Abstract
6-Shogaol (SHO) and 6-gingerol (GIN), naturally derived compounds of ginger (Zingiber officinale Roscoe), have been found to have anti-allergic effects on dermatitis-like skin lesions and rhinitis. Although SHO and GIN have demonstrated a potential in various inflammatory diseases, their efficacy and mechanism in asthma have not been largely examined. Therefore, the present study demonstrated the anti-asthmatic effects of SHO and GIN on the T-helper (Th) 2 cell-mediated allergic response pathway in an ovalbumin (OVA)-induced asthma mouse model. The asthma mouse model was established with an intraperitoneal (i.p.) injection of 50 µg OVA and 1 mg aluminum hydroxide with or without an i.p. injection of SHO and GIN (10 mg/kg) before treatment with OVA. In addition, the current study assessed mast cell degranulation in antigen-stimulated RBL-2H3 cells under different treatment conditions (SHO or GIN at 0, 10, 25, 50 and 100 nM) and determined the mRNA and protein levels of anti-oxidative enzymes [superoxide dismutase (SOD)1, SOD2, glutathione peroxidase-1/2, catalase] in lung tissues. SHO and GIN inhibited eosinophilia in the bronchoalveolar lavage fluids and H&E-stained lung tissues. Both factors also decreased mucus production in periodic acid-Schiff-stained lung tissues and the levels of Th2 cytokines in these tissues. GIN attenuated oxidative stress by upregulating the expression levels of anti-oxidative proteins. In an in vitro experiment, the degranulation of RBL-2H3 rat mast cells was significantly decreased. It was found that SHO and GIN effectively suppressed the allergic response in the mouse model by inhibiting eosinophilia and Th2 cytokine production. Collectively, it was suggested that SHO can inhibit lung inflammation by attenuating the Th2 cell-mediated allergic response signals, and that GIN can inhibit lung inflammation and epithelial cell remodeling by repressing oxidative stress. Therefore, SHO and GIN could be used therapeutically for allergic and eosinophilic asthma.
Keywords: asthma, ginger, allergy, lung inflammation, oxidative stress
Introduction
Asthma is a chronic inflammatory disease with symptoms such as airway remodeling and hyperresponsiveness, mucus hypersecretion, wheezing, dyspnea, cough and chest tightness (1-3). These symptoms are caused by an allergic response mediated by the T-helper (Th) cells. Naive Th cells are induced by antigen-activated dendritic cells to differentiate into Th1 or Th2 cells through exposure to proinflammatory cytokines such as IL-4 and IL-2, respectively (4). After Th cell differentiation, Th2 cells secrete various cytokines, such as IL-4 and IL-5 (2,5,6). Allergic proteins, such as histamines, are released from the IgE-activated mast cells, recruiting eosinophils and causing acute and chronic inflammation (7,8).
Allergic proteins, especially histamines, play a significant role in an allergic response. Therefore, reducing histamines by inhibiting mast cell degranulation may be crucial for suppressing allergic reactions in individuals with asthma. The asthmatic allergic response can be triggered quickly by various environmental stimulants, including cold air, ozone, pollen, strong odors, smoke, house dust mites and particulate matter (2,9). Recently, countries around the world (such as China, Bangladesh, Pakistan, India, Mongolia and South Korea) have undergone rapid industrialization, which has increased the concentration of particulate matter in the environment, thus affecting the incidence of acute asthma and exacerbating its symptoms (10-13).
At present, no treatments exist for asthma itself, only the use of symptom controllers, such as inhaled corticosteroids (14). However, the long-term application of glucocorticoid drugs can severely affect the musculoskeletal, endocrine/metabolic, gastrointestinal, cardiovascular, dermatological, neuropsychiatric, ophthalmological and immunological systems (15,16). Therefore, extensive research has been conducted to develop naturally derived compounds that can treat asthma without causing severe side effects. For example, the sesquiterpene lactones from Saussurea costus (Falc.) have been found to reduce the expression of Th2 cytokine genes and recruit inflammatory cells in an ovalbumin (OVA)-induced asthma mouse model (17). Galangin, a natural flavonoid, has been found to attenuate severe inflammation and airway remodeling by inhibiting the generation of TNF-β1-mediated reactive oxygen species (ROS) and MAPK/Akt phosphorylation in an OVA-sensitized asthma mouse model (18).
Ginger (Zingiber officinale Roscoe) has been used in traditional Chinese and Indian medicine to treat numerous diseases and symptoms, including nausea, diarrhea, gingivitis, arthritis and asthma (19). Ginger contains >400 compounds, including shogaol, gingerol, paradol, gingerdiol and zingerone; 6-shogaol (SHO) and 6-gingerol (GIN) are the major compounds (20). A previous study has shown that ginger powder-containing food and GIN exert anti-rhinitis effects on the major allergic response via the Th2 cell signaling pathway in an OVA-induced allergic rhinitis mouse model (21). In addition, GIN and SHO have been shown to combat asthma by relaxing the airway smooth muscle (ASM) and inhibiting chronic inflammation (22).
However, at present, research is lacking on the mechanism underlying SHO's effects on critical allergic reactions. Therefore, it is necessary to confirm the anti-allergic effects of SHO to verify its efficacy in asthma treatment. Thus, in the present study, we hypothesized that SHO and GIN could inhibit chronic inflammation by suppressing allergic responses and oxidative stress in an OVA-induced asthma mouse model.
Materials and methods
Animals and reagents
A total of 24 male BALB/c mice (age, 5 weeks; weight, 18-20 g) were supplied by RaonBio, Inc. and maintained at 22±2˚C and 50±10% humidity in a 12-h light/dark cycle and were freely provided with tap water and commercial food. The animals' weight was measured once a week to monitoring their conditions. SHO [1-[4-Hydroxy-3-methoxyphenyl]-4-decen-3-one; C17H24O3; purity, >98% as detected via high-performance liquid chromatography (HPLC); cat. no. 555-66-8] and GIN ([5S]-5-Hydroxy-1-[4-hydroxy-3-methoxy-phenyl]decan-3-one; [S]-[6]-Gingerol; and 3-Decanone, 5-hydroxy-1-[4-hydroxy-3-methoxyphenyl]; purity, >98% as detected by HPLC; cat. no. 23513-14-6) were purchased from Chengdu Biopurify Phytochemicals Ltd.
OVA-induced asthma mouse model
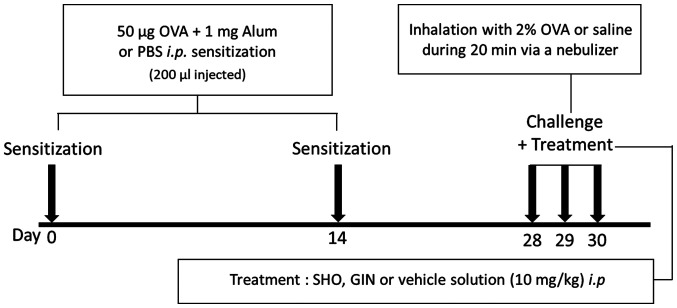
The mice were divided into four groups: Control, OVA, OVA + SHO and OVA + GIN, with n=6 per group. The induction of asthma in the mice treated with OVA was performed as described previously (17). The asthma mouse model was established by sensitizing the mice with an intraperitoneal (i.p.) injection of 50 µg OVA (cat. no. A5503; Sigma-Aldrich; Merck KGaA) and 1 mg aluminum hydroxide (cat. no. 239186; Sigma-Aldrich; Merck KGaA) in 200 µl PBS on days 0 and 14 (Fig. 1). The control group was treated with 200 µl PBS.
Figure 1.
Protocol for the OVA/alum-induced asthma mouse model. The OVA, OVA + SHO and OVA + GIN groups were sensitized with 50 µg OVA and 1 mg alum (i.p.) in 200 µl PBS, and the control group was sensitized with 200 µl PBS on days 0 and 14 (n=6/group). The OVA, OVA + SHO and OVA + GIN groups were challenged with 2% OVA, and the control group received saline solution for 20 min through a nebulizer on days 28, 29 and 30. i.p. injections of SHO or GIN were performed 2 h before each challenge step. i.p., intraperitoneal; OVA, ovalbumin; SHO, 6-shogaol; GIN, 6-gingerol; alum, aluminum hydroxide.
On days 28, 29 and 30, the mice received an i.p. injection of SHO at 10 mg/kg or GIN at 10 mg/kg 2 h before they were challenged with OVA. SHO and GIN were dissolved in 0.5 µl DMSO, and 89.5 µl PBS with 10 µl 50% Tween-20 was added to the 100-µl final dose. The mice were then challenged with 2% OVA in sterile saline through a whole-body exposure system with a nebulizer on days 28, 29 and 30 for 20 min. The control group inhaled sterile saline. The mice were sacrificed at 24 h after the final OVA challenge by cervical dislocation.
Measurement of bronchoalveolar lavage fluid (BALF)
BALF was obtained from the mice at 24 h after the final OVA challenge by flushing 0.5 ml cold PBS through the lungs three times with a tracheal catheter. The BALF was centrifuged at 318 x g for 10 min at 4˚C to collect the pellet, which was resuspended with PBS and stored at 4˚C for a differential cell count. The BALF cells in the pellet were stained with the Diff-Quik stain kit (cat. no. 38721; Sysmex Corporation), and 5 µl BALF cells were smeared onto one end of a glass slide according to the manufacturer's instructions. The cells were stained with Diff quick solution II and I for 30 sec at room temperature each, and rinsed with tap water. Stained cells were counted with a hematocytometer (HSU-0650030; Paul Marienfeld GmbH & Co. KG). The differential cells in the BALFs were classified as lymphocytes, neutrophils, macrophages or eosinophils. The distribution of the cells in the BALFs was expressed as a percentage.
Lung histological analysis
The right lung lobes of the mice were fixed in 4% formaldehyde at 4˚C for 48 h, embedded in paraffin and sliced to 4-µm sections (6 slides/mouse). The sections were stained with hematoxylin at room temperature for 5 min and then with eosin at room temperature for 3 min to analyze the degree of inflammation in the lung tissues. The degree of inflammation was assigned an arbitrary score of 0 (normal = no inflammation), 1 (minimal = perivascular, peribronchial or patchy interstitial inflammation involving <10% of lung volume), 2 (mild = perivascular, peribronchial or patchy interstitial inflammation involving 10-20% of lung volume), 3 (moderate = perivascular, peribronchial, patchy interstitial or diffuse inflammation involving 20-50% of lung volume) and 4 (severe = diffuse inflammation involving >50% of lung volume). The average inflammatory score was analyzed using a total of 18 slides (3 slides/mouse). In addition, the thickness of the lung epithelial cells was measured, calculated as the average value of the top, bottom, left and right of three random alveoli per slide using a light microscope (Leica Microsystems GmbH) with Leica AF6000 modular systems.
The sections also were stained with periodic acid-Schiff (PAS) at room temperature for 15 min to visualize the goblet cells to determine the extent of mucus production. A point-counting method was performed to quantify the number of cells stained positively in three random fields from each slide. The average of the cells was analyzed with a total of 18 slides (3 slides/mouse). These lung sections were observed using light microscope (magnification, x100) and analyzed with LAS AF Ink (Leica Microsystems GmbH) and ImageJ software (v1.53e; National Institutes of Health).
Reverse transcription-quantitative (RT-q)PCR analysis
Total RNA was isolated from the mouse lung tissues using the TRI-Solution (cat. no. TS200-001; Bio Science Technology). The total RNA was synthesized to cDNA using a PrimeScript™ 1st strand cDNA Synthesis kit (cat. no. 6110; Takara Biotechnology Co., Ltd.) with oligo-dT primers. The qPCR reaction mixture contained 8 µl cDNA, 10 µl Power SYBR®-Green PCR Master mix (cat. no. 4367659; Applied Biosystems; Thermo Fisher Scientific, Inc.), 1 µl 0.2-pmol forward primer and 1 µl 0.2-pmol reverse primer. The forward and reverse primers for β-actin (5'-GGCTCTTTTCCAGCCTTCCT-3' and 5'-GTCTTTACGG ATGTCAACGTCACA-3', respectively), IL-4 (5'-CCACGGAT GCGACAAAAATC-3' and 5'-GACGTTTGGCACATCCAT CTC-3'), IL-5 (5'-GATGGACGCAGGAGGATCAC-3' and 5'-GTGTGGCATCCCTCAGCAA-3'), IL-13 (5'-GGCCAG CCCACAGTTCTACA-3' and 5'-ACCACCAAGGCAAGCAA GAG-3'), superoxide dismutase 1 (SOD1; 5'-GACTTGGGC AAAGGTGGAAA-3' and 5'-CAGGGAATGTTTACTGCGC AAT-3'), SOD2 (5'-TGCTCTTGATTGAACATTTTCGTTA-3' and 5'-GCCCCCCAAAACAGAGATG-3'), catalase (5'-CGA CCAGGGCATCAAAAACT-3' and 5'-ATTGGCGATGGCAT TGAAA-3') and glutathione peroxidase-1 (GPx-1; 5'-AGAAAG CGATGCCACGTGAT-3' and 5'-GGAGATGTTGGGACTC AAACG-3') were used. The qPCR was run on the Applied Biosystems real-time PCR program (StepOnePlus™ Real-Time PCR System) at 95˚C for 10 min, followed by 40 cycles of a cycling stage at 95˚C for 15 sec and 60˚C for 1 min, and a melt curve stage at 95˚C for 15 sec, 60˚C for 1 min and 95˚C for 15 sec. All data were analyzed using the 2-ΔΔCq method (23) and expressed as fold change relative to controls. The relative expression levels of IL-4, IL-5, IL-13, SOD1, SOD2, catalase and GPx-1 were normalized to those of β-actin.
Western blotting assay
Lung tissues were lysed using the PRO-PREP for Cell/Tissue Protein Extraction Solution kit (cat. no. 17081; Intron Biotechnology, Inc.) with the Protease Inhibitor Cocktail (cat. no. P3100; GenDEPOT, LLC). The protein concentrations of the tissue lysates were determined using the BCA protein assay kit (cat. no. 23227; Thermo Fisher Scientific, Inc.). A total of 30 µg protein from each sample was separated via 10-12% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skim milk in a 1X TBS-Tween-20 (TBST; containing 0.05% Tween-20) at room temperature for 1 h and incubated with primary antibodies against IL-4 (monoclonal; rat anti-mouse; 1:1,000; cat. no. ab11524; Abcam), IL-5 (monoclonal; mouse anti-mouse; 1:1,000; cat. no. sc-398334; Santa Cruz Biotechnology, Inc.), IL-13 (polyclonal; rabbit anti-mouse; 1:1,000; cat. no. ab106732; Abcam), SOD1 (monoclonal; mouse anti-mouse; 1:1,000; cat. no. sc-101523; Santa Cruz Biotechnology, Inc.), SOD2 (monoclonal; mouse anti-mouse; 1:1,000; cat. no. sc-133134; Santa Cruz Biotechnology, Inc.), catalase (monoclonal; mouse anti-mouse; 1:1,000; cat. no. sc-271803; Santa Cruz Biotechnology, Inc.), GPx-1/2 (monoclonal; mouse anti-mouse; 1:1,000; cat. no. sc-133160; Santa Cruz Biotechnology, Inc.) or β-actin (monoclonal; mouse anti-mouse; 1:1,000; cat. no. sc-47778; Santa Cruz Biotechnology, Inc.) diluted with 3% skim milk in a 1X TBST buffer at 4˚C overnight. Then, the blots were washed three times with 1X TBST buffer and incubated with anti-mouse HRP-conjugated secondary antibodies (goat anti-mouse IgG-HRP; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) diluted at 1:5,000 with 3% skim milk in 1X TBST buffer at room temperature for 1 h. Next, the membranes were washed three times with 1X TBST buffer, and the protein bands were detected with the ECL detection reagents (cat. no. 34580; Thermo Fisher Scientific, Inc.) and semi-quantified with ImageQuant LAS 500 (Cytiva). The protein expression level of β-actin was used as the loading control.
Cell lines and culture
Mast cell degranulation was evaluated by the release of β-hexosaminidase from the mast cell line, RBL-2H3. Rat RBL-2H3 mast cells were obtained from the American Type Culture Collection and maintained in MEM medium (cat. no. 61100061; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (cat. no. A31604; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (cat. no. 15140122; Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2 incubator at 37˚C. RBL-2H3 cells were sensitized via an incubation with 0.2 µg/ml monoclonal anti-dinitrophenyl mouse IgE (cat. no. D8406; Sigma-Aldrich; Merck KGaA) diluted medium overnight at 37˚C. The cells were washed twice with a piperazine-N, N'bis (2-ethanesulfonic acid) (PIPES) buffer (pH 7.2) containing 25 mM PIPES, 0.05 mM NaOH, 110 mM DNP-IgE and 0.1% BSA (cat. no. A0100-010; GenDEPOT LLC). The cells were then incubated in PIPES buffer containing different concentrations of SHO and GIN (0, 10, 25, 50 and 100 nM) at 37˚C for 30 min. Next, the cells were incubated with 1 µg/ml human dinitrophenyl albumin (cat. no. A6661; Sigma-Aldrich; Merck KGaA) to induce degranulation for 15 min at 37˚C. After centrifugation at 125 x g for 5 min at 4˚C, 25 µl supernatant from each reaction was transferred to a 96-well microplate and incubated for 110 min with 5 mM 4-nitrophenyl N-acetyl-β-D-glucosaminide in a 0.1-M citrate buffer (pH 4.5; cat. no. N9376; Sigma-Aldrich; Merck KGaA). The reaction was terminated by adding 0.05 M sodium carbonate buffer (pH 10). The optical density of each reaction was measured at the absorbance wavelength of 405 nm using a microplate reader.
The concentrations of SHO and GIN were selected using a cell viability assay. Cell viability was evaluated to determine cytotoxicity of SHO and GIN using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Inc.) assay, following the manufacturer's instructions. Jurkat cells (human T cell line; 1x104 cells/well) were obtained from American Type Culture Collection and maintained in RPMI-1640 medium (cat. no. 31800022; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS and 1% penicillin/streptomycin in a 5% CO2 incubator at 37˚C. Jurkat cells were incubated at 37 ˚C in 96-well plates with SHO and GIN at 0, 10, 50 and 100 nM for 0, 24, 48 and 72 h. RBL-2H3 cells (5x103 cells/well) were incubated at 37˚C in 96-well plates with SHO (0, 1, 2.5 and 5 µM) and GIN (0, 0.5, 1 and 2 µM) for 0, 24, 48 and 72 h. Next, 10 µl CCK-8 reagent was added to each well, and cells were incubated at 37˚C for an additional 2 h. The absorbance was measured at 450 nm using a microplate reader.
Statistical analysis
Data were collected from ≥3 independent experiments. All results were expressed as the mean ± SD. All analyses were performed using SPSS software (version 20; IBM Corp.). Statistical significance between experimental groups was determined using one-way ANOVA for pair-wise comparisons with Bonferroni's multiple comparisons test. Ordinal data were analyzed using a Kruskal-Wallis test followed by Dunn's test. P<0.05 was considered to indicate a statistically significant difference.
Results
SHO and GIN inhibit eosinophil recruitment in BALFs
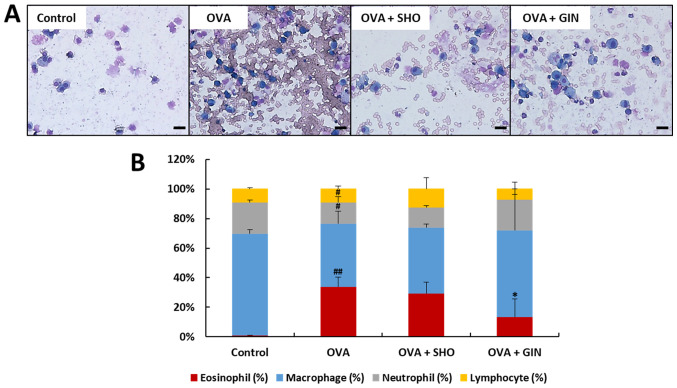
In the OVA group, the eosinophil level was increased significantly compared with that of the control group. In the OVA + GIN group, this was decreased significantly compared with that of the OVA group (Fig. 2). The current study found no significant difference between the OVA + SHO and OVA groups, but did identify a decreasing trend. These data suggest that GIN strongly inhibited the recruitment of eosinophils in asthma; SHO also may have such a potential. Therefore, GIN and SHO likely have anti-inflammatory effects on asthma in a mouse model. Thus, additional experiments were conducted to confirm the anti-allergic effects of both using histological examination.
Figure 2.
Effects of SHO and GIN on inflammatory cell recruitment in BALF. BALF was obtained from the control, OVA, OVA + SHO and OVA + GIN groups. (A) BALF was stained with H&E (magnification, x400; scale bars, 20 µm), and cells were (B) counted with a hematocytometer. Differential cells were classified as lymphocytes (yellow), neutrophils (gray), macrophages (blue) and eosinophils (red). The distribution of inflammatory cells (white blood cells) is expressed as a percentage (mean ± SD, n=6). #P<0.05, ##P<0.01 vs. control group; *P<0.05 vs. OVA group. OVA, ovalbumin; SHO, 6-shogaol; GIN, 6-gingerol.
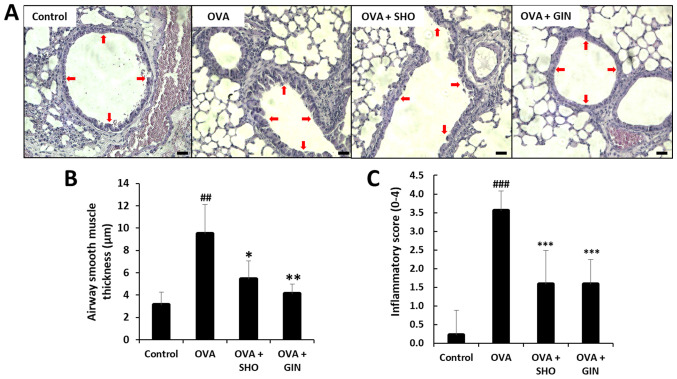
SHO and GIN suppress the airway inflammatory response and inflammatory cell infiltration in lung tissues
The lung sections from the mice in different treatment groups were stained with H&E to confirm the inflammatory cell-counting results (Fig. 2). In chronic inflammation, inflammatory cells infiltrate the ASM cell layer, inducing eosinophilia in the lung tissues (24). The ASM cell thickness was increased in the OVA group compared with that in the control group, suggesting that a chronic inflammatory response, such as asthma, was induced in the OVA group (Fig. 3A and B). In the OVA + SHO and OVA + GIN groups, ASM thickness was significantly decreased compared to that of the OVA group (Fig. 3A and B). Moreover, the inflammatory score was significantly higher in the OVA group and was significantly lower in the OVA + SHO and OVA + GIN groups (Fig. 3C).
Figure 3.
Effects of SHO and GIN on inflammatory cell infiltration and airway inflammation in lung tissues. The right lung lobes were isolated at 24 h after the final OVA challenge. Next, 4-µm lung sections were stained with H&E to analyze inflammatory cell infiltration and inflammatory score. (A) Panels show H&E-stained lung sections obtained from the control (first), OVA (second), OVA + SHO (third) and OVA + GIN (fourth) groups. Magnification, x200; scale bar, 75 µm. (B) ASM thickness was evaluated with LAS AF Ink. (C) Total inflammatory score expressed as an average. Red arrow indicates measured thickness site of epithelial cells. Values are presented as mean ± SD (n=6). ##P<0.01, ###P<0.001 vs. control group; *P<0.05, **P<0.01, ***P<0.001 vs. OVA group. OVA, ovalbumin; SHO, 6-shogaol; GIN, 6-gingerol.
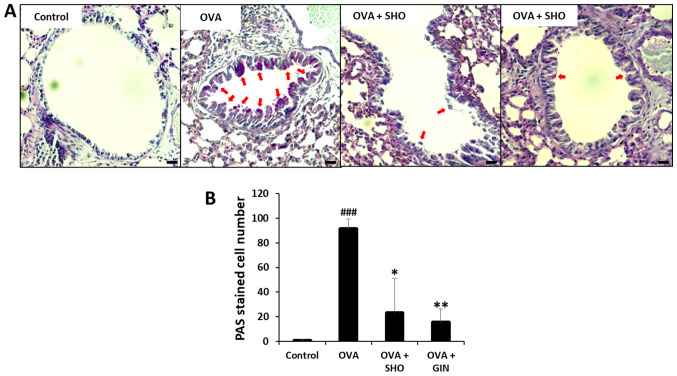
SHO and GIN decrease goblet cell hyperplasia and mucus production in lung tissues
The mucins produced by the goblet cells were stained using PAS (red arrows; Fig. 4A). Mucus production was significantly increased in the OVA group compared with that in the control group; however, it was significantly reduced in the OVA + SHO and OVA + GIN groups as compared with the OVA group (Fig. 4B).
Figure 4.
Effects of SHO and GIN on mucus production in lung tissues. The right lung lobes were isolated 24 h after the final OVA challenge. Then, 4 µm-cut-lung sections were stained with PAS to analyze mucus production. (A) Panels show PAS-stained lung sections obtained from the control (first), OVA (second), OVA + SHO (third) and OVA + GIN (fourth) groups. Magnification, x200; scale bars, 75 µm. Fixed lung tissues were stained with PAS for visualizing mucus production. (B) PAS-stained cells are goblet cells presented with a red arrow. Values are presented as mean ± SD (n=6). ###P<0.001 vs. control group; *P<0.05, **P<0.01 vs. OVA group. OVA, ovalbumin; SHO, 6-shogaol; GIN, 6-gingerol; PAS, periodic acid-Schiff.
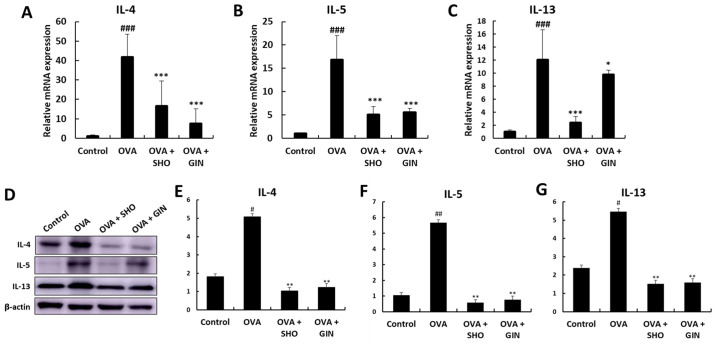
SHO and GIN suppress inflammatory cytokine levels in lung tissues
Th2 cytokines regulate the inflammatory response in allergic diseases such as asthma (25,26). The expression of genes encoding Th2 cell-mediated cytokines, including IL-4, IL-5 and IL-13, in the lung tissues after the final OVA challenge was examined using RT-qPCR (Fig. 5). The mRNA expression levels of IL-4, IL-5 and IL-13 were significantly increased in the OVA group compared with those in the control group (Fig. 5A-C). SHO and GIN significantly decreased the mRNA expression levels of IL-4 and IL-5 (Fig. 5A and B). In addition, both significantly reduced the expression level of IL-13 (Fig. 5C). The protein expression levels of IL-4, IL-5 and IL-13 were determined using a western blotting assay to confirm the mRNA expression data (Fig. 5D-G). The protein expression levels of IL-4, IL-5 and IL-13 were significantly increased in the OVA group. Furthermore, SHO and GIN decreased the protein expression levels of IL-4, IL-5 and IL-13.
Figure 5.
Effects of SHO and GIN on inflammatory cytokines levels in lung tissues. Levels of Th2 cell-related inflammatory cytokines in lung tissues. Total RNA was isolated from lung tissues. (A) IL-4, (B) IL-5 and (C) IL-6 mRNA expression levels were detected using reverse transcription-quantitative PCR with specific primers. The relative mRNA expression levels were calculated based on β-actin mRNA expression in lung tissues. (D) Protein expression levels of cytokines were determined via western blotting, and (E) IL-4, (F) IL-5 and (G) IL-6 protein expression levels were semi-quantified. Values are presented as mean ± SD (n=6). #P<0.05, ##P<0.01, ###P<0.001 vs. control group; *P<0.05, **P<0.01, ***P<0.001 vs. OVA group. OVA, ovalbumin; SHO, 6-shogaol; GIN, 6-gingerol.
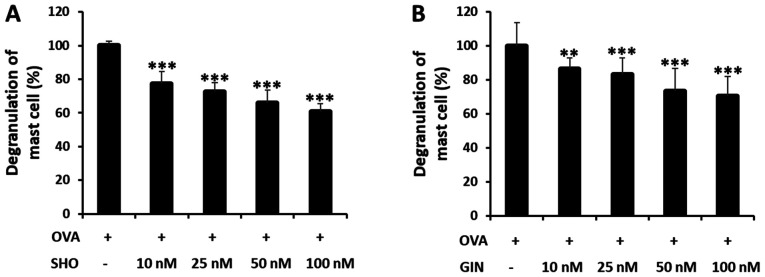
SHO and GIN inhibit mast cell degranulation in RBL-2H3 cells
Cells were treated with SHO or GIN at 0, 10, 25, 50 and 100 nM to determine their dose-dependent effects on the mast cells (Fig. 6). The OVA + SHO group showed a significant dose-dependent decrease in mast cell degranulation, to 61% in the group treated with 100 nM of SHO (Fig. 6A). GIN had the same tendency, decreasing degranulation to 70% in the group treated with 100 nM GIN (Fig. 6B). Additionally, the cytotoxicity of SHO and GIN in the RBL-2H3 and Jurkat cells were determined using CCK-8 assays, which demonstrated that SHO and GIN were not toxic (Fig. S1). The concentrations of SHO and GIN used for cell line treatment were selected based on the results in Fig. S1. These data suggested that both reagents effectively suppressed the allergic response in asthma by inhibiting mast cell degranulation.
Figure 6.
Effects of SHO and GIN on antigen-induced β-hexosaminidase release in the RBL-2H3 cell line. RBL-2H3 cells were sensitized with monoclonal anti-dinitrophenyl mouse IgE, and degranulation was induced. Cells were treated with 0 nM (OVA group treated with DMSO), 10, 25, 50 and 100 nM (A) SHO or (B) GIN. OVA value was used to determine maximum response (100%). Values are presented as mean ± SD. **P<0.01, ***P<0.001 vs. OVA group. OVA, ovalbumin; SHO, 6-shogaol; GIN, 6-gingerol.
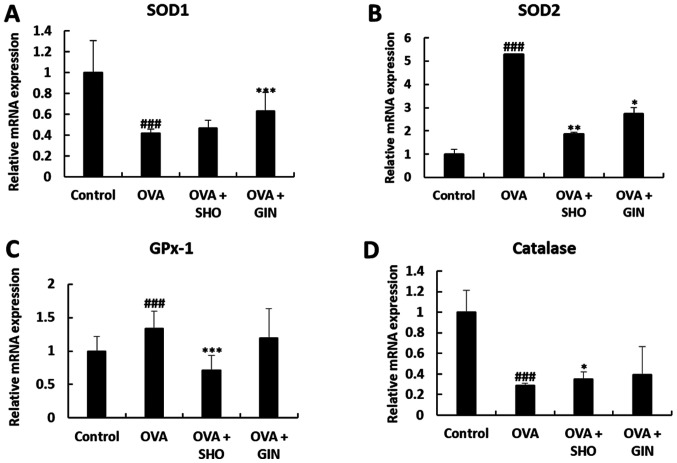
SHO and GIN restore the expression levels of antioxidant factors
Oxidative stress is an important pathological mechanism of asthma. SOD1, SOD2, catalase and GPx-1/2 are critical antioxidant enzymes to reduce oxidative stress (27-29). Total mRNA and proteins were isolated from the lung tissues from different treatment groups to determine the effects of SHO and GIN on the levels of various antioxidant enzymes. The mRNA expression level of SOD1 was significantly decreased in the OVA group compared with that in the control group, and the expression level of SOD1 was restored in the OVA + GIN group. In addition, the current study found no difference between the OVA + SHO and OVA groups, but observed an increasing tendency (Fig. 7A).
Figure 7.
Effects of SHO and GIN on anti-oxidant/oxidant mRNA balance in the OVA-induced asthma mice model. Level of anti-oxidant mRNA in lung tissues. Total RNA was isolated from lung tissues. (A) SOD1, (B) SOD2, (C) GPx-1 and (D) catalase expression levels were detected using reverse transcription-quantitative PCR with specific primers. The relative expression levels of mRNA were calculated based on β-actin mRNA expression in lung tissues. Values are presented as mean ± SD (n=6). ###P<0.001 vs. control group; *P<0.05, **P<0.01, ***P<0.001 vs. OVA group. OVA, ovalbumin; SHO, 6-shogaol; GIN, 6-gingerol; SOD, superoxide dismutase; GPx-1, glutathione peroxidase-1.
The mRNA expression levels of SOD2 and GPx-1 were increased significantly in the OVA group compared with the control group, but were decreased significantly in the OVA + SHO group as compared with the OVA group (Fig. 7B and C). Similarly, SOD2 expression was significantly decreased in the OVA + GIN group compared with the OVA group (Fig. 7B). However, no significant difference was detected in the expression level of GPx-1 between the OVA and OVA + GIN groups (Fig. 7C).
The mRNA expression levels of catalase were significantly decreased in the OVA group compared with those in the control group, and this expression was restored in the OVA + SHO group. However, no difference was observed in catalase expression between the OVA and OVA + GIN groups (Fig. 7D).
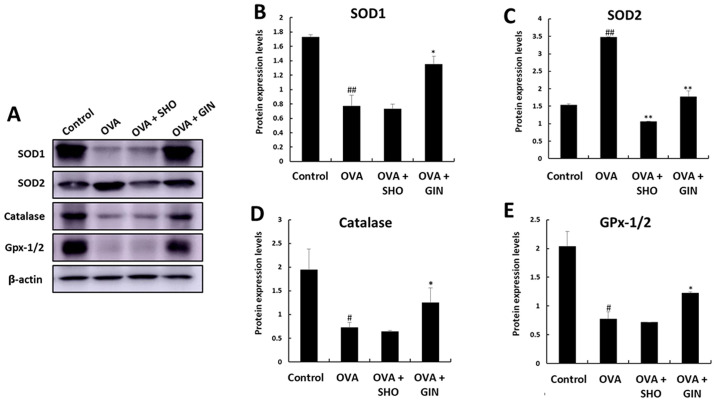
The expression patterns of the antioxidant genes were confirmed by examining the corresponding protein expression levels using western blot analysis (Fig. 8A). The protein expression levels of SOD1, SOD2 and catalase exhibited the same trend as those of the mRNA expression of the corresponding genes (Fig. 8B-D). However, GPx-1/2 expression showed the opposite tendency to the mRNA expression levels of GPx-1 (Fig. 8D). GPx-1/2 was decreased in the OVA group compared with the control group, was increased in the OVA + GIN group, but showed no change in the OVA + SHO group (Fig. 8D).
Figure 8.
Effects of SHO and GIN on anti-oxidant/oxidant protein balance in the OVA-induced asthma mice model. Expression levels of anti-oxidant proteins in lung tissues. Proteins were isolated from lung tissues. (A) Expression levels of (B) SOD1, (C) SOD2, (D) catalase and (E) GPx-1/2 were analyzed using western blotting. Data are expressed as fold changes compared with β-actin values. Values are presented as mean ± SD (n=6). #P<0.05, ##P<0.01 vs. control group; *P<0.05, **P<0.01 vs. OVA group. OVA, ovalbumin; SHO, 6-shogaol; GIN, 6-gingerol; SOD, superoxide dismutase; GPx-1, glutathione peroxidase-1.
Discussion
The present study demonstrated the in vitro and in vivo anti-inflammatory and anti-allergic effects of SHO and GIN. In the OVA-induced asthma mouse model, allergic response and chronic inflammation are mediated via the Th2 cell-signaling pathway (30). The present study administrated 10 mg/kg SHO and GIN via i.p. injection in mice. The dose for i.p. injection was selected based on previous studies (31,32). The current results revealed the anti-asthmatic effects of SHO and GIN on the cells, as determined using inflammatory cell counting and histological examination in BALF samples and lung tissue. The present study also examined the compounds' effects on the balance of antioxidant enzymes in lung tissues and their anti-allergic effects on mast cells RBL-2H3.
The accumulation of inflammatory cells is associated with an allergic response in asthma (33). Among the inflammatory cells, eosinophils are most important in chronic asthma. High levels of eosinophils have been found in asthmatic sputum, blood and BALF (34). Therefore, the current study investigated the effects of SHO and GIN on the recruitment of inflammatory cells, such as lymphocytes, neutrophils, macrophages and eosinophils, in the BALF from different treatment groups. SHO and GIN had a significant impact on the accumulation of inflammatory cells, including eosinophils, in BALF. A significant decrease occurred in the eosinophils in the OVA + SHO and OVA + GIN groups compared with the OVA group. Furthermore, the histological results indicated that the overall inflammation and eosinophilia were substantially inhibited. Eosinophils produce inflammatory cytokines, lipid mediators, eosinophil extracellular traps and ROS that can cause inflammation and asthma symptoms (35,36).
SHO and GIN also significantly reduced mucus production in lung tissues. Mucus production plays a vital role in airway narrowing, obstruction and hyperresponsiveness in asthma (37). Therefore, SHO and GIN could treat allergic asthma by suppressing eosinophil infiltration in the lung tissues and inhibiting eosinophil accumulation. However, it is not sufficient to judge whether SHO and GIN can inhibit eosinophilic asthma. Therefore, the current study also confirmed the levels of inflammatory cytokines, namely IL-4, IL-5 and IL-13, which are associated with allergic responses and eosinophilia (38-40). The present results supported those reported by Tavernier et al (41). It was found that SHO and GIN significantly decreased IL-4, IL-5 and IL-13 levels, according to the inflammatory cell counting results.
Th2 cell cytokines, including IL-4, IL-5 and IL-13, are typical in asthma (42). The main characteristic of the OVA-induced asthma mouse model is the allergic response through IL-4(43). In the present study, the gene expression analysis verified that OVA induced allergic asthma via high levels of IL-4. IL-4 broadly influences the pathogenesis of allergic asthma, including airway inflammation, eosinophilia and bronchial hyperresponsiveness, through Th2 cell proliferation (44,45). Furthermore, IL-4 and IL-13 are cytokines that induce allergic reactions in asthma and worsen lung inflammation (46,47). IL-5 affects the development of eosinophilic inflammation in asthma. Moreover, IL-5 is involved in the production, differentiation, maturation and activation of eosinophils (48). Finally, these reactions can lead to airway inflammation in lung epithelial cells by increasing mucus production and bronchial hyperresponsiveness. Therefore, SHO and GIN likely suppressed the allergic response and eosinophilic inflammation by inhibiting the production of inflammatory cytokines in the OVA-induced asthma mouse model. However, the present study did not provide any evidence regarding the Th2 pathway, such as the changes of Th cells in different groups. Thus, the effect of SHO and GIN on Th cells using the Jurkat cell line should be investigated to confirm this association.
SHO and GIN also caused a significant dose-dependent decrease in mast cell degranulation in the RBL-2H3 cells. Mast cells, which secrete various allergy mediators such as histamines and β-hexosaminidase, have emerged as primary cells in the pathogenesis of allergic asthma (49). IgE-bound mast cells release various allergic mediators, such as histamines, serotonin and β-hexosaminidase, which can induce inflammation. Among the mediators of mast cell degranulation, β-hexosaminidase is central in airway remodeling and inflammation (50). Thus, the current study measured the release of β-hexosaminidase to verify mast cell degranulation. RBL-2H3 cells were sensitized with IgE to induce degranulation. Treatment with SHO or GIN reduced the degranulation of these cells, thereby confirming them in vitro anti-allergic effects and supporting previous results.
The current study also examined the cytotoxicity of SHO and GIN in the RBL-2H3 and Jurkat cells using CCK-8 assays, which revealed that SHO and GIN were not toxic.
In asthma, oxidative stress causes inflammation of the epithelial cells (51-53). The present study determined the expression levels of the antioxidant genes in lung tissues to verify the mechanism underlying SHO's and GIN's inhibition of inflammation. Both have been considered to possess antioxidant and anti-inflammatory properties, and SHO has exhibited more potency (54). SHO can attenuate inflammation and oxidative stress by modulating nuclear factor-erythroid factor 2-related factor 2 signaling in human epidermal keratinocytes (55), as well as has been shown to ameliorate oxidative stress and inflammation in an induced middle-cerebral-artery occlusion mouse model (56). Moreover, GIN has the potential to protect against arsenic-induced oxidative stress in the pancreas by increasing the levels of antioxidant proteins, such as GPx, catalase and SOD (57).
However, GPx-1/2 was found to have different mRNA and protein expression levels. It was predicted that GPx-1/2 mRNA was produced to protect cells against ROS in the OVA group but was not converted to protein. Numerous factors come into play at the translation from mRNA to protein (58). Moreover, the difference in mRNA and protein levels may be observed due to time differences. Protein and mRNA cannot always present equally (59); therefore, future studies should confirm the relationship and reason for the different expression levels. The present study demonstrated that GPx-1 was upregulated by GIN, as the protein is the last form of the gene that performs the final function. SHO and GIN were found to reduce oxidative stress by regulating SOD1, SOD2, catalase and GPx-1. SOD1, GPx-1 and catalase expression levels were increased in the OVA + GIN group and may regulate the balance of antioxidant proteins in lung epithelial cells. However, SOD2 expression was decreased in the OVA + SHO and OVA + GIN groups, but the levels of antioxidant proteins in these groups were similar to those of the control group. These data indicate that SHO and GIN can suppress oxidative stress.
In summary, the present study identified that SHO and GIN effectively suppressed the allergic response in an OVA-induced asthma mouse model by inhibiting inflammatory cell infiltration, airway mucus production and Th2 cell-mediated inflammatory cytokine production in lung tissues. In addition, both reagents can regulate the oxidant/antioxidant proteins to suppress oxidative stress. Therefore, the current findings support the therapeutic application of SHO and GIN for patients with allergic and eosinophilic asthma.
Supplementary Material
Acknowledgements
The authors thank Professor Jong-Hwan Park (Laboratory Animal Medicine, Chonnam National University, South Korea) and Professor Dong-Soon Im (College of Pharmacy, Pusan National University, South Korea) for technical assistance with this research.
Funding Statement
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (grant nos. 2020R1I1A2075315, 2020R1I1A1A01074542 and 2020R1A4A1018280).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
EK and SJ designed and performed the experiments. EK, HH, HK, HJK, NEC, HI and HZ performed formal analysis, including statistical analysis and other techniques to analyze or synthesize study data. YSC and YS designed the methods and contributed to animal experiments. SHK, JKY and SL contributed to analysis and interpretation of data and drafted the manuscript. EK, SJ and HJK confirmed the authenticity of raw data. MOK and ZYR contributed to conception and design and supervised the experiments and reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Kyungpook National University Industry Foundation approved this study for animal experiments (approval no. 2018-0140).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Okuyama K, Ohwada K, Sakurada S, Sato N, Sora I, Tamura G, Takayanagi M, Ohno I. The distinctive effects of acute and chronic psychological stress on airway inflammation in a murine model of allergic asthma. Allergol Int. 2007;56:29–35. doi: 10.2332/allergolint.O-06-435. [DOI] [PubMed] [Google Scholar]
- 2.Meyer EH, DeKruyff RH, Umetsu DT. T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med. 2008;59:281–292. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Li C, Guo S. Effects of inhaled inactivated Mycobacterium phlei on airway inflammation in mouse asthmatic models. J Aerosol Med Pulm Drug Deliv. 2012;25:96–103. doi: 10.1089/jamp.2011.0904. [DOI] [PubMed] [Google Scholar]
- 4.O'Garra A. Commitment factors for T helper cells. Curr Biol. 2000;10:R492–R494. doi: 10.1016/s0960-9822(00)00556-x. [DOI] [PubMed] [Google Scholar]
- 5.Williams AS, Eynott PR, Leung SY, Nath P, Jupp R, De Sanctis GT, Resnick R, Adcock IM, Chung KF. Role of cathepsin S in ozone-induced airway hyperresponsiveness and inflammation. Pulm Pharmacol Ther. 2009;22:27–32. doi: 10.1016/j.pupt.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Walker JA, McKenzie AN. TH2 cell development and function. Nat Rev Immunol. 2018;18:121–133. doi: 10.1038/nri.2017.118. [DOI] [PubMed] [Google Scholar]
- 7.Asano T, Kume H, Taki F, Ito S, Hasegawa Y. Thalidomide attenuates airway hyperresponsiveness and eosinophilic inflammation in a murine model of allergic asthma. Biol Pharm Bull. 2010;33:1028–1032. doi: 10.1248/bpb.33.1028. [DOI] [PubMed] [Google Scholar]
- 8.Heo JY, Im DS. Anti-allergic effects of salvianolic acid A and tanshinone IIA from Salvia miltiorrhiza determined using in vivo and in vitro experiments. Int Immunopharmacol. 2019;67:69–77. doi: 10.1016/j.intimp.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Ishii T, Niikura Y, Kurata K, Muroi M, Tanamoto K, Nagase T, Sakaguchi M, Yamashita N. Time-dependent distinct roles of Toll-like receptor 4 in a house dust mite-induced asthma mouse model. Scand J Immunol. 2018;87(e12641) doi: 10.1111/sji.12641. [DOI] [PubMed] [Google Scholar]
- 10.White MC, Etzel RA, Wilcox WD, Lloyd C. Exacerbations of childhood asthma and ozone pollution in Atlanta. Environ Res. 1994;65:56–68. doi: 10.1006/enrs.1994.1021. [DOI] [PubMed] [Google Scholar]
- 11.Perez L, Declercq C, Iñiguez C, Aguilera I, Badaloni C, Ballester F, Bouland C, Chanel O, Cirarda FB, Forastiere F, et al. Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network) Eur Respir J. 2013;42:594–605. doi: 10.1183/09031936.00031112. [DOI] [PubMed] [Google Scholar]
- 12.Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect. 1999;107:489–493. doi: 10.1289/ehp.99107489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett AG, Williams GM, Schwartz J, Neller AH, Best TL, Petroeschevsky AL, Simpson RW. Air pollution and child respiratory health: A case-crossover study in Australia and New Zealand. Am J Respir Crit Care Med. 2005;171:1272–1278. doi: 10.1164/rccm.200411-1586OC. [DOI] [PubMed] [Google Scholar]
- 14.Lipworth BJ. Clinical pharmacology of corticosteroids in bronchial asthma. Pharmacol Ther. 1993;58:173–209. doi: 10.1016/0163-7258(93)90049-j. [DOI] [PubMed] [Google Scholar]
- 15.Bjermer L, Diamant Z. Current and emerging nonsteroidal anti-inflammatory therapies targeting specific mechanisms in asthma and allergy. Treat Respir Med. 2004;3:235–246. doi: 10.2165/00151829-200403040-00004. [DOI] [PubMed] [Google Scholar]
- 16.Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15:457–465. doi: 10.1517/14740338.2016.1140743. [DOI] [PubMed] [Google Scholar]
- 17.Lee BK, Park SJ, Nam SY, Kang S, Hwang J, Lee SJ, Im DS. Anti-allergic effects of sesquiterpene lactones from Saussurea costus (Falc.) Lipsch. determined using in vivo and in vitro experiments. J Ethnopharmacol. 2018;213:256–261. doi: 10.1016/j.jep.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Liu YN, Zha WJ, Ma Y, Chen FF, Zhu W, Ge A, Zeng XN, Huang M. Galangin attenuates airway remodelling by inhibiting TGF-β1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci Rep. 2015;5(11758) doi: 10.1038/srep11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grzanna R, Lindmark L, Frondoza CG. Ginger - an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125–132. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 20.Prasad S, Tyagi AK. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract. 2015;2015(142979) doi: 10.1155/2015/142979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamoto Y, Ueno Y, Nakahashi E, Obayashi M, Sugihara K, Qiao S, Iida M, Kumasaka MY, Yajima I, Goto Y, et al. Prevention of allergic rhinitis by ginger and the molecular basis of immunosuppression by 6-gingerol through T cell inactivation. J Nutr Biochem. 2016;27:112–122. doi: 10.1016/j.jnutbio.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Yocum GT, Hwang JJ, Mikami M, Danielsson J, Kuforiji AS, Emala CW. Ginger and its bioactive component 6-shogaol mitigate lung inflammation in a murine asthma model. Am J Physiol Lung Cell Mol Physiol. 2020;318:L296–L303. doi: 10.1152/ajplung.00249.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Shi H, Qin S, Huang G, Chen Y, Xiao C, Xu H, Liang G, Xie Z, Qin X, Wu J, et al. Infiltration of eosinophils into the asthmatic airways caused by interleukin 5. Am J Respir Cell Mol Biol. 1997;16:220–224. doi: 10.1165/ajrcmb.16.3.9070605. [DOI] [PubMed] [Google Scholar]
- 25.Deo SS, Mistry KJ, Kakade AM, Niphadkar PV. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India. 2010;27:66–71. doi: 10.4103/0970-2113.63609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes PJ. Th2 cytokines and asthma: An introduction. Respir Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci Ö. Oxidative stress in asthma: Part of the puzzle. Pediatr Allergy Immunol. 2018;29:789–800. doi: 10.1111/pai.12965. [DOI] [PubMed] [Google Scholar]
- 28.Powell CV, Nash AA, Powers HJ, Primhak RA. Antioxidant status in asthma. Pediatr Pulmono. 1994;18:34–38. doi: 10.1002/ppul.1950180109. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad A, Shameem M, Husain Q. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Ann Thorac Med. 2012;7:226–232. doi: 10.4103/1817-1737.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar RK, Herbert C, Foster PS. The ‘classical’ ovalbumin challenge model of asthma in mice. Curr Drug Targets. 2008;9:485–494. doi: 10.2174/138945008784533561. [DOI] [PubMed] [Google Scholar]
- 31.Park G, Kim HG, Ju MS, Ha SK, Park Y, Kim SY, Oh MS. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson's disease models via anti-neuroinflammation. Acta Pharmacol Sin. 2013;34:1131–1139. doi: 10.1038/aps.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MO, Lee MH, Oi N, Kim SH, Bae KB, Huang Z, Kim DJ, Reddy K, Lee SY, Park SJ, et al. [6]-shogaol inhibits growth and induces apoptosis of non-small cell lung cancer cells by directly regulating Akt1/2. Carcinogenesis. 2014;35:683–691. doi: 10.1093/carcin/bgt365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogaert P, Tournoy KG, Naessens T, Grooten J. Where asthma and hypersensitivity pneumonitis meet and differ: Noneosinophilic severe asthma. Am J Pathol. 2009;174:3–13. doi: 10.2353/ajpath.2009.071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brussino L, Heffler E, Bucca C, Nicola S, Rolla G. doi: 10.1155/2018/7582057. Eosinophils target therapy for severe asthma: Critical points. Biomed Res Int: Oct 25, 2018 (Epub ahead of print). doi: 10.1155/2018/7582057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patadia MO, Murrill LL, Corey J. Asthma: Symptoms and presentation. Otolaryngol Clin North Am. 2014;47:23–32. doi: 10.1016/j.otc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Silveira JS, Antunes GL, Kaiber DB, da Costa MS, Marques EP, Ferreira FS, Gassen RB, Breda RV, Wyse ATS, Pitrez P, et al. Reactive oxygen species are involved in eosinophil extracellular traps release and in airway inflammation in asthma. J Cell Physiol. 2019;234:23633–23646. doi: 10.1002/jcp.28931. [DOI] [PubMed] [Google Scholar]
- 37.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 40.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50:975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Tavernier J, Plaetinck G, Guisez Y, van der Heyden J, Kips J, Peleman R, Devos R. The role of interleukin 5 in the production and function of eosinophils. In: Hematopoietic cell Growth Factors and their Receptors. Whetton AD and Gordon J (eds). Plenum Press, New York, NY, pp321-361, 1996. [Google Scholar]
- 42.Kips JC. Cytokines in asthma. Eur Respir J Suppl. 2001;34:24s–33s. doi: 10.1183/09031936.01.00229601. [DOI] [PubMed] [Google Scholar]
- 43.Debeuf N, Haspeslagh E, van Helden M, Hammad H, Lambrecht BN. Mouse models of asthma. Curr Protoc Mouse Biol. 2016;6:169–184. doi: 10.1002/cpmo.4. [DOI] [PubMed] [Google Scholar]
- 44.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999;162:6233–6237. [PubMed] [Google Scholar]
- 45.Trautmann A, Krohne G, Bröcker EB, Klein CE. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol. 1998;160:5053–5057. [PubMed] [Google Scholar]
- 46.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 47.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75:68–78. doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matucci A, Maggi E, Vultaggio A. Eosinophils, the IL-5/IL-5Rα axis, and the biologic effects of benralizumab in severe asthma. Respir Med. 2019;160(105819) doi: 10.1016/j.rmed.2019.105819. [DOI] [PubMed] [Google Scholar]
- 49.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 50.Tomasiak MM, Tomasiak M, Zietkowski Z, Skiepko R, Bodzenta-Lukaszyk A. N-acetyl-beta-hexosaminidase activity in asthma. Int Arch Allergy Immunol. 2008;146:133–137. doi: 10.1159/000113516. [DOI] [PubMed] [Google Scholar]
- 51.Mishra V, Banga J, Silveyra P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol Ther. 2018;181:169–182. doi: 10.1016/j.pharmthera.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erzurum SC. New insights in oxidant biology in asthma. Ann Am Thorac Soc. 2016;(Suppl 1): 13:S35–S9. doi: 10.1513/AnnalsATS.201506-385MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Comhair SA, Erzurum SC. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Chen F, Tang Y, Sun Y, Veeraraghavan VP, Mohan SK, Cui C. 6-shogaol, a active constiuents of ginger prevents UVB radiation mediated inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes (HaCaT cells) J Photochem Photobiol B. 2019;197(111518) doi: 10.1016/j.jphotobiol.2019.111518. [DOI] [PubMed] [Google Scholar]
- 56.Na JY, Song K, Lee JW, Kim S, Kwon J. Pretreatment of 6-shogaol attenuates oxidative stress and inflammation in middle cerebral artery occlusion-induced mice. Eur J Pharmacol. 2016;788:241–247. doi: 10.1016/j.ejphar.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 57.Chakraborty D, Mukherjee A, Sikdar S, Paul A, Ghosh S, Khuda-Bukhsh AR. [6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol Lett. 2012;210:34–43. doi: 10.1016/j.toxlet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci Rep. 2015;5(10775) doi: 10.1038/srep10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fortelny N, Overall CM, Pavlidis P, Freue GV. Can we predict protein from mRNA levels? Nature. 2017;547:E19–E20. doi: 10.1038/nature22293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.