: 45–49. [DOI] [PubMed] [Google Scholar]
- 83.Bouskela E, Donyo KA, Verbeuren TJ. Effects of Daflon 500 mg on increased microvascular permeability in normal hamsters. Int J Microcirc 1995; 15: 22–26. [DOI] [PubMed] [Google Scholar]
- 84.Batchvarov IV, Batselova MG, Damyanov II. One-year Diosmin therapy (600 mg) in patients with chronic venous insufficiency—results and analysis. J Biomed Clin Res 2010; 3: 51–54. [Google Scholar]
- 85.Carballo-Villalobos AI, González-Trujano ME, Pellicer F, et al. Antihyperalgesic effect of hesperidin improves with Diosmin in experimental neuropathic pain. Biomed Res Int 2016; 2016: 8263463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ehrlich C, Iker E, Herbst KL, et al. Lymphedema and lipedema nutrition guide. Foods, vitamins, minerals, and supplements. San Francisco: Lymph Notes, 2015. [Google Scholar]
- 87.Di Renzo L, Cinelli G, Romano L, et al. Potential effects of a modified Mediterranean diet on body composition in lipoedema. Nutrients 2021; 13: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keith L, Seo CA, Rowsemitt C, et al. Ketogenic diet as a potential intervention for lipedema. Med Hypotheses 2021; 146: 110435–112020. [DOI] [PubMed] [Google Scholar]
- 89.Kosinski C, Jornayvaz FR. Effects of ketogenic diets on cardiovascular risk factors: evidence from animal and human studies. Nutrients 2017; 9: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pereira-Santos M, Costa PR, Assis AM, et al. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev 2015; 16: 341–349. [DOI] [PubMed] [Google Scholar]
- 91.Greene AK, Sudduth CL. Lower extremity lymphatic function predicted by body mass index: a lymphoscintigraphic study of obesity and lipedema. Int J Obes 2021; 45: 369–373. [DOI] [PubMed] [Google Scholar]
- 92.Lymphology Association of North America®, https://www.clt-lana.org/ (2020, 2020).
- 93.Wittlinger H, Wittlinger D, Wittlinger A, et al. Complementary techniques. In: Dr Vodder's manual lymph drainage: a practical guide. New York/Stuttgart: Thieme Publishers, 2018, 153 pp.
- 94.Szolnoky G, Borsos B, Barsony K, et al. Complete decongestive physiotherapy with and without pneumatic compression for treatment of lipedema: a pilot study. Lymphology 2008; 41: 40–44. [PubMed] [Google Scholar]
- 95.Schwahn-Schreiber C, Breu FX, Rabe E, et al. [S1 guideline on intermittent pneumatic compression (IPC)]. Hautarzt 2018; 69: 662–673. [DOI] [PubMed] [Google Scholar]
- 96.Hodge LM, King HH, Williams AG, Jr, et al. Abdominal lymphatic pump treatment increases leukocyte count and flux in thoracic duct lymph. Lymphat Res Biol 2007; 5: 127–133. [DOI] [PubMed] [Google Scholar]
- 97.Huff JB, Schander A, Downey HF, et al. Lymphatic pump treatment augments lymphatic flux of lymphocytes in rats. Lymphat Res Biol 2010; 8: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gautam AP, Maiya AG, Vidyasagar MS. Effect of home-based exercise program on lymphedema and quality of life in female postmastectomy patients: pre-post intervention study. J Rehabil Res Dev 2011; 48: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 99.Reed RK, Townsley MI, Zhao Z, et al. Lymphatic hyaluronan flux from skin increases during increased lymph flow induced by intravenous saline loading. Int J Microcirc 1994; 14: 56–61. [DOI] [PubMed] [Google Scholar]
- 100.Herbst KL, Ussery C, Eekema A. Pilot study: whole body manual subcutaneous adipose tissue (SAT) therapy improved pain and SAT structure in women with lipedema. Horm Mol Biol Clin Investig 2017; 33: 2018–2033. [DOI] [PubMed] [Google Scholar]
- 101.Ibarra M, Eekema A, Ussery C, et al. Subcutaneous adipose tissue therapy reduces fat by dual X-ray absorptiometry scan and improves tissue structure by ultrasound in women with lipoedema and Dercum disease. Clin Obes 2018; 8: 398–406. [DOI] [PubMed] [Google Scholar]
- 102.Wenczl E, Daróczy J. [Lipedema, a barely known disease: diagnosis, associated diseases and therapy]. Orv Hetil 2008; 149: 2121–2127. [DOI] [PubMed] [Google Scholar]
- 103.Paling I, Macintyre L. Survey of lipoedema symptoms and experience with compression garments. Br J Community Nurs 2020; 25: S17–S22. [DOI] [PubMed] [Google Scholar]
- 104.Szolnoky G, Varga E, Varga M, et al. Lymphedema treatment decreases pain intensity in lipedema. Lymphology 2011; 44: 178–182. [PubMed] [Google Scholar]
- 105.Hardy D, Williams A. Best practice guidelines for the management of lipoedema. Br J Community Nurs 2017; 22: S44–S48. [DOI] [PubMed] [Google Scholar]
- 106.Linnitt N, Davies R. Fundamentals of compression in the management of lymphoedema. Br J Nurs 2007; 16: 588–592. [DOI] [PubMed] [Google Scholar]
- 107.Schaverien MV, Moeller JA, Cleveland SD. Nonoperative treatment of lymphedema. Semin Plast Surg 2018; 32: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilburn O, Wilburn P, Rockson SG. A pilot, prospective evaluation of a novel alternative for maintenance therapy of breast cancer-associated lymphedema. BMC Cancer 2006; 6: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bellini E, Grieco MP, Raposio E. A journey through liposuction and liposculture: review. Ann Med Surg (Lond) 2017; 24: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peled AW, Slavin SA, Brorson H. Long-term outcome after surgical treatment of lipedema. Ann Plast Surg 2012; 68: 303–307. [DOI] [PubMed] [Google Scholar]
- 111.Baumgartner A, Hueppe M, Schmeller W. Long-term benefit of liposuction in patients with lipoedema. A follow-up study after an average of 4 and 8 years. Br J Dermatol 2015; 174: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 112.Schmeller W, Hueppe M, Meier-Vollrath I. Tumescent liposuction in lipoedema yields good long-term results. Br J Dermatol 2012; 166: 161–168. [DOI] [PubMed] [Google Scholar]
- 113.Stutz JJ. Liposuction in lipedema to prevent later joint complications. Vasomed 2011; 23: 6. [Google Scholar]
- 114.Witte T, Dadras M, Heck FC, et al. Water-jet-assisted liposuction for the treatment of lipedema: standardized treatment protocol and results of 63 patients. J Plast Reconstr Aesthet Surg 2020; 73: 1637–1644. [DOI] [PubMed] [Google Scholar]
- 115.van de Pas CB, Boonen RS, Stevens S, et al. Does tumescent liposuction damage the lymph vessels in lipoedema patients? Phlebology 2020; 35: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmeller W, Meier-Vollrath I. Tumescent liposuction: a new and successful therapy for lipedema. J Cutan Med Surg 2006; 10: 7–10. [DOI] [PubMed] [Google Scholar]
- 117.Stutz JJ, Krahl D. Water jet-assisted liposuction for patients with lipoedema: histologic and immunohistologic analysis of the aspirates of 30 lipoedema patients. Aesthetic Plast Surg 2009; 33: 153–162. [DOI] [PubMed] [Google Scholar]
- 118.Rapprich S, Loehnert M, Hagedorn M. Therapy of lipoedema syndrome by liposuction under tumescent local anaesthesia. Ann Dermatol Venereol 2002; 129: 1S711. [Google Scholar]
- 119.Rapprich S, Baum S, Kaak I, et al. Treatment of lipoedema using liposuction. Results of our own surveys. Phlebologie 2015; 44: 121–132. [Google Scholar]
- 120.Dadras M, Mallinger PJ, Corterier CC, et al. Liposuction in the treatment of lipedema: a longitudinal study. Arch Plast Surg 2017; 44: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dean SM, Valenti E, Hock K, et al. The clinical characteristics of lower extremity lymphedema in 440 patients. J Vasc Surg Venous Lymphat Disord 2020; 8: 851–859. [DOI] [PubMed] [Google Scholar]
- 122.Chang SL, Huang YL, Lee MC, et al. Association of varicose veins with incident venous thromboembolism and peripheral artery disease. JAMA 2018; 319: 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dua A, Neiva S, Sutherland A. Does previous varicose vein surgery alter deep vein thrombosis risk after lower limb arthroplasty? Orthop Surg 2012; 4: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dixit VV, Wagh MS. Unfavourable outcomes of liposuction and their management. Indian J Plast Surg 2013; 46: 377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wollina U, Heinig B. Treatment of lipedema by low-volume micro-cannular liposuction in tumescent anesthesia: results in 111 patients. Dermatol Ther 2019; 32: e12820. [DOI] [PubMed] [Google Scholar]
- 126.Klein JA. Tumescent technique. Tumescent anesthesia and microcannular liposuction. New York: Mosby, 2000. [Google Scholar]
- 127.Kinugawa K, Nuri T, Iwanaga H, et al. Lymph vessel mapping using indocyanine green lymphography in the nonaffected side of lower leg. Plast Reconstr Surg Glob Open 2020; 8: e2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Triana L, Triana C, Barbato C, et al. Liposuction: 25 years of experience in 26,259 patients using different devices. Aesthet Surg J 2009; 29: 509–512. [DOI] [PubMed] [Google Scholar]
- 129.Wollina U, Heinig B, Nowak A. Treatment of elderly patients with advanced lipedema: a combination of laser-assisted liposuction, medial thigh lift, and lower partial abdominoplasty. Clin Cosmet Investig Dermatol 2014; 7: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Olin JW, White CJ, Armstrong EJ, et al. Peripheral artery disease: evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol 2016; 67: 1338–1357. [DOI] [PubMed] [Google Scholar]
- 131.Bonham PA. Steps for determining the toe brachial pressure index. Adv Skin Wound Care 2004; 17: 44–45. [DOI] [PubMed] [Google Scholar]
- 132.Vowden K, Vowden P. Managing leg ulcers: a review of the clinical guidelines. Nurs Times 2000; 96: 19–20. [PubMed] [Google Scholar]
- 133.Mutnal S, Rangappa P, Jacob I, et al. A case report of lipedema with acute bilateral lower limb ischemia. Indian J Vasc Endovasc Surg 2019; 6: 132–134. [Google Scholar]
- 134.Cronin M, Dengler N, Krauss ES, et al. Completion of the updated Caprini risk assessment model (2013 version). Clin Appl Thromb Hemost 2019; 25: 1076029619838052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sandhofer M, Schauer P, Sandhofer M, et al. Lipödem. J Ästhet Chir 2017; 10: 61–70. [Google Scholar]
- 136.Evans CJ, Fowkes FG, Ruckley CV, et al. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health 1999; 53: 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation 2014; 130: 333–346. [DOI] [PubMed] [Google Scholar]
- 138.Stemmer R. Ein klinisches zeichen zur früh-und differentialdiagnose des lymphödems. Vasa 1976; 5: 261–262. [PubMed] [Google Scholar]
- 139.Kistner RL, Eklof B, Masuda EM. Diagnosis of chronic venous disease of the lower extremities: the “CEAP” classification. Mayo Clin Proc 1996; 71: 338–345. [DOI] [PubMed] [Google Scholar]
- 140.Naouri M, Samimi M, Atlan M, et al. High-resolution cutaneous ultrasonography to differentiate lipoedema from lymphoedema. Br J Dermatol 2010; 163: 296–301. [DOI] [PubMed] [Google Scholar]
- 141.Bootun R, Lane TR, Davies AH. The advent of non-thermal, non-tumescent techniques for treatment of varicose veins. Phlebology 2016; 31: 5–14. [DOI] [PubMed] [Google Scholar]
- 142.Koramaz İ, El Kılıç H, Gökalp F, et al. Ablation of the great saphenous vein with nontumescent n-butyl cyanoacrylate versus endovenous laser therapy. J Vasc Surg Venous Lymphat Disord 2017; 5: 210–215. [DOI] [PubMed] [Google Scholar]
- 143.Weiss RA, Munavalli G. Endovenous ablation of truncal veins. Semin Cutan Med Surg 2005; 24: 193–199. [DOI] [PubMed] [Google Scholar]
- 144.Winokur RS, Khilnani NM. Superficial veins: treatment options and techniques for saphenous veins, perforators, and tributary veins. Tech Vasc Interv Radiol 2014; 17: 82–89. [DOI] [PubMed] [Google Scholar]
- 145.Angst F, Lehmann S, Aeschlimann A, et al. Cross-sectional validity and specificity of comprehensive measurement in lymphedema and lipedema of the lower extremity: a comparison of five outcome instruments. Health Qual Life Outcomes 2020; 18: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blome C, Augustin M, Heyer K, et al. Evaluation of patient-relevant outcomes of lymphedema and lipedema treatment: development and validation of a new benefit tool. Eur J Vasc Endovasc Surg 2014; 47: 100–107. [DOI] [PubMed] [Google Scholar]