Abstract
Background
Chronic rhinitis is a prevalent condition with a significant impact on quality of life. Posterior nasal nerve and vidian neurectomy are surgical options for treating the symptoms of chronic rhinitis but are invasive procedures.
Objective
To determine the outcomes of patients diagnosed with refractory chronic rhinitis and treated with temperature-controlled radiofrequency neurolysis of the posterior nasal nerve area in a minimally invasive procedure.
Methods
A prospective, single-arm multicenter study with follow-up through 52 weeks. Eligible adult patients had chronic rhinitis symptoms of at least 6 months duration with inadequate response to at least 4 weeks usage of intranasal steroids and an overall 12-h reflective total nasal symptom score (rTNSS) ≥ 6 with subscores 2 to 3 for rhinorrhea, 1 to 3 for nasal congestion, and 0 to 3 for each of nasal itching and sneezing. Temperature-controlled radiofrequency energy was delivered to the nasal cavity mucosa overlying the posterior nasal nerve region with a novel single-use, disposable, handheld device.
Results
A total of 50 patients were treated (42.0% male; mean age 57.9 ± 11.9 years), and 47 completed the study through 52 weeks. Mean rTNSS significantly improved from 8.5 (95% CI 8.0, 9.0) at baseline to 3.6 (95% CI 3.0, 4.3) at 52 weeks (P < .001), a 57.6% improvement. Similar trends in improvement were noted for rTNSS subscores (rhinorrhea, nasal congestion, itching, sneezing), postnasal drip scores, and chronic cough scores. Subgroup analysis demonstrated the treatment was effective regardless of rhinitis classification (allergic or nonallergic). No serious adverse events with a relationship to the device/procedure occurred.
Conclusions
Temperature-controlled radiofrequency neurolysis of the posterior nasal nerve area for the treatment of chronic rhinitis is safe and resulted in a durable improvement in the symptoms of chronic rhinitis through a 52-week follow-up. Data suggest that this novel device could be considered a minimally invasive option in the otolaryngologist's armamentarium for the treatment of chronic rhinitis.
Keywords: rhinitis, rTNSS, rhinorrhea, congestion, posterior nasal nerve, neurectomy, temperature-controlled, radiofrequency ablation, allergic, neurolysis
Introduction
Chronic rhinitis is a common medical problem affecting the quality of life of tens of millions of Americans. 1 Allergic and nonallergic rhinitis are associated with different triggers, and patients may present with either trigger or a mix of triggers.2,3 Pharmacological therapies for the treatment of chronic rhinitis include intranasal antihistamines, corticosteroid or anticholinergic sprays, oral antihistamines, and immunotherapy. 4
Patients may consider surgical procedures when medical management does not offer sufficient relief. 4 Vidian neurectomy is an option, but it can be a challenging procedure and may lead to dry eyes and numbness of the palate, upper front teeth, or cheek. 5 Posterior nasal nerve (PNN) neurectomy has gained acceptance as it is less likely to cause keratoconjunctivitis sicca.6,7 Less invasive office-based procedures for PNN ablation via cryoablation or diode laser ablation have been reported.8–11
Radiofrequency (RF) devices are commonly used in otolaryngology,12–14 and most regulate power output based on the impedance, or resistance, of the treated tissue as it desiccates. Temperature-controlled RF neurolysis is different from most RF devices in that the energy delivered into the tissue is controlled by continually measuring the temperature of the treatment area and varying output to maintain a desired tissue temperature of 60°C. The objective of this study was to evaluate safety and efficacy through 52 weeks in adults diagnosed with chronic rhinitis and treated with a temperature-controlled RF device.
Methods
This study was a nonsignificant risk, prospective, single-arm, open-label, multicenter study with enrollment at 5 centers in the United States. The study was approved by the Western Institutional Review Board (reference 20182161) and registered at clincaltrials.gov (NCT03727347). All investigators are board-certified otolaryngologists.
Patients were enrolled and treated between October 2018 and June 2019 and gave written informed consent. Key inclusion criteria were chronic rhinitis symptoms of at least 6 months duration, dissatisfaction with medical management defined as an inadequate response (as judged by the patient) to at least 4 weeks usage of intranasal steroids, a reflective total nasal symptom score (rTNSS) score ≥6, as well as ≥2 for rhinorrhea, and ≥1 for congestion. Patients were excluded if they had nasal anatomic abnormalities or obstructions that could restrict access to the treatment site, rhinitis medicamentosa, active nasal/sinus infection, history of nose bleeds, ocular allergic symptoms, or history of dry eye.
Patients were not compensated for enrollment in the study, however, they were compensated with a gift card when attending follow-up visits, as approved by the Institutional Review Board approving the study.
The protocol did not dictate that the patients should be tested to determine the underlying cause of rhinitis as part of the study, but the cause was recorded if known to the patient or investigator.
The RhinAer System (Aerin Medical, Inc.) comprises the Aerin Console and the RhinAer Stylus (Figure 1A and B). The console powers the stylus, which is a single-use disposable handheld device that delivers 60°C temperature-controlled bipolar RF energy to the PNN region. The target tissue was the portion of the nasal cavity mucosa overlying the PNN region in the posterior middle meatus and along the posterior inferior turbinate (Figure 1C).
Figure 1.
(A) Aerin Console, which delivers low doses of temperature-controlled RF energy. (B) The tip of the RhinAer Stylus, a single-use disposable handheld device that delivers bipolar RF energy to tissue. (C) The RhinAer Stylus is placed at the target tissue, the nasal cavity mucosa overlying the PNN region (the posterior middle meatus and posterior inferior turbinate). The stylus delivers bipolar RF energy maintained at ∼60°C.
Abbreviations: RF, radiofrequency; PNN, posterior nasal nerve.
Patients were seated partially reclined in an office setting. Topical anesthetic (tetracaine, oxymetazoline, lidocaine, epinephrine per investigator preference) was applied to the middle meatus and the posterior/superior portion of the inferior turbinate, followed by submucosally infiltrated 1% to 4% lidocaine (with or without epinephrine) at the treatment sites, with the dose and volume per investigator preference. Both nostrils were treated at 1–3 nonoverlapping positions, depending on the size of each target treatment area. Settings were 60°C at 4 W for 12 s of treatment time at each position. No repeat/touch-up procedures were performed.
At baseline, patients completed the rTNSS questionnaire and recorded postnasal drip and chronic cough symptom scores on a 6-point scale (0 = no problem and 5 = severe symptoms). Immediately after treatment, patients marked a standard 100-mm visual analog scale (VAS) reflecting their pain level. Patients returned for follow-up visits at 2, 4, 12, 26, and 52 weeks posttreatment, and the rTNSS, postnasal drip and chronic cough scores, and the pain VAS were repeated. Medications for rhinitis were not controlled during the study; however, concomitant medication use at all follow-up visits was recorded. Adverse events were captured throughout.
The primary efficacy endpoint was the change in rTNSS from baseline through 12 weeks, with the hypothesis that improvement exceeding 1 point would constitute a minimal clinically important difference (MCID) that is more stringent than MCIDs derived from anchor-based and distribution-based methodologies.15–17 Responder rates were also calculated using an even more stringent definition (≥30% improvement relative to baseline).16,18
The minimum sample size was 36, based on an expected change of –2 and standard deviation of 2, 90% power at α = .05 (1 sided), and assuming a 10% dropout/nonevaluable rate. Descriptive statistics were calculated with no data imputation. Unless otherwise stated, continuous data are presented as mean and 95% confidence intervals (CI), and categorical data as numbers and percentages of total. Outcomes were assessed using a linear mixed-effect model to test for an overall change over time, with Dunnett's test used for comparison of follow-up visits to baseline. Rhinitis subtype analyses used linear mixed models with Tukey-Cramer multiple comparisons adjustments.
Results
A total of 50 patients were enrolled and treated and 47 (94%) completed the 52-week visit (Figure 2). The group included slightly more women than men, with an average age of 57.9 ± 11.9 years (Table 1). The majority of patients (80%) were overweight or obese. All patients had been suffering from the symptoms of rhinitis for more than 1 year and approximately half (42%) were reported to suffer from allergic rhinitis, based on prior knowledge (patient report or physician assessment). The anesthesia regimen was most commonly topical tetracaine and submucosal lidocaine plus epinephrine in the region of the sphenopalatine foramen. The majority of patients were treated bilaterally at 2 or 3 sites per side (Table 1). Immediately after the treatment, mean pain was 18.1 (95% CI 12.3, 23.9) out of a maximum of 100. After 4 weeks, mean pain intensity had decreased to 5.0 (95% CI 2.1, 8.0) and 67.3% of patients (n = 49) had no pain (rating of 0).
Figure 2.
Patient disposition: 94% of patients completed a 52-week follow-up.
Table 1.
Patient Demographics, Baseline, and Procedural Information.
| N = 50 | ||
|---|---|---|
| Male sex | 21 | (42) |
| Female sex | 29 | (58) |
| Age (years) | 57.9 | ± 11.9 |
| Race | ||
| White | 47 | (94) |
| Asian | 2 | (4) |
| American Indian or Alaska Native | 1 | (2) |
| Body mass index | 29.8 | ± 6.4 |
| Rhinitis type | ||
| Allergic | 21 | (42) |
| Nonallergic | 17 | (34) |
| Unknown | 12 | (24) |
| Topical anesthetic | ||
| Tetracaine (4%) alone | 26 | (52) |
| Tetracaine plus other agents | 24 | (48) |
| Injected anesthetic | ||
| Lidocaine alone | 1 | (2) |
| Lidocaine plus epinephrine (1:100,000 mg/mL) | 49 | (98) |
| Volume (mL), per nostril | 1.9 | ± 1.2 |
| Treatment applications per nostril | ||
| 1 each side | 1 | (2) |
| 2 each side | 24 | (48) |
| 3 each side | 22 | (44) |
| 6 each side (unusually large anatomy) | 1 | (2) |
| Unequal numbers each side (1, 2, or 3) | 2 | (4) |
| Procedure time (minutes) | ||
| Mean | 21.9 | ± 19.1 |
| Median | 16.5 | |
| Range | 3–105 | |
Continuous data are presented as mean ± standard deviation and categorical data as number (percent of total).
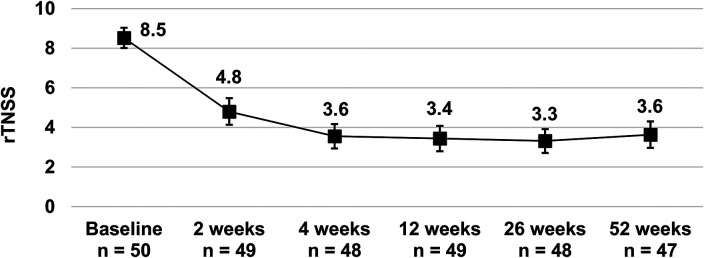
Treatment improved the mean rTNSS from 8.5 (95% CI 8.0, 9.0) at baseline to 3.4 (95% CI 2.8, 4.1) at 12 weeks and showed sustained improvement to 3.6 (95% CI 3.0, 4.3) at 52 weeks (Figure 3). Relative to baseline, the improvement was statistically significant at each time point (all P < .001). The mean change in rTNSS from baseline at 12 weeks was − 5.1 (95% CI − 5.8, − 4.4), P < .001, and therefore the study met its primary efficacy endpoint of ≥1 point improvement at 12 weeks postprocedure. The mean percent change in rTNSS from baseline was −59.2% (95% CI − 66.9%, − 51.6%) and − 57.7% (95% CI − 65.3%, − 50.0%) at 12 and 52 weeks, respectively. The potential effect of missing data on the primary efficacy endpoint was assessed by imputing the missing change in rTNSS as 0, but it made no difference to the conclusion (mean change in rTNSS from baseline to 12 weeks − 5.0 [95% CI − 5.8, − 4.2], P < .001).
Figure 3.
Mean reflective total nasal symptom score (rTNSS) at baseline and follow-up. Bars represent 95% confidence intervals. All values were statistically significantly improved throughout follow-up (P < .001 at all time points based on a linear mixed-effect model).
The proportion of patients considered responders (≥1-point improvement in rTNSS) at each follow-up were 93.9% (95% CI 83.5%, 97.9%) at 12 weeks, 95.8% (95% CI 86.0%, 98.8%) at 26 weeks, and 100% (95% CI 92.4%, 100%) at 52 weeks. Using a more stringent definition of a responder (≥30% improvement relative to baseline), 87.8% (95% CI 75.8%, 94.3%), 91.7% (95% CI 80.4%, 96.7%), and 80.9% (95% CI 67.5%, 89.6%) of patients were considered responders at 12, 26, and 52 weeks, respectively.
All rTNSS subscores (rhinorrhea, nasal congestion, nasal itching, and sneezing, all rated 0-3) as well as postnasal drip and chronic cough symptom scores (rated 0-5), improved with treatment (Figures 4 and 5). The rTNSS subscores and postnasal drip and chronic cough symptom scores remained statistically significantly improved from baseline at all follow-up time points (all P < .001).
Figure 4.
Mean reflective total nasal symptom score (rTNSS) subscores at baseline and follow-up. Bars represent 95% confidence intervals. All values were statistically significantly improved from baseline throughout follow-up (P < .001 at all time points based on a linear mixed-effect model).
Figure 5.
Mean postnasal drip and chronic cough scores at baseline and follow-up. Bars represent 95% confidence intervals. All values were statistically significantly improved from baseline throughout follow-up (P < .001 at all time points based on a linear mixed-effect model).
An improvement in rTNSS from baseline was observed at all time points for patients with allergic and nonallergic rhinitis, as well as rhinitis of unknown etiology (Table 2, P < .001 for all time points, based on subgroup analyses using linear mixed models with Tukey-Cramer multiple comparisons adjustments). The overall effect of rhinitis trigger was not significant (P = .696) and there was no significant interaction of visit and rhinitis trigger (P = .491), indicating the amount of improvement in rTNSS was not significantly different among the rhinitis triggers and the effect was consistent across follow-up visits.
Table 2.
Rhinitis Subtype Subgroup Analysis of rTNSS at Baseline and Over Time.
| Baseline | 2 weeks | 4 weeks | 12 weeks | 26 weeks | 52 weeks | |
|---|---|---|---|---|---|---|
| Allergic—rTNSS | ||||||
| n | 21 | 21 | 20 | 20 | 21 | 21 |
| Meana | 8.2 | 5.4 | 3.6 | 3.9 | 3.7 | 3.7 |
| 95% CI | (7.4, 9.0) | (4.4, 6.4) | (2.6, 4.6) | (2.9, 4.9) | (2.7, 4.6) | (2.7, 4.7) |
| Nonallergic—rTNSS | ||||||
| n | 17 | 16 | 16 | 17 | 15 | 15 |
| Meana | 8.9 | 3.8 | 3.6 | 3.4 | 3.2 | 3.6 |
| 95% CI | (8.1, 9.8) | (2.7, 4.9) | (2.5, 4.6) | (2.3, 4.5) | (2.1, 4.3) | (2.5, 4.8) |
| Unknown—rTNSS | ||||||
| n | 12 | 12 | 12 | 12 | 12 | 11 |
| Meana | 8.5 | 5.1 | 3.5 | 2.8 | 2.8 | 3.5 |
| 95% CI | (7.4, 9.6) | (3.7, 6.4) | (2.2, 4.8) | (1.4, 4.1) | (1.6, 4.1) | (2.1, 4.9) |
rTNSS, reflective total nasal symptom score.
aLeast square mean.
The proportion of patients who self-reported “never” or “rarely” used oral medications or nasal sprays for rhinitis increased from ∼20% at baseline (oral medications 21.7%, nasal sprays 19.6%) to >35% at follow-up (all time points P < .05), while the use of nasal breathing strips was uncommon at both baseline and follow-up (Figure 6A). Based on concomitant medication reporting at 52 weeks, more patients decreased their usage of antihistamines/decongestants, decongestant nasal sprays, and steroid nasal sprays relative to baseline than increased their use of these products (Figure 6B). Ipratropium bromide was used by 8 patients at baseline. During follow-up, 1 of these patients withdrew after the 12-week evaluation as a ≥30% responder, 3 patients discontinued use and were ≥30% responders at 52 weeks, 1 continued use and was a ≥30% responder at 52 weeks, and 3 who continued use were ≥30% nonresponders at 52 weeks. In addition, 1 patient began using ipratropium bromide 1 month after the procedure and was a ≥30% nonresponder at 52 weeks. Because ipratropium bromide use could be a confounding variable, primary endpoint analysis was repeated excluding the 9 patients who used ipratropium bromide during the study (and imputing the missing change in rTNSS data as zero of 1 patient that did not reach 12 weeks) and the overall device treatment effect was unchanged from that of the entire cohort. Specifically, at 12 weeks the mean change in rTNSS from baseline was − 5.1 (95% CI − 6.0, − 4.3), P < .001. The proportion of patients not using ipratropium bromide who were responders at 12 weeks (with data imputation as outlined above) was 92.7% (95% CI 80.6%, 97.5%) (≥1-point improvement) and 87.8% (95% CI 74.5%, 94.7%) (≥30% improvement). The result at 52 weeks in the cohort not using ipratropium bromide (n = 39 at 52 weeks, no data imputation) was similar to that of the entire cohort. Specifically, the mean change in rTNSS from baseline at 52 weeks was − 5.3 (95% CI − 6.2, − 4.3), P < .001. The proportion of patients not using ipratropium bromide who were responders at 52 weeks was 100% (95% CI 91.0%, 100%) (≥1-point improvement) and 87.2% (95% CI 73.3%, 94.4%) (≥30% improvement).
Figure 6.
(A) The proportion of patients who rarely or never used oral rhinitis medications, nasal sprays, or nasal breathing strips increased relative to baseline at each of the posttreatment follow-ups. (B) Concomitant medication tracking through the study indicated that more patients decreased their usage of antihistamines/decongestants, decongestant nasal sprays, and steroid nasal sprays during the study than increased use.
Two serious adverse events occurred during the study (pneumonia and cholecystitis); however, they were not considered device- or procedure-related by the site investigator treating the patient and study sponsor. A total of 16 adverse events that were considered possibly device- or procedure-related occurred in 8 patients (Table 3). The majority of adverse events occurred within the first 2 weeks and all except 2 adverse events (sinusitis and worsening dry eye) resolved (Table 3). At 52 weeks, the dry eye was stable, and the sinus congestion was weather-dependent.
Table 3.
Adverse Events that Were at Least Possibly Related to the Device or Procedure.
| Event | Number | Discovery window (weeks) a | Days to resolution |
|---|---|---|---|
| Eye dryness (worsening) | 1 | 0–2 | Ongoing |
| Internal nasal swelling/edema (severe) | 1 | 2–4 | 158 |
| Nasal soreness/pain (severe) | 1 | 0–2 | 27 |
| Nasal inflammation/redness (severe) | 1 | 4–12 | 88 |
| Nasal mucosa changes | 1 | 2–4 | 158 |
| Postnasal drip | 2 | 0–2 | 23, 28 |
| Sneezing | 1 | 0–2 | 4 |
| Nasal drainage (increased) | 1 | 0–2 | 23 |
| Nasal bleeding | 1 | 0–2 | 1 |
| Nasal septum irritation | 1 | 0–2 | 4 |
| Teeth pain | 1 | 0–2 | 1 |
| Sinusitis | 1 | 0–2 | Ongoing |
| Mouth—roof irritation | 1 | 0–2 | 23 |
| Oropharyngeal pain | 2 | 0–2 | 1, 2 |
| Overall total adverse events | 16 | 0–12 | — |
Weeks postprocedure.
Discussion
The data from this prospective study demonstrated temperature-controlled RF neurolysis of the PNN area results in a durable, statistically significant, improvement in rhinorrhea, nasal congestion, itching, sneezing, postnasal drip, and chronic cough symptoms over baseline conditions. Following a single bilateral treatment session, symptom severity as measured by rTNSS and other scales statistically significantly decreased in the first 2 weeks after treatment and remained low through 52 weeks follow-up.
The efficacy of Vidian and PNN neurectomy is believed to result from the interruption of efferent parasympathetic stimulation of the nasal mucosa. Blocking parasympathetic innervation has been shown to reduce submucosal glands secretion, blood flow in the submucosa, and stromal edema. 19 Temperature-controlled RF energy allows for targeted neurolysis while limiting damage to overlying mucosa and adjacent tissues.
All patients who reached 52 weeks had reductions of at least 1 point on the rTNSS relative to baseline, and 80.9% of patients improved ≥30% relative to baseline. For context, a report of a minimally invasive cryosurgical PNN ablation technique quoted an rTNSS ≥30% responder rate of 70.7% at 270 days (∼39 weeks), the longest-term data point reported. 9 A report on short-term outcomes following cryosurgical PNN ablation reported a ≥1-point responder rate of 86.7% at 3 months, 10 compared to 93.9% in our study using the same criterion and timepoint.
Reports on cryosurgical ablation studies have included changes in rTNSS from baseline to 270 days of 6.1 ± 1.9 to 3.0 ± 2.4 9 and from baseline to 3 months of 7.0 (95% CI 5.0, 9.0) to 2.5 (95% CI 2.0, 5.0) 10 compared to this study's mean change in rTNSS from baseline to 52 weeks of 8.5 ± 1.8 to 3.6 ± 2.3. A laser ablation study included changes in rTNSS from baseline to 90 days of 6.0 ± 0.7 to 2.3 ± 0.4. 11
Subgroup analysis showed temperature-controlled RF neurolysis of the PNN area improved the symptoms of chronic rhinitis in patients whose history indicated allergic, nonallergic, and unknown subtypes, pointing to broad applicability.
The treatment was completed in-office under local anesthesia in all patients. The treatment was well-tolerated and postprocedural pain was generally mild and resolved quickly. There were no serious adverse events related to the device or treatment and a low rate of adverse events, most comprising mild-to-moderate oropharyngeal discomfort, was reported. Importantly, no device- or procedure-related headaches were reported through 52 weeks. Temperature-controlled RF is designed to maintain the tissue temperature at a therapeutic level at the treatment site without causing rapid tissue desiccation or destruction and, therefore, no issues with healing were noted at the treatment sites. This is in contrast to standard RF devices, which can cause rapid destruction of tissue and are best suited to ablation or debulking of tissue.
The pragmatic nature of the study meant that concomitant medication was not controlled during the study. However, on the whole, medication use for rhinitis symptoms decreased during the study, indicating medication use is unlikely to have contributed to the observed symptom improvement. Nasal spray use, in particular, decreased after the procedure. Ipratropium bromide is an anticholinergic drug that inhibits parasympathetic nerve conduction by antagonizing the action of acetylcholine. Although the protocol did not dictate that patients had to have tried anticholinergic spray prior to inclusion in the study, 8 patients were using it at baseline and 1 patient started using it during the study, as outlined above. To help ensure that ipratropium bromide was not a major confounding variable in this study, the primary endpoint analysis was repeated excluding the 9 patients who used ipratropium during the study with no effect on the overall outcome. Medications are an inherent part of rhinitis management, and therefore, the pragmatic design and results of this study reflect those to be expected in real-world clinical use.
Limitations of the study include the fact that the study was not controlled nor blinded. While the effect sizes of the primary endpoint were sufficiently large as to demonstrate intra-patient baseline-to-follow-up changes, placebo effects may have contributed to the reported outcomes. Furthermore, autonomic testing prior to inclusion would more thoroughly characterized autonomic dysfunction and prospective allergy testing would have resulted in a more robust assignment of the rhinitis types. Lastly, control of medication use would have reduced any confounding effects of medication changes.
Conclusion
In-office, temperature-controlled RF neurolysis of the PNN area resulted in significant and durable improvement in the symptoms of chronic rhinitis (rhinorrhea, congestion, itching, sneezing, postnasal drip, and chronic cough) through 52 weeks postprocedure. Treatment was well-tolerated and adverse events generally resolved quickly. Larger, controlled studies will further demonstrate the efficacy of the device.
Acknowledgments
The authors thank study investigator Dr Bryan Davis. The authors thank Jeff F. Doerzbacher, MS for assistance in the statistical analysis, Julie Perkins PhD for writing support, and Allison Foster PhD of Foster Medical Communications, Auckland, New Zealand for writing support, all consultants to Aerin Medical.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dale Ehmer and V. Vasu Kakarlapudi are consultants for Aerin Medical. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Aerin Medical Inc.
ORCID iD: V. Vasu Kakarlapudi https://orcid.org/0000-0001-6694-4598
References
- 1.Settipane RA, Charnock DR. Epidemiology of rhinitis: allergic and nonallergic. Clin Allergy Immunol. 2007;19:23–34. [PubMed] [Google Scholar]
- 2.Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI Guideline for the diagnosis and management of allergic and non-allergic rhinitis (revised edition 2017; first edition 2007). Clin Exp Allergy. 2017;47(7):856–889. [DOI] [PubMed] [Google Scholar]
- 3.Greiwe JC, Bernstein JA. Allergic and mixed rhinitis: diagnosis and natural evolution. J Clin Med. 2019;8(11). doi: 10.3390/jcm8112019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel GB, Kern RC, Bernstein JA, Hae-Sim P, Peters AT. Current and future treatments of rhinitis and sinusitis. J Allergy Clin Immunol Pract. 2020;8(5):1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshak T, Yun WK, Hazout C, Sacks R, Harvey RJ. A systematic review of the evidence base for vidian neurectomy in managing rhinitis. J Laryngol Otol. 2016;130(Suppl 4):S7–S28. [DOI] [PubMed] [Google Scholar]
- 6.Kanaya T, Kikawada T. Endoscopic posterior nasal neurectomy: an alternative to vidian neurectomy. Clin Exp Allergy. 2009;9(1):24–27. [Google Scholar]
- 7.Takahara D, Takeno S, Hamamoto T, Ishino T, Hirakawa K. Management of intractable nasal hyperreactivity by selective resection of posterior nasal nerve branches. Int J Otolaryngol. 2017;2017:1907862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang PH, Lin B, Weiss R, Atkins J, Johnson J. Cryosurgical posterior nasal tissue ablation for the treatment of rhinitis. Int Forum Allergy Rhinol. 2017;7(10):952–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang MT, Song S, Hwang PH. Cryosurgical ablation for treatment of rhinitis: a prospective multicenter study. Laryngoscope. 2019;130(8):1877–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yen DM, Conley DB, O’Malley EM, Byerly TA, Johnson J. Multiple site cryoablation treatment of the posterior nasal nerve for treatment of chronic rhinitis: an observational feasibility study. Allergy Rhinol (Providence). 2020;11. doi: 10.1177/2152656720946996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krespi YP, Wilson KA, Kizhner V. Laser ablation of posterior nasal nerves for rhinitis. Am J Otolaryngol. 2020;41(3):102396. [DOI] [PubMed] [Google Scholar]
- 12.Hytonen ML, Back LJ, Malmivaara AV, Roine RP. Radiofrequency thermal ablation for patients with nasal symptoms: a systematic review of effectiveness and complications. Eur Arch Otorhinolaryngol. 2009;266(8):1257–1266. [DOI] [PubMed] [Google Scholar]
- 13.Sapci T, Sahin B, Karavus A, Akbulut UG. Comparison of the effects of radiofrequency tissue ablation, CO2 laser ablation, and partial turbinectomy applications on nasal mucociliary functions. Laryngoscope. 2003;113(3):514–519. [DOI] [PubMed] [Google Scholar]
- 14.Hultcrantz E, Ericsson E. Pediatric tonsillotomy with the radiofrequency technique: less morbidity and pain. Laryngoscope. 2004;114(5):871–877. [DOI] [PubMed] [Google Scholar]
- 15.Barnes ML, Vaidyanathan S, Williamson PA, Lipworth BJ. The minimal clinically important difference in allergic rhinitis. Clin Exp Allergy. 2010;40(2):242–250. [DOI] [PubMed] [Google Scholar]
- 16.Meltzer EO, Wallace D, Dykewicz M, Shneyer L. Minimal clinically important difference (MCID) in allergic rhinitis: agency for healthcare research and quality or anchor-based thresholds? J Allergy Clin Immunol Pract. 2016;4(4):682–688. e686. [DOI] [PubMed] [Google Scholar]
- 17.Brixner D, Meltzer EO, Morland K, Carroll CA, Munzel U, Lipworth BJ. Implication of alternative minimal clinically important difference threshold estimation methods on technology assessment. Int J Technol Assess Health Care. 2016;32(6):371–375. [DOI] [PubMed] [Google Scholar]
- 18.Glacy J, Putnam K, Godfrey S, et al. In: AHRQ comparative effectiveness reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013: Report No.: 13-EHC098-EF.
- 19.Konno A. Historical, pathophysiological, and therapeutic aspects of vidian neurectomy. Curr Allergy Asthma Rep. 2010;10(2):105–112. [DOI] [PubMed] [Google Scholar]