Abstract
Background:
Acute stress is thought to reduce goal-directed behaviour, an effect purportedly associated with stress-induced release of catecholamines. In contrast, experimentally increased systemic catecholamine levels have been shown to increase goal-directed behaviour. Whether experimentally increased catecholamine function can modulate stress-induced reductions in goal-directed behaviour and its neural substrates, is currently unknown.
Aim:
To assess whether and how experimentally induced increases in dopamine and noradrenaline contribute to the acute stress effects on goal-directed behaviour and associated brain activation.
Methods:
One hundred participants underwent a stress induction protocol (Maastricht acute stress test; MAST) or a control procedure and received methylphenidate (MPH) (40 mg, oral) or placebo according to a 2 × 2 between-subjects design. In a well-established instrumental learning paradigm, participants learnt stimulus–response–outcome associations, after which rewards were selectively devalued. Participants’ brain activation and associated goal-directed behaviour were assessed in a magnetic resonance imaging scanner at peak cortisol/MPH concentrations.
Results:
The MAST and MPH increased physiological measures of stress (salivary cortisol and blood pressure), but only MAST increased subjective measures of stress. MPH modulated stress effects on activation of brain areas associated with goal-directed behaviour, including insula, putamen, amygdala, medial prefrontal cortex, frontal pole and orbitofrontal cortex. However, MPH did not modulate the tendency of stress to induce a reduction in goal-directed behaviour.
Conclusion:
Our neuroimaging data suggest that MPH-induced increases in dopamine and noradrenaline reverse stress-induced changes in key brain regions associated with goal-directed behaviour, while behavioural effects were absent. These effects may be relevant for preventing stress-induced maladaptive behaviour like in addiction or binge eating disorder.
Keywords: Dopamine, stress, goal-directed behaviour, neuroimaging
Introduction
Stress plays an important role in the development and maintenance of maladaptive behaviours like addiction (Brewer et al., 1998; Sinha, 2001; Sinha and Jastreboff, 2013). Particularly the hypothesis of stress-induced relapse has received substantial attention (Sinha, 2001; Sinha and Jastreboff, 2013), which has been linked to deficits in instrumental learning more generally (Schwabe et al., 2011), and reduced goal-directed behaviour, that is, increased habitual responding, specifically. Habitual behaviour has been described using a wide range of theoretical frameworks, including similarity based retrieval of episodic memory (Logan, 1988), contingency encoding models (Van Dessel et al., 2019) and dual-process models (Evans, 2008). A commonly shared characteristic of such frameworks is that habitual behaviour refers to actions/choices that are not driven by their current reward value, but rather by (rewarding) characteristics of the action/choice experienced in the past.
In individuals suffering from addiction, behaviour that was originally goal-directed has progressively become more habitual and may dominate the behavioural repertoire; that is, behaviour is no longer sensitive to outcome value and is assumed to be no longer under cognitive control (Everitt and Robbins, 2005, 2016). In the context of stress, a similar shift in instrumental learning can be observed in the form of rigid and computationally frugal habits in lieu of flexible yet cognitively demanding goal-directed behaviour (Goldfarb et al., 2017; Quaedflieg et al., 2019; Schwabe and Wolf, 2009, 2010; Smeets et al., 2019; for reviews see Schwabe and Wolf, 2013; Schwabe et al., 2010b; Wirz et al., 2018).
The neural correlates of goal-directed behaviour involve a wide range of regions associated with reward value (e.g. orbitofrontal cortex; Graybiel and Grafton, 2015; Quaedflieg et al., 2019; Valentin et al., 2007), instrumental behaviour (e.g. caudate and putamen; de Wit et al., 2012; Tricomi et al., 2009; Watson et al., 2018) and cognitive control (e.g. anterior cingulate; Holroyd and Yeung, 2012). In individuals suffering from addiction, a lack of goal-directed behaviour is associated with increased activation of brain regions underlying habitual behaviour (e.g. dorsal striatum) and decreased activation of brain regions underlying goal-directed behaviour (e.g. ventral striatum and frontal cortex), compared with healthy controls (Belin et al., 2013; Vollstädt-Klein et al., 2010). This ventral-to-dorsal activation shift might reflect a neurobiological mechanism associated with stress-induced relapse. Indeed, acute stress is associated with a shift in brain activation from ventral-to-dorsal striatum, and an impaired outcome processing in prefrontal cortex can be observed too (e.g. Arnsten, 2009a; Dedovic et al., 2009; Schwabe, 2017; Schwabe et al., 2012).
It is thought that catecholamines such as dopamine and noradrenaline are crucially involved in goal-directed behaviour and habit formation (Gasbarri et al., 2014; Nelson and Killcross, 2013; Schwabe et al., 2010a). First, dopamine is known to signal positive reward prediction errors (Maes et al., 2020; Sharpe et al., 2020) and catecholamines more generally underlie motivational processes associated with approach behaviour (Berridge and Robinson, 2016; Bouret et al., 2012). Second, reductions in brain-wide catecholamine availability through acute phenylalanine/tyrosine depletion increased the habitual behaviour in healthy female participants (de Wit et al., 2011b). Similarly, increased catecholamine availability by administering L-DOPA enhanced model-based control (akin to goal-directed behaviour) (Wunderlich et al., 2012), particularly in individuals with high working memory capacity (Kroemer et al., 2019), suggesting that catecholaminergic involvement in goal-directed behaviour might involve modulation of frontal cortex functional capacity. In contrast, de Wit et al. (2011a) did not observe differences in goal-directed behaviour between Parkinson’s disease patients on- and off-dopamine agonist medication (L-DOPA). However, the patients on medication also showed higher disease severity, which was associated with worse performance. Third, such modulation of cognitive control and frontal cortex functional capacity has also been reported for catecholamine enhancement using methylphenidate (MPH) both in healthy volunteers (Pauls et al., 2012) as well as in individuals with attention deficit hyperactivity disorder (Rubia et al., 2014).
Catecholaminergic involvement in goal-directed behaviour is also relevant in the context of stress, relapse and addiction. Acute stress exposure is associated with increased cortical and striatal dopamine release, both in humans (ventromedial prefrontal cortex (vmPFC): Lataster et al., 2011; Pruessner et al., 2004, for review see: Vaessen et al., 2015) and rodents (e.g. Jackson and Moghaddam, 2004; King et al., 1997; Tidey and Miczek, 1996). Stress induced dopamine release is likely the end-result of increased phasic firing of ventral tegmental area (e.g. Holly and Miczek, 2016; Ungless et al., 2010) and locus coeruleus (Kawahara et al., 2000; Valentino and Van Bockstaele, 2008) neurones, two regions with the highest density of dopamine and noradrenaline neurones, respectively. Moreover, stress and rewards engage the same populations of ventral tegmental area dopamine neurones (Del Arco et al., 2020), and stress-induced dopamine release may trigger relapse-like behaviour in animal models of addiction (Wang et al., 2005, 2007). Thus, importantly, these studies indicate that stress-induced shifts in goal-directed behaviour may involve catecholaminergic mechanism that are typically activated by acute stress and that may contribute to stress-induced relapse. However, a definitive demonstration of the role of catecholamines (specifically, dopamine and noradrenaline) in orchestrating a shift in goal-directed behaviour under stress is currently lacking.
Here, we aimed to unravel the functional role of catecholamines in the effect of acute stress on goal-directed behaviour and associated brain activation. One hundred healthy participants underwent a stress-induction protocol, or a control procedure (Maastricht acute stress test (MAST); Smeets et al., 2012), and received oral methylphenidate (MPH) to increase synaptic catecholamine levels, or placebo (PLC), after which we assessed goal-directed behaviour and associated brain activation using a previously validated instrumental learning paradigm in combination with outcome devaluation (Hartogsveld et al., 2020). Based on previous work (Fournier et al., 2017; Hartogsveld et al., 2020; Schwabe and Wolf, 2010; Smeets et al., 2019), we expected that acute stress would reduce goal-directed behaviour, and that this would be reflected in the corresponding neural activation (e.g. increased activation in the dorsal striatum and putamen, decreased activation in the orbitofrontal cortex and anterior cingulate cortex). In light of the well-known enhancing effects of MPH on dopamine and noradrenaline release (Volkow et al., 2002), we also expected MPH to increase habitual behaviour at the expense of goal-directed behaviour. Most importantly, however, we expected that administration of MPH would modulate the effect of acute stress on goal-directed behaviour and associated neural activity (i.e. a stress-by-MPH interaction), thereby providing evidence for catecholaminergic regulation of goal-directed behaviour under stress.
Materials and methods
Participants
All participants were recruited and tested between January 2017 and May 2019. Healthy male and female young adults were recruited from the general population. Conservative power analyses (GPower; Faul et al., 2007: α = 0.05 two-tailed; 1 − β = 0.80) based on previous effects size of MAST observed in our previous studies in a 2 (MAST: stress and control) × 2 (Value: valuable, devalued) repeated measure design (η2p = 0.10; Smeets et al., 2019), indicate that the required sample size is 80 participants. One hundred participants entered the study (56 female, range 18–35, = 22.47 years, SE = 0.34) after having provided written informed consent and following a medical examination. Potential participants received a full physical examination to determine their suitability. First, exclusion criteria were assessed by means of a questionnaire. Participants were excluded in case of regular intoxications (i.e. substance/drug use in the 3 weeks prior, >24 alcoholic units per week), Body mass index outside the range of 18–28 m2/kg, pregnancy, the presence of non-removable metal objects in or on the body, and current or past medical condition. Subsequently, a standard physical medical examination, including a reassessment of the medical questionnaire, assessment of vital signs, electrocardiogram, drug screen, blood biochemistry, haematology, serology and urine-analysis, was performed by a licenced, independent physician. Any abnormalities in the results of the medical examination were evaluated at the discretion of the physician and if indicative of a medical condition (e.g. blood pressure diastolic >90 mmHg, systolic >140 mmHg, renal, pulmonary, gastrointestinal, cardiovascular, hepatic, psychiatric or neurological disease/disorder), participants were excluded from further participation. Upon full participation, participants received financial compensation for their time investment. Of the 100 participants that entered the study, data from two participants were removed due to high baseline cortisol levels (i.e. >3 SD), three participants discontinued due to personal reasons, one participant exhibited chance-level performance on the stimulus–response–outcome (S–R–O) associations (i.e. <50% correct on the final assessment during training and reminder phase), and one due to technical reasons. The study was approved by the local Medical Ethics Committee Academic Hospital/Maastricht University (nr.METC163021) and conducted in accordance with the Declaration of Helsinki and its amendments (World-Medical-Association, 1964, 1996, 2008, 2013).
Experimental design and manipulations
The study was conducted according to a 2(drug: MPH vs. PLC) × 2(MAST: stress vs. control) between-subjects partially blind design. Both participant and experimenter were blind with respect to drug administration. For practical reasons, the experimenter guided the whole test day and administered the MAST, thus the experimenter was not blind to the MAST condition. Participants were randomly allocated to one of the conditions using a computerised block-randomisation procedure taking sex and age into account for equal distribution across conditions, which resulted in the following groups: stress/MPH (n = 24, 14 female, = 22.25 years, SE = 0.63), stress/PLC (n = 21, 15 female, = 22.81 years, SE = 0.65), control/MPH (n = 24, 14 female, = 21.37 years, SE = 0.52), control/PLC (n = 24, 14 female, = 23.83 years, SE = 0.88).
Stress
The MAST (Smeets et al., 2012) was used to induce acute stress and is a reliable method to induce strong autonomic, glucocorticoid and subjective stress responses (Quaedflieg et al., 2017). The MAST combines physical stress induction, unpredictability, uncontrollability and social-evaluative nature of other stress induction protocols. In short, participants alternated between putting their hand in 2°C water for a period between 45 and 90 s and doing mental arithmetic (counting back from 2043 in steps of 17) while their faces were recorded and social-evaluative pressure (i.e. negative feedback) was provided by an experimenter unfamiliar to the participant. The control procedure was similar to the experimental procedure with the difference that water was lukewarm (36℃) and participants had to count from 1 to 25 at their own pace while no social pressure was applied. To determine individuals’ responses to the stressor, salivary cortisol samples and vital signs (heart rate (HR), systolic (SBP) and diastolic (DBP) blood pressure) were obtained prior to and following the MAST (see Figure 1 panel (b)). Subjective stress was assessed after performance of the MAST using visual analogue scales (VAS). Participants placed a vertical mark on three 10 cm horizontal lines indicating how they felt at that moment. Anchors were ‘not at all stressful’, ‘extremely stressful’; ‘not at all painful’, ‘worst pain imaginable’; ‘extremely pleasant’, ‘extremely unpleasant’.
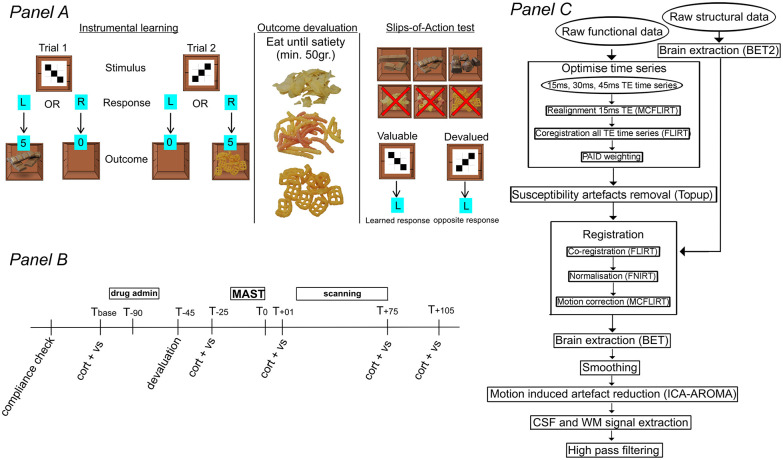
Figure 1.
Overview of the instrumental learning task, procedure and imaging processing pipeline. Panel (a) Overview of the instrumental learning task. Upon presentation of a stimulus, participants responded with a left hand or right hand button as fast as possible. If a ‘correct’ response was provided, a box opened and a virtual reward was inside (chocolate or crisp; 75% contingency) and points were collected. All ‘Incorrect responses’ resulted in an empty box and no points. Explicit S–R–O association knowledge was assessed after every two blocks. After each block, participants received a small snack (chocolate and crisp) to incentivize learning. For the slips-of-action phase, devalued rewards with a red cross superimposed on an image of potential outcomes were shown before every block. Panel (b) Day 2 overview. Vital signs and saliva samples were collected at baseline (Tbase), post-devaluation (T−25), post-MAST (T+01), post-scanning (T+75), and at the end of the test day (T+105). Tx: time in minutes relative to the end of the MAST; cort + vs: salivary cortisol sample and vital signs collection; drug admin.: administration of MPH 40 mg orally or placebo. Panel (c) fMRI processing pipeline. BET: brain extraction tool; CSF: cerebrospinal fluid; FLIRT: FMRIB linear registration tool; FNIRT: FMRIB nonlinear registration tool; ICA-AROMA: independent component analysis-based automatic removal of motion artefacts; MCFLIRT: motion correction; PAID: parallel acquired inhomogeneity desensitisation; TE: time of echo; WM: white matter.
Neuroendocrine stress response
Salivary cortisol was used to assess the neuroendocrine stress response and was collected through synthetic Salivette (Sarstedt, Etten-Leur, The Netherlands) devices. Saliva samples were stored at −20°C immediately after collection and kept until data collection was completed. Salivary cortisol levels were determined in singlet using a chemiluminescence immunoassay with high sensitivity (IBL International, Hamburg, Germany). This method produces intra- and inter-assay coefficients for cortisol below 9%. Concentration values at indicated time points are in reference to the end of MAST (see Figure 1 panel (b)). Next to these concentration values, areas under the curve were calculated by AUCg = (((Tbase + T−25)/2) × 90) + (((T−25 + T+01)/2) × 26) + (((T+01 + T+75)/2) × 76) + (((T+75 + T+105)/2) × 30) to obtain a measure of total cortisol and AUCi = (AUCg) − (Tbase × (90 + 26 + 75 + 105)) to obtain a measure for cortisol increase (Pruessner et al., 2003).
Methylphenidate
MPH (40 mg, oral) was administered to increase synaptic dopamine and noradrenaline levels in the brain during task performance in the MR scanner (i.e. 105 min after administration). Orally administered MPH immediate release formulation reaches Cmax between 60 and 120 min after administration, occupies approximately 70% of striatal dopamine transporters and plasma levels decrease to 50% after approximately 6 h (Volkow et al., 2002). Existing data suggest a wide margin of a safe dose–response range 10–90 mg (Mehta et al., 2000; Volkow et al., 2002). MPH’s actions on extracellular dopamine and noradrenaline levels among others involve blockade of dopamine and noradrenaline transporters in the striatum and frontal cortex (Montgomery et al., 2007). Furthermore, MPH has been shown to increase cognitive control (Pauls et al., 2012; Rubia et al., 2014), which is crucial for successful goal-directed behaviour. Both MPH and acute stress are associated with changes in ventral tegmental area and locus coeruleus cell firing (e.g. Holly and Miczek, 2016; Karim et al., 2017), suggesting a degree of overlap in neurochemical mechanisms associated with modulation of goal-directed behaviour under stress and in response to stimulants.
Instrumental learning task
A four-stage instrumental learning task administered across 2 days was used to assess goal-directed and habitual behaviour (Smeets et al., 2019, see Figure 1 panel (a)). Participants learned six S–R–O associations by trial-and-error on day one (stage 1). Visual stimuli consisted of abstract black and white block figures in a 3 × 3 grid on the outside of a box to which participants pressed a left- or right-hand button as fast as possible. From a collection of eight chocolate and eight crisps type rewards presented as images on a single A4-sized paper, participants selected six preferred food type outcomes beforehand (three chocolate and three crisp) that served as rewards/outcomes. Following a ‘correct’ response, the box opened, and both a virtual reward (chocolate or crisp type reward) and points (ranging from 5 to 1, depending on reaction time) were presented. ‘Incorrect’ responses lead to an empty box and no points. Left/right button presses were associated with the optimal outcome for 3/6 stimuli. A contingency rate of 75% was implemented; that is, in 25% of all correct button presses no reward was presented and no points were collected. In the learning stage, participants completed eight blocks of 24 trials, totalling 192 trials. After each block of trials, participants consumed a small snack (chocolate or crisp) to make the receipt of rewards ‘feel’ more realistic, and to incentivize learning of the S–R–O associations, explicit knowledge of which was assessed after every second block of trials. Participants were instructed to collect as many rewards and points as possible.
On day 2, participants performed a reminder task with the same S–R–O associations (stage 2, two blocks of 24 trials), after which explicit knowledge of the S–R–O associations was assessed. Following the reminder task, one reward type (crisp or chocolate) was selectively devalued (stage 3). Fifty grams of chocolate or crisps were initially presented, which participants were required to eat. A subsequent 200 g were presented, and participants were urged to eat as much as possible until satiety was reached. The number of grams eaten by the participant was recorded. Following this devaluation approach, two 100 mm VASs measured the extent to which participants felt hungry (anchors: not at all hungry–very hungry) and felt like eating something tasty (anchors: not at all–very much so). Distance from the start of the line to the markings were measured and converted to a percentage of the total line.
Finally, the slips-of-action (SOA) task (six blocks of 24 trials, totalling 144 trials) was performed in the MR scanner (stage 4; day 2). For non-devalued outcomes, participants were instructed to press buttons associated with presentations of a food reward; for stimuli that were associated with food rewards that participants sampled until they were satiated in stage 3 (i.e. devalued outcomes), participants were instructed to press the opposite button (compared with previously learnt actions). Within the context of our paradigm, opposite button presses for these particular stimuli are considered goal-directed responses since they are indicative of an action associated with avoidance of the devalued reward and is in accordance with the new instructions that contrasts the presumed dominant learned response. In addition to appetitive devaluation of the rewards during stage 3, we aimed to cognitively devalue the outcomes during stage 4 by showing images of all outcomes at the start of every block of trials, and selectively superimposing a red cross on the devalued outcomes. Learned responses served as the primary outcome measure, which were defined as the response to a stimulus that leads to a valuable or devalued outcome (i.e. a food reward). Feedback was not provided during stage 4 to prevent relearning of associations. Inter-trial-intervals were jittered by applying a variable interval between 6 and 10 s following stimulus offset.
Procedures and testing
Participants were instructed on each day of the 2 day procedure to visit our facilities well rested, not having performed any strenuous exercise within the previous 24 h, not having used any over-the-counter drugs in the past 2 days or prescribed drugs in the past 3 weeks, not having consumed alcohol since 19.00 h. the day before or caffeine-containing products or food the last 3 h, or smoked during the last 2 h. No participant reported any violations of these requirements upon enquiry at the start of the test day. On day 1, participants learned the S–R–O associations. On day 2 (i.e. the following day) participants received either oral capsules of PLC or MPH (T−90 relative to end of MAST) that had to be swallowed whole assisted by plain water. Selective devaluation (T−45) was achieved by having participants eat until satiety. At T−15 the MAST was performed (between 13.30 or 14.30 h to avoid high cortisol levels observed during morning hours) where after subjective levels of stress, pain and unpleasantness were assessed. MR scanning (T+15) was performed lasting 1 h. Participants performed the SOA during the peak cortisol and MPH levels. Structural, resting state brain activation and arterial spin labelling images were also recorded (all to be reported elsewhere). Finally, three cognitive tasks were performed typically sensitive to frontal lobe dysfunctions (i.e. N-back task, stop signal task, and Iowa gambling task; all to be reported elsewhere).
Imaging data acquisition and processing
Functional imaging was performed using a Siemens 3-Tesla Prisma MRI scanner. Each volume consisted of 42 slices, consisting of 3 mm isotropic voxels in a 224 mm field of view. Slice thickness was 3 mm with no gap between the slices. TR = 1000 ms and a multi-echo sequence was used to optimise the signal for each voxel offline (TE 1 = 5 ms; TE 2 = 29.93 ms; TE 3 = 44.86 ms). Flip angle was 60° and a multi-band acceleration factor of three was used. For co-registration, high-resolution T1-weighted structural images were obtained using an MPRAGE sequence resulting in 256 slices and 0.7 mm isotropic voxels in a 224 mm field of view. TR = 2400 ms, TE = 2.34 ms and flip angle = 8°.
Analyses were performed using custom and FMRIB’s Software Library (FSL; Jenkinson et al., 2012) scripts (see Figure 1 panel (c) for an overview). First, images from the three echo data were combined to construct a single optimised 4D image in which the TE with the best signal-to-noise ratio was determined and used per voxel. Therefore, realignment was performed using motion correction FMRIB linear registration tool (MCFLIRT) (Jenkinson et al., 2002) for the first echo data and applied to other two echo data by registering them using FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002). Next, parallel-acquired inhomogeneity desensitisation (PAID; Poser et al., 2006) weighting was performed by splitting the first 30 volumes from the time series and minimally smoothing them using a 2 mm FWHM Gaussian kernel to assist in PAID weight estimation. Of the 30 volumes a mean image and standard deviation were calculated, and the mean was multiplied by the echo time and divided by the standard deviation. Values were then adjusted such that the value per voxel is 1. Then, individual images are divided by the sum of the images and the weights are applied to the individual echoes. Finally, the weighted echoes are summed to generate a weighted time series. The same processing was applied to reverse phase encoded acquired images with distortions in opposite directions and which were used to determine susceptibility-induced off-resonance field to correct for the distortions using FSL’s top up (Andersson et al., 2003; Smith et al., 2004). These PAID weighted images were then subjected to a pre-processing pipeline using FSL6.0. The pipeline included brain extraction of the anatomical images using brain extraction tool (BET2; Smith, 2002) assisted by using coordinates of the massa intermedia for accurate extraction and manual parameter setting for each brain for high quality results.
Functional data were further pre-processed using FSL FMRI expert analysis tool (FEAT) and consisted of the following steps: removal of the first three volumes of the functional data, a four pass rigid-body motion correction (MCFLIRT; Jenkinson et al., 2002), co-registration of the functional data with the anatomical data using FLIRT and normalisation to MNI space using FNIRT (Andersson et al., 2010). The pre-processed functional data were inspected manually for exceedance of motion limits (framewise displacement >1 mm), which none of the participants exceeded. Subsequently the data were smoothed with a 5 mm FWHM Gaussian kernel, and independent component analysis-based automatic removal of motion artefacts (ICA-AROMA) (Pruim et al., 2015) was applied to remove head motion related noise. ICA-AROMA is an automatic procedure that uses independent component analysis to identify components representing head motion generated noise. Subsequently it removes the components from the data using least squares regression. Next, data were high pass filtered (>0.008 Hz). Finally, to account for signal from white matter and cerebrospinal fluid (CSF), white matter and CSF masks were created by segmenting the anatomical data. The masks were co-registered with the functional data using the inverse of the previously created transformation matrix and used to extract the signal from the time series.
Neuroimaging data analysis
For one participant data were lost due to a technical error. Therefore, imaging data were analysed for 92 participants. For every participant a statistical model of the BOLD response was constructed that consisted of regressors for baseline epochs, Valuablecorrect (action associated with a non-devalued outcome), Valuableincorrect (action associated with avoiding a non-devalued outcome), Devaluedcorrect (action associated with avoiding a devalued outcome), Devaluedlearned-response (action associated with a devalued outcome), white matter and CSF signal averaged over voxels, and first derivatives. Subsequently, the BOLD signal associated with two trial types (Valuablecorrect and Devaluedlearned-response) was contrasted with the BOLD signal associated with baseline epochs. Baseline epochs for every trial were defined as the final time period of the presentation of the inter-trial fixation cross following the presentation of the stimulus. The duration of the baseline epoch for every individual trial was the same duration as the reaction time (RT) on the previous trial. Therefore, every trial epoch is compared with a baseline epoch of identical duration, but never overlapped. To determine significant trial-baseline activation differences, FEAT (Woolrich et al., 2001, 2004) was used with a p < 0.05 family wise error (FWE) corrected as threshold and threshold free cluster enhancement (TFCE) applied. The resulting contrast parameters of estimate (COPEs) were normalised to MNI space (FNIRT) and merged into a 4D image. A second level analysis was performed in which the interaction between MAST and drug was determined on activation representing goal-directed behaviour (Valuablecorrect − Devaluedlearned-response), i.e. trials in which the participant erroneously responded with a learned response to a devalued outcome (Devaluedlearned-response) contrasted with trials in which participants correctly responded with a learned response to a non-devalued outcome (Valuablecorrect). For this contrast, positive values suggest greater activity for valuable outcome trials, while negative values indicate greater activity for Devaluedlearned-response trials.
A drug-by-MAST interaction was assessed within a mask of whole brain grey matter with permutation testing using FSL’s randomise with 10,000 permutations and p < 0.01 FWE-corrected as threshold and TFCE applied. Significant clusters were determined by thresholding the resulting statistical map using a minimum cluster size of 25 voxels. To further determine how MPH modulates MAST-induced brain activation associated with (reduced) goal-directed behaviour, follow-up analyses of the interaction were performed to assess the effect of MAST separately in PLC and in MPH groups using the same permutation testing procedure (p < 0.05, FWE corrected, TFCE applied). The simple main effect of MAST in PLC was specified by stress < control and stress > control t-contrasts indicating less and more activation in the stress condition, respectively. Finally, to further characterise the interaction and detect potential modulation of the effect of stress by MPH, conjunction statistical maps were formed between the p-values statistical maps associated with t-contrasts for stress > control and for the bidirectional F-test of MAST in the MPH-groups, and between the p-values statistical maps associated with stress < control t-contrast and bidirectional F-test of MAST in the MPH-groups (cluster threshold 25 voxels). Beta values for Valuablecorrect − Devaluedlearned-response in statistically significant clusters were extracted and displayed.
In the main text, we confine our results to regions previously associated with instrumental behaviour (de Wit et al., 2012; Tricomi et al., 2009; Valentin et al., 2007; Watson et al., 2018), that is, orbitofrontal cortex (OFC), vmPFC, anterior cingulate (ACC), paracingulate, premotor cortex (PMC), middle temporal gyrus, putamen, caudate, operculum, amygdala and insula, as determined using Harvard-Oxford cortical structural, Harvard-Oxford subcortical structural and Jeulich Histological atlases as implemented in FSLeyes, FSL6.0.3. A complete overview of the drug-by-MAST interaction on brain activation is reported in the Supplemental Material (Table S2).
Finally, for significant clusters average COPE values for Valuablecorrect − Devaluedlearned-response were correlated with a devaluation sensitivity index (DSI: e.g. Watson et al., 2018) defined as the difference between percentage learned response on valuable and devalued trials for every participant-group separately. Positive correlations between positive difference in activation (Valuablecorrect − Devaluedlearned-response) and DSI is interpreted as that brain area being associated with the tendency to respond more for valuable outcomes compared with devalued outcomes. Only participants with an SOA score of at least 1 were considered for this analysis (n = 82). Given the skewed distribution of the Valuablecorrect − Devaluedlearned-response Spearman’s correlation was used and Bonferroni’s multiple comparisons correction was applied per group (p = 0.05/11, α = 0.005).
Data and statistical analysis
Behavioural data were checked for outliers (±3SD) and non-normality using the Shapiro-Wilk tests and transformed by taking the natural log of the values whenever needed. Presence of outliers in the number of learned responses in the instrumental learning task were determined per condition (stress, drug and value), per block (1–6). No outliers in the number of learned responses were detected. As there were no significant differences between blocks and the blocks did not interact with any other factor, all blocks were concatenated by averaging the scores over blocks. α < 0.05 was regarded as statistically significant. In case of violations of the sphericity assumptions as shown by significant Mauchly’s test, Greenhouse–Geisser corrected values are reported. For all significant analyses of variance (ANOVAs) partial eta squared (η2p) are reported as a measure of effect size (Fritz et al., 2012).
MAST (stress and control), drug (MPH and PLC) and time (Tbase, T−25, T+01, T+75 and T+105) effects on cortisol levels and physiological stress measures (HR, SBP and DBP) and the interaction between these factors were assessed using a repeated measures ANOVA with time as repeated measure. Physiological data for one participant were not recorded prior to MAST onset and therefore unavailable. Drug and MAST effects on subjective stress were assessed in a MAST (stress and control) × drug (MPH and PLC) model using univariate ANOVAs. Data for seven participants for the subjective measures were missing. The amount of food (weighted in gram) consumed, and measures of feeling hungry (%VAS line length), and feeling like eating something tasty (%VAS line length) during stage 3 (outcome devaluation) were compared between conditions using a univariate ANOVA with MAST (stress and control) and drug (PLC and MPH) as between-subject factors. For learned responses during the SOA test, a repeated measures ANOVA was performed with MAST (stress and control ) and drug (MPH and PLC) as between-subject factors and value (valuable and devalued) as repeated measure. Only significant ANOVAs were followed up by post-hoc tests. All data except fMRI data were analysed using IBM SPSS statistics 24.
Results
Physiological and subjective stress
Physiological stress
For statistical details of all simple effects see Figure 2 and Table 1. Elevated cortisol levels were observed following the MAST in the stress relative to the control condition until 75 min after the stress induction (MAST × time: F(2.898,255.003) = 3.26, p = 0.023 η2 p = 0.036). Drug did not change the effect of MAST on cortisol levels over time or across all time points (drug × MAST × time: F(2.898,255.003) = 0.714, p = 0.540; drug × MAST: F(1,88) = 3.46, p = 0.066). Elevated cortisol levels were observed following MPH administration until 195 min after (i.e. 105 min after stress induction) compared with PLC (drug × time: F(2.898,255.003) = 3.18, p = 0.026, η2 p = 0.035). Finally, sex did not modulate any of the effects of drug or MAST or their interaction (all Fs(2,64) < 0.40, p > 0.671).
Figure 2.
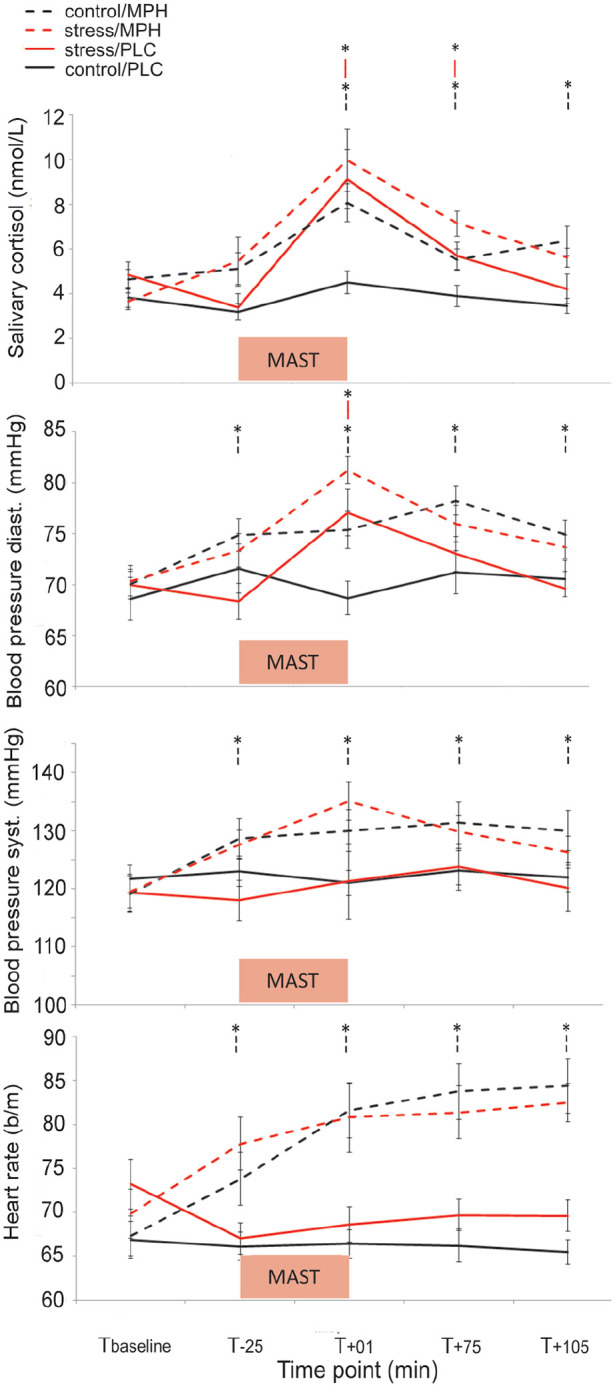
Effectiveness of stress induction. Data show untransformed means (±SE) of salivary cortisol (nmol/L), diastolic- and systolic blood pressure (mmHg), and heart rate (b/min) over time (Tbaseline, T−25, T+01, T+75, T+105). Both acute stress (red lines) and MPH (dashed lines) increased cortisol levels and diastolic blood pressure, but no drug-by-MAST interactions were observed. MPH additionally increased systolic blood pressure and heart rate, while stress did not. Comparisons are simple effects following significant MAST-by-time and drug-by-time interactions.
*p < 0.05 compared with control/PLC.
Table 1.
Inferential statistics of MAST and drug per time point.
| MAST | Drug | |||||||
|---|---|---|---|---|---|---|---|---|
| F | df | p | η2 p | F | df | p | η2 p | |
| Cortisol | ||||||||
| Tbase | 0.05 | 1,90 | 0.816 | – | 0.01 | 1,90 | 0.972 | – |
| T−25 | 0.03 | 1,91 | 0.854 | – | 7.50 | 1,91 | 0.007* | 0.076 |
| T+01 | 7.96 | 1,91 | 0.006* | 0.080 | 8.10 | 1,91 | 0.005* | 0.082 |
| T+75 | 10.69 | 1,91 | 0.002* | 0.105 | 12.84 | 1,91 | <0.001* | 0.124 |
| T+105 | 0.11 | 1,91 | 0.736 | – | 25.39 | 1,91 | <0.001* | 0.218 |
| Blood pressure diastolic | ||||||||
| Tbase | 0.26 | 1,91 | 0.611 | – | 0.37 | 1,91 | 0.543 | – |
| T−25 | 1.366 | 1,90 | 0.246 | – | 4.67 | 1,90 | 0.033* | 0.049 |
| T+01 | 15.24 | 1,91 | 0.001* | 0.143 | 8.77 | 1,91 | 0.004* | 0.088 |
| T+75 | <0.01 | 1,91 | 0.954 | – | 8.05 | 1,91 | 0.006* | 0.081 |
| T+105 | 0.39 | 1,91 | 0.532 | – | 7.98 | 1,91 | 0.006* | 0.081 |
| Blood pressure systolic | ||||||||
| Tbase | 0.13 | 1,91 | 0.724 | – | 0.19 | 1,91 | 0.661 | – |
| T−25 | 0.75 | 1,90 | 0.390 | – | 6.08 | 1,90 | 0.016* | 0.063 |
| T+01 | 0.57 | 1,91 | 0.451 | – | 8.00 | 1,91 | 0.006* | 0.081 |
| T+75 | <0.01 | 1,91 | 0.945 | – | 5.01 | 1,91 | 0.028* | 0.052 |
| T+105 | 0.63 | 1,91 | 0.428 | – | 4.73 | 1,91 | 0.032* | 0.049 |
| Heart rate | ||||||||
| Tbase | 2.93 | 1,91 | 0.090 | – | 0.25 | 1,91 | 0.620 | – |
| T−25 | 1.09 | 1,90 | 0.299 | – | 14.18 | 1,90 | <0.001* | 0.136 |
| T+01 | 0.12 | 1,91 | 0.735 | – | 23.23 | 1,91 | <0.001* | 0.203 |
| T+75 | 0.10 | 1,91 | 0.755 | – | 31.73 | 1,91 | <0.001* | 0.259 |
| T+105 | 0.30 | 1,91 | 0.583 | – | 50.34 | 1,91 | <0.001* | 0.356 |
p < 0.05.
DBP was elevated immediately following the MAST, but not following the control condition (MAST × time: F(3.522,309.931) = 9.24, p < 0.001, η2 p = 0.095; see Figure 2 and Table 1), irrespective of drug (drug × MAST × time: F(3.522,309.931) = 0.81, p = 0.506). Drug did not affect DBP changes over time (drug × time: F(3.522,309.931) = 2.04, p = 0.097), but MPH did increase DBP values after administration (drug: F(1,88) = 8.26, p = 0.005, η2 p = 0.086).
Stress did not affect SBP over time (MAST × time: F(2.951,259.730) = 1.39, p = 0.248) or across time points (MAST: F(1,88) = 0.14, p = 0.707). Drug did not modulate the effect of stress on SBP over time (drug × MAST × time: F(2.951,259.730) = 0.721, p = 0.538) or across time (drug × MAST: F(1,88) = 0.11, p = 0.746). SBP was higher on all time points after MPH administration compared with placebo, but not before MPH (drug × time: F(2.951,259.730) = 5.90, p = 0.001, η2 p = 0.063).
Stress did not affect HR over time (MAST × time: (F(3.138,276.108) = 0.87, p = 0.461), independent of drug (drug × MAST × time: F(3.138,276.108) = 1.40, p = 0.243) nor across all time points (MAST: F(1,88) = 0.75, p = 0.390), independent of drug (MAST × drug: F(1,88) = 0.55, p = 0.461). HR was higher on all time points after MPH compared with placebo, but not before drug administration (drug × time: F(3.138,276.108) = 19.98, p < 0.001, η2 p = 0.185).
All in all, both the stress and MPH increased cortisol levels and diastolic blood pressure significantly, but MPH did not modulate the effect of stress. MPH also increased systolic blood pressure and heart rate, while stress did not.
Subjective stress
Participants reported increased feelings of stress (F(1,84) = 162.16, p < 0.001, η2 p = 0.659), pain (F(1,84) = 356.13, p < 0.001, η2 p = 0.809) and unpleasantness (F(1,84) = 107.79, p < 0.001, η2 p = 0.562) post-MAST, compared with the control condition, irrespective of drug (drug × MAST: all Fs < 2.61, p > 0.110). MPH reduced feelings of pain (F(1,84) = 4.58, p = 0.035, η2 p = 0.052), but did not affect feelings of stress or unpleasantness (F(1,84) < 0.03, p = 0.954 and F(1,84) = 3.59, p = 0.061, respectively).
Goal-directed behaviour
S–R–O Learning
As expected, participants in all conditions learned the S–R–O associations as demonstrated by increased performance over blocks for the correct (rewarded) button press and reaching asymptotic levels. Instrumental learning increased over the course of the eight learning phase blocks (Block: F(3.763,334.882) = 111.76, p < 0.001, η2 p = 0.557; See Supplemental Material (Figure S1)). No drug-by-MAST interaction on instrumental learning over blocks was observed (block × drug × MAST: F(3.763,334.882) = 0.90, p = 0.462). Correct button presses did not differ over blocks between participants in the stress versus control condition (block × MAST: F(3.763,334.882) = 1.34, p = 0.257), similar to participants in both drug conditions (block × drug: F(3.763,334.882) = 0.87, p = 0.477). No drug-by-MAST interaction was observed when collapsing performance across blocks (MAST × drug: F(1,89) = 0.52, p = 0.473).
S–R–O reminder
Groups did not differ on the percentage of correct responses during the reminder phase (see Supplemental Material (Figure S1)). The drug-by-MAST interaction was not significant (F(1,88) = 0.08, p = 0.773), nor were the main effects of MAST (F(1,88) = 1.17, p = 0.283) or drug (F(1,88) = 0.07, p = 0.797), indicating that all groups performed similarly during this phase of the task.
Devaluation
The amount of food consumed during the devaluation procedure (in grams) did not differ between groups (all main and interaction effects involving drug and MAST: Fs(1,89) < 1.34, all ps > 0.251). Moreover, no differences in the feeling of being hungry were observed between groups (drug × MAST: F(1,85) = 0.63, p = 0.431), drug: F(1,85) = 0.03, p = 0.875, MAST: F(1,85) = 0.23, p = 0.636).
A drug-by-MAST interaction was observed for the feeling of ‘wanting to eat something’ (F(1,84) = 4.01, p = 0.048, η2 p = 0.046). Follow-up analysis revealed that while no drug effect was observed in the control condition (F(1,44) = 0.13, p = 0.726), in the stress condition, stressed participants who received MPH felt more like eating something than stressed participants who received PLC (F(1,40) = 6.10, p = 0.018, η2 p = 0.132).
Slips-of-action test
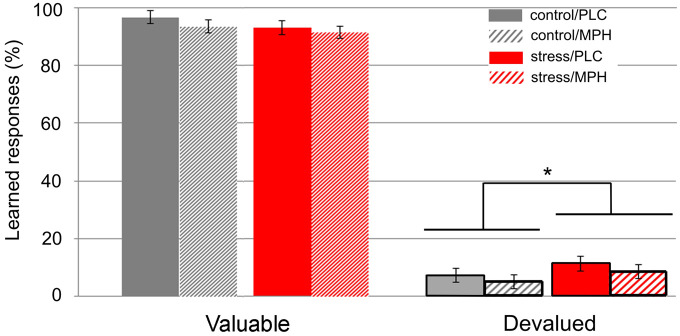
MPH did not modulate the effect of MAST on learned responses for valued versus devalued outcomes (value × MAST × drug: F(1,89) = 0.03, p = 0.873). The differential effect of stress on learned responses for valuable versus devalued outcomes approached significance (value × MAST: F(1,89) = 3.91, p = 0.051, η2 p = 0.042). If stress reduces goal-directed behaviour, then these effects should be most pronounced for learned responses to devalued outcomes. Indeed, exploratory simple effects analyses revealed that participants in the stress condition exhibited an increased tendency to provide learned responses for devalued outcomes compared with controls (F(1,91) = 5.45, p = 0.022 η2 p = 0.056), while stress versus control participants did not differ in learned responses for valuable outcomes (F(1,91) = 1.83, p = 0.180). MPH did not affect learned responses for valuable versus devalued outcomes (value × drug: F(1,89) = 0.15, p = 0.700; Figure 3).
Figure 3.
Performance on the slips-of-action task. Stress increased learned responses (%) to devalued, but not to valuable outcomes independent of drug. Note that learned responses to devalued rewards indicate reduced goal-directed behaviour.
*p < 0.05.
Imaging results
Task related network: Valuable correct – Devalued learned-response
To explore the network of brain areas involved in goal-directed behaviour (i.e. positive or negative activity difference for Valuablecorrect – Devaluedlearned-response), a second level ANOVA was performed with these first level contrasts as input. Main effects of MAST, drug, their interaction, and the variance not explained by the main or interaction effects were modelled. Brain areas in sensorimotor and executive control networks were identified, including bilateral frontal pole/middle frontal gyrus, anterior cingulate, primary motor cortex, bilateral lateral occipital cortex, precuneous, insula, cerebellum and putamen, which were all consistent with previous work (Watson et al., 2018). See Supplemental Material (Figure S2 and Table S1).
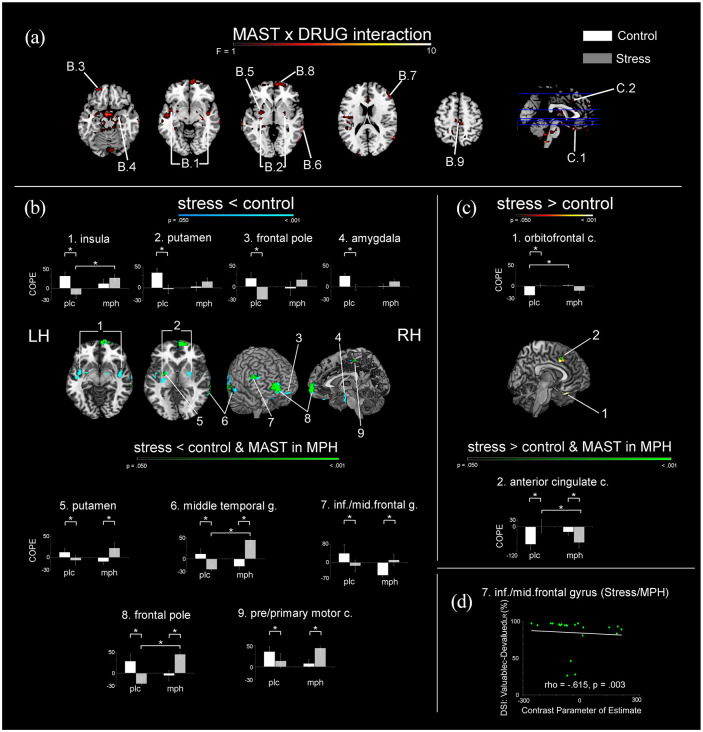
Drug-dependent effects of stress on activation in regions implicated in goal-directed control
The analysis of the MAST-by-drug interaction for the Valuablecorrect − Devaluedlearned-response contrast revealed several significant clusters (Figure 4 panel (a), Table 2). The subsequent post-hoc analyses per level of the factor drug (PLC and MPH) revealed nine clusters in which stress reduced the positive activation difference (i.e. reduced Valuablecorrect > Devaluedlearned-response; Figure 4 panel (b)). In two clusters, stress reduced the negative activation difference (i.e. reduced Valuablecorrect < Devaluedlearned-reponse; Figure 4 panel (c)).
Figure 4.
Drug-by-MAST interactions for brain activation related to goal-directed behaviour. Panel (a) Clusters of activation showing a significant drug-by-MAST interaction. Panel (b) Stress-induced reductions for positive Valuablecorrect – Devaluedlearned-responses activation differences. Significant clusters in blue indicate reduced positive activation difference only in participants who received placebo; green colours indicate the conjunction with the effect of stress after MPH administration. Panel (c) Stress-induced diminished negative difference following Valuablecorrect – Devaluedlearned-response in participants who received PLC (hot colours) and the conjunction with effects of stress after MPH (green). All contrasts are thresholded at p < 0.05, FWE, TFCE, minimal cluster size 25 voxels. Bar graphs display extracted cluster beta values ( ± SE). Panel (d) Negative correlation between the devaluation sensitivity index (DSI: %Valuablecorrect − %Devaluedlearned-response) and the extracted beta values for the inferior/middle frontal gyrus of stressed participants who received MPH, which indicates that a larger positive activation difference between Valuablecorrect and Devaluedlearned-response is associated with less goal-directed behaviour.
COPE: Contrast parameter of estimate.
Table 2.
Drug dependent effects of stress on brain activation.
| Area number Figure 4 | Brain area | Lat. | BA | Cluster size (voxels) | Peak p-value (FWE corrected) | Peak coordinates (MNI) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| B | Stress reduced activation after PLC | |||||||
| 1 | Insula | L | 48 | 207 | <0.001 | −42 | −4 | −6 |
| R | 48 | 81 | <0.001 | 42 | −8 | −14 | ||
| 2 | Putamen | L | 48 | 93 | <0.001 | −30 | −18 | 2 |
| R | 35 | 53 | <0.001 | 33 | 61 | 36 | ||
| 3 | Frontal pole | L | 11 | 39 | 0.002 | −24 | 52 | −16 |
| 4 | Amygdala | R | 50 | <0.001 | 20 | −8 | −20 | |
| Stress reduced activation after PLC and affected activation after MPH | ||||||||
| 5 | Putamen | L | 34 | 88 | 0.002 | −28 | −4 | −8 |
| 6 | Middle temporal gyrus | R | 21/37 | 85 | 0.005 | 68 | −48 | −4 |
| 7 | Inferior/Middle frontal gyrus | R | 45 | 62 | 0.002 | 54 | 36 | 20 |
| 8 | Frontal pole | R | 10 | 274 | <0.001 | 8 | 64 | −2 |
| 9 | Pre/primary motor cortex | R | 4 | 38 | 0.002 | 8 | −28 | 54 |
| C | Stress increased activation after PLC | |||||||
| 1 | Orbitofrontal cortex | L | 11 | 45 | <0.001 | −20 | 18 | −26 |
| Stress increased activation after PLC and affected activation after MPH | ||||||||
| 2 | Anterior cingulate cortex | R | 32 | 39 | 0.018 | 4 | 22 | 38 |
Clusters of activation showing MPH-dependent activation differences between stress and control. Section B lists clusters in which stress reduced brain activation associated with goal-directed behaviour (Valuablecorrect − Devaluedlearned-response), both after PLC administration only and in conjunction with effects of stress following MPH. Section C lists clusters in which stress increased activation associated with goal-directed behaviour, both after PLC only and in conjunction with effects of stress after MPH administration.
L: left; R: right; Lat.: laterality; BA: Brodmann area; FWE: familywise error; MNI: Montreal Neurological Institute; PLC: placebo; MPH: methylphenidate
With regard to the positive activation difference, in 4/9 clusters (bilateral insula, bilateral putamen, left frontal pole and right amygdala; Figure 4 panel (b), top row) stress reduced brain activation associated with goal-directed behaviour only in participants who received placebo. In these regions, no effect of stress on brain activation associated with goal-directed behaviour was observed in participants who received MPH. For the remaining five clusters (left putamen, right middle temporal gyrus, right inferior/middle frontal gyrus, frontal pole and right pre-/primary motor cortex; Figure 4 panel (b), bottom row), stress increased brain activation associated with goal-directed behaviour in participants who received MPH.
Importantly, using t-contrasts, we observed that MPH reversed the effect of acute stress (relative to the effect of stress observed in the PLC group) in right middle temporal gyrus and frontal pole; in these regions, the positive activation difference for Valuablecorrect versus Devaluedlearned-reponse trials was greater for participants in the MPH/stress condition compared with participants in the PLC/stress condition, and participants in the MPH/stress condition did not statistically differ from participants in the PLC/control condition. We observed a similar pattern in the bilateral insula, but in contrast, here MPH/stress and MPH/control participants did not statistically differ from each other.
With regard to the two negative activation difference clusters, we observed that stress was associated with a reduction in the activation difference between Valuablecorrect and Devaluedlearned-response in orbitofrontal cortex and anterior cingulate cortex. In the right anterior cingulate, MPH successfully reversed the effect of stress on goal-directed behaviour associated activation: the negative activation difference for Valuablecorrect versus Devaluedlearned-reponse trials was smaller for PLC/stress than for MPH/stress participants, and PLC/control and MPH/stress participants did not differ from each other.
For exploratory purposes, a full list of all peak voxels that were not a priori associated with instrumental behaviour (goal-directed or habitual), but that did show significant effects of MAST (in PLC and/or MPH groups) is presented in Supplemental Material (Table S2). The effect of MPH in control (i.e. no-stress) participants is reported in Supplemental Material (Figure S3 and Table S3).
Associations between brain activity and goal-directed behaviour
Correlations between brain activation associated with goal-directed behaviour (contrast Valuablecorrect − Devaluedlearned-responses) and the percentage of learned responses for valuable−devalued trials, which is considered to be a measure of sensitivity to outcome devaluation (DSI; Watson et al., 2018), were performed per group, per significant cluster. Only the negative correlation for the inferior/middle frontal gyrus in stressed participants who received MPH remained significant after correcting for multiple comparisons (ρ = −0.615, p = 0.003; see Figure 4 panel (d)). All other correlations were not significant (p’s > 0.006)
Discussion
We aimed to investigate how catecholamines contribute to stress-induced changes in goal-directed behaviour and associated brain activation, using a combination of experimental stress induction and MPH administration. The MAST successfully increased subjective ratings and objective (i.e. salivary cortisol levels and vital signs) measures of stress, and MPH increased salivary cortisol levels, vital signs and decreased feelings of pain, suggesting that both manipulations were successful.
Acute stress seemed to increase the tendency of participants to exert actions (button presses) associated with obtaining a devalued outcome (i.e. ‘learned responses’), representing a reduction in goal-directed behaviour. Performance on trials involving non-devalued outcomes, however, were similar across all groups, suggesting that acute stress was not associated with more general impairments in instrumental behaviour. The observation that stress tended to reduce goal-directed behaviour corroborates previously observed effects of stress on instrumental behaviour (e.g. Quaedflieg et al., 2019; Smeets et al., 2019, for review see Schwabe and Wolf, 2011), and action-outcome learning specifically (e.g. Otto et al., 2013).
Contrasting trials that presumably involve more (learned responses for non-devalued outcomes) and less (learned responses for devalued outcomes) goal-directed behaviour, we observed activity differences in, among others, insula, putamen, anterior cingulate and lateral prefrontal cortex, consistent with previous work (e.g. de Wit et al., 2012; Watson et al., 2018). Activation of the putamen and insula has been consistently observed in studies using outcome devaluation paradigms (Balleine and Dickinson, 2000; de Wit et al., 2012; Watson et al., 2018). The putamen has been argued to track outcome probabilities (Brovelli et al., 2011) and putamen and insula both encode aspects of reward value (Peterson and Seger, 2013; Smith et al., 2009). As part of the salience network, the insula moreover plays a role in assigning incentive value to outcomes based on saliency (Balleine and Dickinson, 2000), facilitates action selection (Oldham et al., 2018) and has previously been implicated in habitual behaviour control (Watson et al., 2018). These reports are in line with our observed finding of greater activation in these regions during learned responses for valuable compared with devalued outcomes.
Acute stress was associated with widespread reductions in activation differences in regions associated with goal-directed behaviour, including bilateral putamen, insula, inferior/middle frontal gyrus and right amygdala. It is thought that less successful goal-directed behaviour is associated with relatively high putamen activation for devalued rewards (Graybiel and Grafton, 2015). Reduced activity in OFC for actions with greater outcome value is also consistent with stress-induced reductions in reward-related medial PFC responses (Ossewaarde et al., 2011), and changes in OFC activation during simultaneous modulation of glucocorticoid and noradrenaline systems (Schwabe et al., 2012). Differences in stress-induced reductions in OFC and ACC activation associated with goal-directed behaviour may contribute to reduced differentiation in judgement of reward/outcome (Graybiel and Grafton, 2015; Quaedflieg et al., 2019), a reduced ability to inhibit learned responses (Verbruggen and Logan, 2008) and impaired response conflict resolution (Botvinick et al., 2009) under stress, which may thus result in the use of ‘habitual’ strategies.
Importantly, although MPH did not modulate task performance, pre-treatment with MPH did prevent a stress-induced shift in brain activation associated with goal-directed behaviour. In the insula, middle temporal gyrus, frontal pole and anterior cingulate cortex, stressed participants who received MPH displayed similar activation levels compared with non-stressed participants who received PLC (while activation levels differed from participants in the stress/PLC and no-stress/MPH conditions). It is well known that acute stress increases dopamine release in cortical and striatal regions (Hernaus et al., 2015; Nagano-Saito et al., 2013; Vaessen et al., 2015). Moreover, L-DOPA administration modulates model-based control of behaviour (Wunderlich et al., 2012), and administration of dopaminergic agonists and antagonists have been linked to selective changes in sensitivity to positive and negative outcomes (Frank et al., 2004). On the other hand, MPH effects on brain activation may also be partly noradrenergic, since MPH has a higher binding potential for noradrenaline transporters compared with the dopamine transporter (Hannestad et al., 2010; Volkow et al., 2002). Moreover, noradrenaline increases seem to normalise dorsal striatum-mPFC connectivity following rewards preceded by cues, thereby enhancing the discrimination between reward and non-rewarded cues in ADHD (Furukawa et al., 2020). Finally, the negative correlation between the DSI as a measure of goal-directed tendencies and the activation difference between Valuablecorrect and Devaluedlearned-response in the inferior/middle frontal gyrus in stressed participants who received MPH may be indicative of MPH contributing to goal-directed behaviour under stress. Taken together, these results suggest that both MPH-induced dopamine and noradrenaline increases may have contributed to normalisation of brain activation associated with goal-directed behaviour under stress.
One potential mechanism-of-action of the observed MPH effects under stress may involve modulation of signal-to-noise ratio in cortical networks. Dopamine and noradrenaline jointly control the signal-to-noise ratio of neural activity in frontal cortical networks, associated with optimal cognitive performance (e.g. Arnsten, 2009a; Vijayraghavan et al., 2007), and acute stress reduces PFC signal-to-noise ratio (Arnsten, 2009b), which, in the current study, could be reflected by reduced activation differences between learned responses for valuable and devalued outcomes under stress. The administration of MPH, via changes in phasic dopamine/noradrenaline firing (Evers et al., 2017), may have thus prevented stress-induced changes in neuronal signal-to-noise ratio. This interpretation would also align with interactions between MPH- and stress-induced frontal cortex dopamine release in rodents (Marsteller et al., 2002).
The observed pattern in the four treatment groups seemed to follow a quadratic trend in the middle temporal gyrus, frontal pole and anterior cingulate cortex. Here, the pattern of brain activation associated with goal-directed behaviour was similar for participants in no-stress/PLC and stress/MPH conditions on the one hand, and for participants in the stress/PLC and no-stress/MPH conditions on the other hand. The observation of an (inverted) U curve has been well-established in the context of cognitive performance and dopamine function (Arnsten, 2009b; Arnsten and Goldman-Rakic, 1998; Cools and D’Esposito, 2011; Goldman-Rakic et al., 2000). To our knowledge, this is the first study to demonstrate in humans how acute stress and stimulants might jointly facilitate shifts in the position along this U curve, via changes in putative dopaminergic mechanisms. Future studies may aim to further explore how varying levels of dopamine agonism (via acute stress or psychopharmacological agents) may induce shifts along this U curve. This will also contribute to a better understanding of interindividual differences in dopamine levels, and their association with cognitive processes and associated brain activation under stress.
Some limitations of the current study should be acknowledged. First, our conclusions are derived from a relatively small behavioural effect. The low number of SOA in all conditions may signal the relative absence of habit formation and limited the sensitivity to detect stress-induced reductions in goal-directed behaviour, and its potential reversal by MPH on a behavioural level. Similarly, the low number of SOA may have affected the signal-to-noise ratio of the fMRI measurement. However, the number of trials associated with a habitual response is similar to that in Watson et al. (2018) who presented comparable results. In addition, Steele et al. (2016) have shown that the 4–10 trials with a sample size of 20 participants produces reliable signals in an error processing task. Another limitation concerns potential boundary conditions associated with stress effects on goal-directed behaviour. One such condition is that stress-induced changes in instrumental behaviour may be limited to participants characterised by low working memory capacity (Quaedflieg et al., 2019). Our sample consisted mostly of academic students who are expected to have relatively high working memory capacity, which may protect against performance impairments under stress. Next, hormonal contraceptives and variation in menstrual cycle may have affected the stress response (Kirschbaum et al., 1999). As data from the current experiment unfortunately do not allow for a sufficiently powered analysis of effects of the menstrual cycle or hormonal contraceptives, future studies could systematically examine potential menstrual cycle phase effects.
The current findings may be relevant to our understanding of stress-associated relapse behaviour in addiction (Sinha, 2001); stress may reduce goal-directed behaviour and thus could prompt reliance on old habits, such as drug-taking behaviour, in individuals suffering from addiction. Our observation that dopamine and noradrenaline contribute to changes in brain activation associated with goal-directed behaviour under stress aligns well with the role of dopamine, particularly in the dorsal striatum, in habit formation (Belin et al., 2013; Gasbarri et al., 2014; Nelson and Killcross, 2013), which are thought to be D2 receptor-mediated (Kwak et al., 2014; Volkow et al., 2006, 2013). Administration of MPH to individuals suffering from addiction may enhance cortico-striatal dopamine function, ultimately enhancing frontal cortex based goal-directed behaviour. This idea is supported by the observation that MPH administration to cocaine-dependent individuals normalises anterior cingulate activation and increases inhibitory control (Li et al., 2010). The use of stimulants in these populations, however, should be closely monitored given that stimulants also increase the motivation to gamble (Zack and Poulos, 2004), and have been reported to increase striatal dopamine release in pathological gamblers (Boileau et al., 2014).
To conclude, stress-induced reductions in brain activation associated with goal-directed behaviour may involve diminished differentiation between valuable and devalued rewards. These effects may be driven by both changes in expected value associated with OFC and ACC activation, and in action selection associated with activation of the putamen and insula. MPH seemed to reverse this stress-induced reduction in activation differences in the insula, middle temporal gyrus, frontal pole and ACC implying that dopamine and noradrenaline may drive stress-induced changes in representations of reward value. However, MPH did not impact goal-directed behaviour. Future studies could be conducted to examine the many boundary conditions related to the stress-induced shift in goal-directed behaviour (e.g. working memory capacity, oral contraceptives, baseline dopamine levels) and further disentangle the association between catecholamine function, stress and brain activation underlying goal-directed behaviour.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_02698811211044679 for Dopaminergic and noradrenergic modulation of stress-induced alterations in brain activation associated with goal-directed behaviour by Peter van Ruitenbeek, Conny WEM Quaedflieg, Dennis Hernaus, Bart Hartogsveld and Tom Smeets in Journal of Psychopharmacology
Acknowledgments
We are thankful to Kemala Cut Nurul, Catalina Duta, Iris Bras, Lichelle Matau and Lonne Heijmans for their help in collecting the data.
Footnotes
Author contributions: P. van Ruitenbeek has designed the experiment, collected, analysed and interpreted the data and drafted the manuscript. C.W.E.M. Quaedflieg has contributed to data collection and contributed substantially to analysing and interpreting the data and revising the manuscript.
D. Hernaus has contributed substantially to revising the manuscript. D. Hernaus has received financial compensation as a consultant for P1vital Products Ltd. These activities are unrelated to the current manuscript. B. Hartogsveld has contributed to collection, analysing and interpreting of the data and revising the manuscript. T. Smeets has contributed substantially to designing the experiment and to analysing and interpreting the data and revising the manuscript. All authors approve of the final version to be published and agree to be accountable for all aspects of the paper by appropriately addressing any questions related to the accuracy or integrity of the paper.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Dutch Research Council (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, NWO) to Dr Smeets (grant number 452-14-003) and Dr Quaedflieg (grant number 446-15-003).
ORCID iD: Peter van Ruitenbeek  https://orcid.org/0000-0002-3802-6551
https://orcid.org/0000-0002-3802-6551
Supplemental material: Supplemental material for this article is available online.
References
- Andersson JL, Skare S, Ashburner J. (2003) How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 20: 870–888. [DOI] [PubMed] [Google Scholar]
- Andersson JLRJ, Jenkinson M, Smith S. (2010) Non-linear registration, aka spatial normalisation. FMRIB Technical Report TR07JA2, 28 June. Oxford: FMRIB Centre. [Google Scholar]
- Arnsten AF. (2009. a) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. (1998) Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry 55: 362–368. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. (2009. b) Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: An important role for prefrontal cortex dysfunction. CNS Drugs 23: 33–41. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. (2000) The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory. J Neurosci 20: 8954–8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, et al. (2013) Addiction: Failure of control over maladaptive incentive habits. Curr Opin Neurobiol 23: 564–572. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. (2016) Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol 71: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Payer D, Chugani B, et al. (2014) In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: A positron emission tomography study with [(11)C]-(+)-PHNO. Mol Psychiatry 19: 1305–1313. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. (2009) Effort discounting in human nucleus accumbens. Cogn Affect Behav Neurosci 9: 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Ravel S, Richmond BJ. (2012) Complementary neural correlates of motivation in dopaminergic and noradrenergic neurons of monkeys. Front Behav Neurosci 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, et al. (1998) A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction 93: 73–92. [PubMed] [Google Scholar]
- Brovelli A, Nazarian B, Meunier M, et al. (2011) Differential roles of caudate nucleus and putamen during instrumental learning. Neuroimage 57: 1580–1590. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69: e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, D’Aguiar C, Pruessner JC. (2009) What stress does to your brain: A review of neuroimaging studies. J Psychiatry 54: 6–15. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Park J, Moghaddam B. (2020) Unanticipated stressful and rewarding experiences engage the same prefrontal cortex and ventral tegmental area neuronal populations. eNeuro 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S, Barker RA, Dickinson AD, et al. (2011. a) Habitual versus goal-directed action control in Parkinson disease. J Cogn Neurosci 23: 1218–1229. [DOI] [PubMed] [Google Scholar]
- de Wit S, Standing HR, DeVito EE, et al. (2011. b) Reliance on habits at the expense of goal-directed control following dopamine precursor depletion. Psychopharmacology (Berl) 219: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S, Watson P, Harsay HA, et al. (2012) Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci 32: 12066–12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JS. (2008) Dual-processing accounts of reasoning, judgment, and social cognition. Annu Rev Psychol 59: 255–278. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. (2005) Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci 8: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. (2016) Drug addiction: Updating actions to habits to compulsions ten years on. Annu Rev Psychol 67: 23–50. [DOI] [PubMed] [Google Scholar]
- Evers EA, Stiers P, Ramaekers JG. (2017) High reward expectancy during methylphenidate depresses the dopaminergic response to gain and loss. Soc Cogn Affect Neurosci 12: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, et al. (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191. [DOI] [PubMed] [Google Scholar]
- Fournier M, D’Arripe-Longueville F, Radel R. (2017) Effects of psychosocial stress on the goal-directed and habit memory systems during learning and later execution. Psychoneuroendocrinology 77: 275–283. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’reilly RC. (2004) By carrot or by stick: Cognitive reinforcement learning in Parkinsonism. Science 306: 1940–1943. [DOI] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, Richler JJ. (2012) Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 141: 2–18. [DOI] [PubMed] [Google Scholar]
- Furukawa E, da Costa RQM, Bado P, et al. (2020) Methylphenidate modifies reward cue responses in adults with ADHD: An fMRI study. Neuropharmacol 162: 107833. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Pompili A, Packard MG, et al. (2014) Habit learning and memory in mammals: behavioral and neural characteristics. Neurobiol Learn Mem 114: 198–208. [DOI] [PubMed] [Google Scholar]
- Goldfarb EV, Mendelevich Y, Phelps EA. (2017) Acute stress time-dependently modulates multiple memory systems. J Cogn Neurosci 29: 1877–1894. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. (2000) D-1 receptors in prefrontal cells and circuits. Brain Res Rev 31: 295–301. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Grafton ST. (2015) The striatum: where skills and habits meet. Cold Spring Harb Perspect Biol 7: a021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Planeta-Wilson B, et al. (2010) Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry 68: 854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartogsveld B, van Ruitenbeek P, Quaedflieg CWEM, et al. (2020) Balancing between goal-directed and habitual responding following acute stress. Exp Psychol 67: 99–111. [DOI] [PubMed] [Google Scholar]
- Hernaus D, Collip D, Kasanova Z, et al. (2015) No evidence for attenuated stress-induced extrastriatal dopamine signaling in psychotic disorder. Transl Psychiatry 5: e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Miczek KA. (2016) Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology 233: 163–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. (2012) Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci 16: 122–128. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. (2004) Stimulus-specific plasticity of prefrontal cortex dopamine neurotransmission. J Neurochem 88: 1327–1334. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, et al. (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, et al. (2012) FSL. Neuroimage 62: 782–790. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Karim TJ, Reyes-Vazquez C, Dafny N. (2017) Comparison of the VTA and LC response to methylphenidate: a concomitant behavioral and neuronal study of adolescent male rats. J Neurophysiol 118: 1501–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. (2000) The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of noradrenaline in the medial prefrontal cortex: A dual-probe microdialysis study in the rat brain. Eur J Pharmacol 387: 279–286. [DOI] [PubMed] [Google Scholar]
- King D, Zigmond MJ, Finlay JM. (1997) Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience 77: 141–153. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, et al. (1999) Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61: 154–162. [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Lee Y, Pooseh S, et al. (2019) L-DOPA reduces model-free control of behavior by attenuating the transfer of value to action. Neuroimage 186: 113–125. [DOI] [PubMed] [Google Scholar]
- Kwak S, Huh N, Seo JS, et al. (2014) Role of dopamine D2 receptors in optimizing choice strategy in a dynamic and uncertain environment. Front Behav Neurosci 8: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lataster J, Collip D, Ceccarini J, et al. (2011) Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: A positron emission tomography study using [¹⁸F]fallypride. Neuroimage 58: 1081–1089. [DOI] [PubMed] [Google Scholar]
- Li CS, Morgan PT, Matuskey D, et al. (2010) Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci U S A 107: 14455–14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. (1988) Toward an Instance Theory of Automatization. Psychol Rev 95: 492–527. [Google Scholar]
- Maes EJP, Sharpe MJ, Usypchuk AA, et al. (2020) Causal evidence supporting the proposal that dopamine transients function as temporal difference prediction errors. Nat Neurosci 23: 176–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsteller DA, Gerasimov MR, Schiffer WK, et al. (2002) Acute handling stress modulates methylphenidate-induced catecholamine overflow in the medial prefrontal cortex. Neuropsychopharmacology 27: 163–170. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, et al. (2000) Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci 20: RC65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AJ, Asselin MC, Farde L, et al. (2007) Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab 27: 369–377. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Dagher A, Booij L, et al. (2013) Stress-induced dopamine release in human medial prefrontal cortex–18F-fallypride/PET study in healthy volunteers. Synapse 67: 821–830. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Killcross S. (2013) Accelerated habit formation following amphetamine exposure is reversed by D1, but enhanced by D2, receptor antagonists. Front Neurosci 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, et al. (2018) The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Hum Brain Mapp 39: 3398–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Qin S, Van Marle HJ, et al. (2011) Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage 55: 345–352. [DOI] [PubMed] [Google Scholar]
- Otto AR, Raio CM, Chiang A, et al. (2013) Working-memory capacity protects model-based learning from stress. Proc Natl Acad Sci U S A 110: 20941–20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls AM, O’Daly OG, Rubia K, et al. (2012) Methylphenidate effects on prefrontal functioning during attentional-capture and response inhibition. Biol Psychiatry 72: 142–149. [DOI] [PubMed] [Google Scholar]
- Peterson EJ, Seger CA. (2013) Many hats: intratrial and reward level-dependent BOLD activity in the striatum and premotor cortex. J Neurophysiol 110: 1689–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA, Versluis MJ, Hoogduin JM, et al. (2006) BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: Parallel-acquired inhomogeneity-desensitized fMRI. Magn Reson Med 55: 1227–1235. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, et al. (2004) Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: A positron emission tomography study using [C-11]raclopride. J Neurosci 24: 2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, et al. (2003) Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28: 916–931. [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, et al. (2015) ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112: 267–277. [DOI] [PubMed] [Google Scholar]
- Quaedflieg CWEM, Meyer T, van Ruitenbeek P, et al. (2017) Examining habituation and sensitization across repetitive laboratory stress inductions using the MAST. Psychoneuroendocrinology 77: 175–181. [DOI] [PubMed] [Google Scholar]
- Quaedflieg CWEM, Stoffregen H, Sebalo I, et al. (2019) Stress-induced impairment in goal-directed instrumental behaviour is moderated by baseline working memory. Neurobiol Learn Mem 158: 42–49. [DOI] [PubMed] [Google Scholar]
- Rubia K, Alegria AA, Cubillo AI, et al. (2014) Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Biol Psychiatry 76: 616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Höffken O, et al. (2010. a) Concurrent glucocorticoid and noradrenergic activity shifts instrumental behavior from goal-directed to habitual control. J Neurosci 30: 8190–8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Oitzl MS. (2010. b) Memory formation under stress: Quantity and quality. Neurosci Biobehav Rev 34: 584–591. [DOI] [PubMed] [Google Scholar]
- Schwabe L. (2017) Memory under stress: From single systems to network changes. Eur J Neurosci 45: 478–489. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Dickinson A, Wolf OT. (2011) Stress, habits, and drug addiction: A psychoneuroendocrinological perspective. Exp Clin Psychopharmacol 19: 53–63. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Höffken O, et al. (2012) Simultaneous glucocorticoid and noradrenergic activity disrupts the neural basis of goal-directed action in the human brain. J Neurosci 32: 10146–10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. (2009) Stress prompts habit behavior in humans. J Neurosci 29: 7191–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. (2010) Socially evaluated cold pressor stress after instrumental learning favors habits over goal-directed action. Psychoneuroendocrinology 35: 977–986. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. (2011) Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behav Brain Res 219: 321–328. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. (2013) Stress and multiple memory systems: from ‘thinking’ to ‘doing’. Trends Cogn Sci 17: 60–68. [DOI] [PubMed] [Google Scholar]
- Sharpe MJ, Batchelor HM, Mueller LE, et al. (2020) Dopamine transients do not act as model-free prediction errors during associative learning. Nat Commun 11: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158: 343–359. [DOI] [PubMed] [Google Scholar]
- Sinha R, Jastreboff AM. (2013) Stress as a common risk factor for obesity and addiction. Biol Psychiatry 73: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Cornelisse S, Quaedflieg CW, et al. (2012) Introducing the Maastricht Acute Stress Test (MAST): A quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology 37: 1998–2008. [DOI] [PubMed] [Google Scholar]
- Smeets T, van Ruitenbeek P, Hartogsveld B, et al. (2019) Stress-induced reliance on habitual behavior is moderated by cortisol reactivity. Brain Cogn 133: 60–71. [DOI] [PubMed] [Google Scholar]
- Smith BW, Mitchell DG, Hardin MG, et al. (2009) Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage 44: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. (2002) Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- Steele VR, Anderson NE, Claus ED, et al. (2016) Neuroimaging measures of error-processing: Extracting reliable signals from event-related potentials and functional magnetic resonance imaging. Neuroimage 132: 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. (1996) Social defeat stress selectively alters mesocorticolimbic dopamine release: An in vivo microdialysis study. Brain Res 721: 140–149. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O’Doherty JP. (2009) A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci 29: 2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Argilli E, Bonci A. (2010) Effects of stress and aversion on dopamine neurons: Implications for addiction. Neurosci Biobehav Rev 35: 151–156. [DOI] [PubMed] [Google Scholar]
- Vaessen T, Hernaus D, Myin-Germeys I, et al. (2015) The dopaminergic response to acute stress in health and psychopathology: A systematic review. Neurosci Biobehav Rev 56: 241–251. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. (2008) Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin VV, Dickinson A, O’Doherty JP. (2007) Determining the neural substrates of goal-directed learning in the human brain. J Neurosci 27: 4019–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dessel P, Hughes S, De Houwer J. (2019) How do actions influence attitudes? An inferential account of the impact of action performance on stimulus evaluation. Pers Soc Psychol Rev 23: 267–284. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. (2008) Response inhibition in the stop-signal paradigm. Trends Cogn Sci 12: 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, et al. (2007) Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10: 376–384. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G, et al. (2002) Mechanism of action of methylphenidate: Insights from PET imaging studies. J Atten Disord 6: 31–43. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, et al. (2013) Predominance of D2 receptors in mediating dopamine’s effects in brain metabolism: Effects of alcoholism. J Neurosci 33: 4527–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, et al. (2006) Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26: 6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, et al. (2010) Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105: 1741–1749. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, et al. (2005) Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: A role in stress-induced relapse to drug seeking. J Neurosci 25: 5389–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, et al. (2007) Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology 193: 283–294. [DOI] [PubMed] [Google Scholar]
- Watson P, van Wingen G, de Wit S. (2018) Conflicted between goal-directed and habitual control, an fMRI Investigation. eNeuro 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz L, Bogdanov M, Schwabe L. (2018) Habits under stress: mechanistic insights across different types of learning. Curr Opin Behav Sci 20: 9–16. [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, et al. (2004) Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21: 1732–1747. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, et al. (2001) Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Smittenaar P, Dolan RJ. (2012) Dopamine enhances model-based over model-free choice behavior. Neuron 75: 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M, Poulos CX. (2004) Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology 29: 195–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_02698811211044679 for Dopaminergic and noradrenergic modulation of stress-induced alterations in brain activation associated with goal-directed behaviour by Peter van Ruitenbeek, Conny WEM Quaedflieg, Dennis Hernaus, Bart Hartogsveld and Tom Smeets in Journal of Psychopharmacology