Abstract
Tropical ophiuroid fauna belonging to the family Ophiolepididae are almost unknown. This study deals with the relative growth and morphometric traits of the ophiuroid Ophiolepis crassa from the Gulf of California, Mexico. Specimens examined in this study came from the Colección Nacional de Equinodermos, Universidad Nacional Autónoma de México, and were collected over soft bottoms off Punta Gorda. Thirteen anatomical features were measured in a total of 152 specimens, including disk diameter, arm length, as well as length and width of dorsal and ventral arm plates, and radial, oral, and adoral shields. Based on the range of values of the disk diameter, varying from 4 to 19 mm, we provided quantitative data on each anatomical measurement considering three size classes. Morphometric data were adjusted to a power equation to detect the degree of allometry in the growth of anatomical traits. Results indicated that all the ventral and dorsal plates, as well as the radial, oral, and adoral shields, suffer changes in shape during growth, but these changes are stronger in the plates. In addition, an analysis of symmetry applied to both right and left radial shields revealed that these structures remain nearly symmetrical during growth. The disk diameter vs arm/disk relationship indicated that the species is a surface dweller inhabitant of the seafloor. This study, based on a single sample collected in a restricted area of the eastern Pacific, provides useful quantitative information for further taxonomic, systematic, or biogeographic studies.
Keywords: Allometric relationships, Symmetry, Disk, Microstructures, Gulf of California
BACKGROUND
The class Ophiuroidea is the largest group among the phylum Echinodermata with 2,096 recognized species (Stöhr et al. 2020). Morphologically, the body of ophiuroids is characterized by a fivefold symmetry. The typical body plan is composed of a pentagonal to round central disk and five arms that sharply depart from the disk. However, some species exhibit six, seven, or up to ten arms which can branch once or many times (Stöhr et al. 2012). Commonly, species with branched arms are known as basket stars and those with no branched arms, as brittle stars. The maximum size of disk diameter in adults mostly varies between 3 and 50 mm, and the length of arms is commonly referred to as n times the disk diameter. This relationship, usually varying from 2 to 20, represents a useful parameter to compare morphologies and may be indicative of the life habits of species (Twitchett et al. 2005; Stöhr et al. 2012).
Ophiuroids occupy marine benthic habitats and can be found in all the oceans, from the poles to the equator and from the intertidal to the hadal zones (Stöhr et al. 2012); although some species can tolerate brackish environments (Hendler 1996). These animals constitute an important portion of both epi-and infaunal communities showing diverse life habits. They can be either surface or burrowing species, but they can also be found hidden in crevices and holes or living over a variety of benthic organisms, such as gorgonians, corals, sponges, and worm tubes (Stöhr et al. 2012; Mills and O’Hara 2013). The ecological significance of ophiuroids in benthic ecosystems can be verified regarding their distribution and abundance. At places and times, epifaunal ophiuroids form dense carpets over the seafloor, whereas burrowing species may account for thousands in infaunal communities (Davoult et al. 1994; Hendler 1996; Turon et al. 2000; Metaxas and Giffin 2004).
Ophiuroids exhibit diverse feeding strategies and a wide spectrum of food. They are mainly carnivores, deposit and suspension feeders, and even browsers. Carnivorous ophiuroids can be predators or scavengers and usually trap large particles, whereas deposit and suspension feeders trap small particles from the sediment and water (Warner 1982). Food items include small living fishes, squids, and crustaceans, dead animals, phyto-and zooplankton, and detritus (Warner 1982; O’Hara 2017). In turn, ophiuroids are eaten by fishes, crabs, shrimps, sea stars as well as other ophiuroids (Hendler 1996).
Allometric studies deal with changes in the morphological characters of living organisms during growth. These morphological features could be the size of a portion of an organism, its shape, and also its physiological, ecological, and behavioral characteristics (Ibáñez et al. 2018). In marine invertebrates, the study of morphometric traits along with allometric relationships of organisms constitute useful tools to identify groups of individuals morphologically similar, characterize sexual dimorphism, and can help explain behavioral and structural adaptations of organisms to the environment (Sanvicente-Añorve et al. 2010 2018; Alitto et al. 2019; Cepeda et al. 2019). Despite their utility, allometric studies concerning ophiuroids are very scarce (Borges et al. 2015).
Ophiolepis crassa is a brittle star belonging to the family Ophiolepididae. This species has been registered in the eastern tropical Pacific, from the Gulf of California to Ecuador, from 6 to 230 m deep, mostly over sandy and muddy bottoms (Maluf 1988; Laguarda-Figueras et al. 2011). In situ observations aboard an underwater vehicle revealed that the species forms dense aggregations in the seamounts of the Gulf of California (Aburto-Oropeza et al. 2010). As with other ophiolepidids, biological and ecological information concerning this species is scant. Taking a sample belonging to a single O. crassa population from the Gulf of California, in this work we present the first effort to examine the relative growth of the species, to quantify the inter-individual anatomical variability regarding different size classes, and to provide quantitative data that could help identify geographical variations in growth or anatomical features across the distributional range of the species or to distinguish O. crassa from morphologically similar species.
MATERIALS AND METHODS
Specimens analyzed in this study came from the Colección Nacional de Equinodermos M.E. Caso, from the Universidad Nacional Autónoma de México. Ophiuroids were collected off Punta Gorda, Gulf of California (Mexico) at 23.13°N, 109.45°W, at 75 m deep over soft bottom (Fig. 1).
Fig. 1.
Study area and location of sampling station.
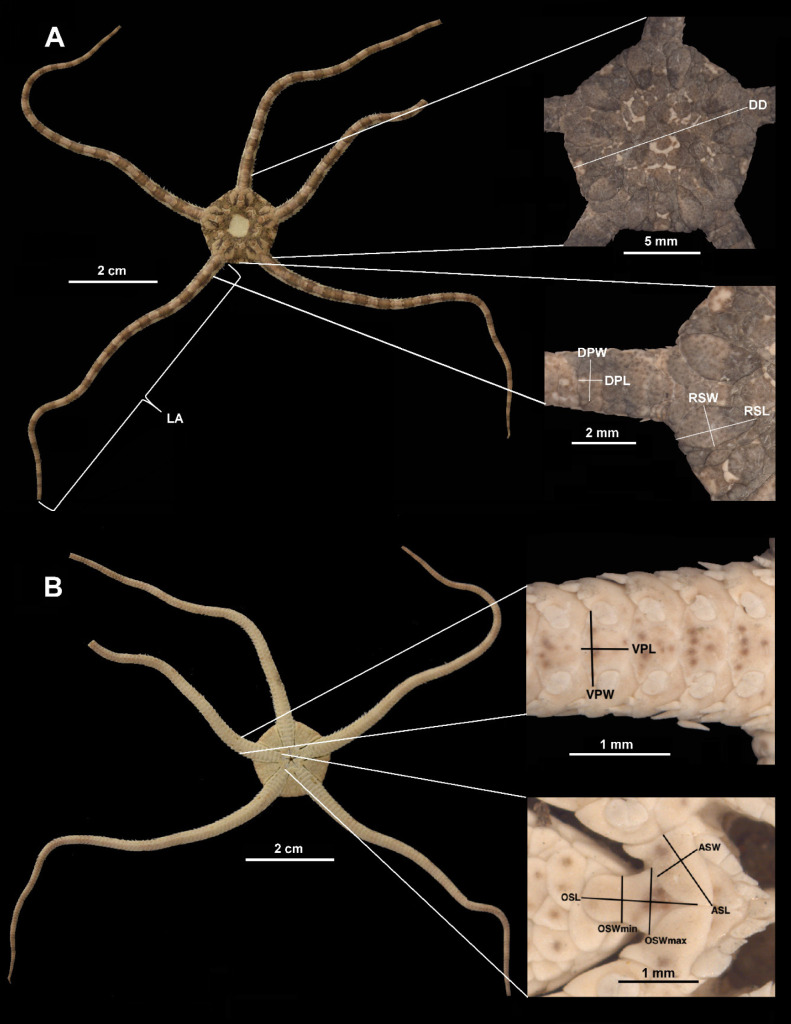
A total of 152 specimens were measured in 13 dimensions (Fig. 2, list of abbreviations). The length of arms was taken with a flexible tape measure, the disk diameter was measured with a digital Vernier caliper, and dimensions of microstructures (plates and shields) were obtained from a digital camera adapted to a stereoscopic microscope. For each individual, we considered the length of the longest arm (Márquez-Borrás et al. 2020), dimensions of the fourth ventral arm plate, and both right and left radial shields. We used the madreporite plate position to locate the measured radial shields, they were always located on the right side of the madreporite plate. Microstructure measurements were taken following Alitto et al. (2019 2020). All measurements were performed by only one person and checked with bivariate graphs to detect wrong data.
Fig. 2.
Ophiolepis crassa. Dorsal (A) and ventral (B) views. Anatomical measurements taken for each individual.
Afterward, three size classes were defined and the mean and standard deviation of each anatomical feature were calculated for each class. The size classes were chosen to evidence differences among structures, each size class having the same length. The coefficient of variation,
, was also calculated in some cases to evaluate the relative variability of the standard deviation related to the mean.
To analyze the changes in the growth of arms related to disk size and that of plates and shields, data were adjusted fitted to a power equation, y = axk, where x is the independent variable, y is the measurement under analysis, and k represents the growth parameter (Gould 1966). This non-linear equation becomes linear when log-transformed, from which the k parameter was estimated for comparison with the theoretical value of 1. The value of k indicates the allometry level in growth: if the k parameter is equal to 1, the growth is isometric, a value greater than 1 indicates positive allometric growth, and if k is lower than 1, the growth is negatively allometric. To detect if the k parameter differed significantly from 1, a
test was applied, where
and ,
, S* equal to the standard deviation of X or Y, r2 the coefficient of determination, and n the number of data (Zar 1999). These tests were carried out with a significance level of α = 0.05.
To prove if both right and left radial shields were symmetric, the asymmetry index (AI) proposed by van Valen (1962) was employed. For each individual, the index was calculated as
, where R and L are the dimensions of the right and left radial shields. Values of AI range from -1 (when the left measurement is larger) to 1 (the right one larger), and the zero value indicates a perfect mirror-symmetry.
RESULTS
Thirteen anatomical features were measured in a total of 152 specimens of Ophiolepis crassa. Disk diameters ranged from 4 to 19 mm, and based on them, three size classes were defined to give a summary (mean and standard deviation of each structure) of the data set (Table 1).
Table 1.
Morphometric data (mm) (mean ± SD) of Ophiolepis crassa specimens collected off Punta Gorda, Gulf of California, considering three size classes
| Anatomical trait | Disk diameter | |||||||||
| 4 to 8.9 | 9 to 13.9 | 14 to 19 | ||||||||
| Arm length | 25.96 | ± | 8.67 | 50.28 | ± | 13.29 | 71.64 | ± | 6.86 | |
| Dorsal arm plate length | 0.69 | ± | 0.13 | 0.82 | ± | 0.06 | 0.94 | ± | 0.06 | |
| Dorsal arm plate width | 1.12 | ± | 0.23 | 1.63 | ± | 0.26 | 2.20 | ± | 0.16 | |
| Ventral arm plate length | 0.68 | ± | 0.09 | 0.80 | ± | 0.06 | 0.93 | ± | 0.05 | |
| Ventral arm plate width | 0.94 | ± | 0.17 | 1.29 | ± | 0.19 | 1.67 | ± | 0.11 | |
| Radial shield length (right) | 1.57 | ± | 0.41 | 2.43 | ± | 0.45 | 3.31 | ± | 0.32 | |
| Radial shield width (right) | 1.02 | ± | 0.23 | 1.45 | ± | 0.24 | 1.94 | ± | 0.16 | |
| Radial shield length (left) | 1.58 | ± | 0.37 | 2.45 | ± | 0.45 | 3.32 | ± | 0.31 | |
| Radial shield width (left) | 1.03 | ± | 0.22 | 1.45 | ± | 0.25 | 1.92 | ± | 0.19 | |
| Oral shield length | 1.07 | ± | 0.18 | 1.38 | ± | 0.21 | 1.73 | ± | 0.16 | |
| Oral shield width (maximum) | 0.57 | ± | 0.09 | 0.72 | ± | 0.11 | 0.88 | ± | 0.12 | |
| Oral shield width (minimum) | 0.49 | ± | 0.07 | 0.62 | ± | 0.08 | 0.77 | ± | 0.08 | |
| Adoral shield length | 0.77 | ± | 0.11 | 1.01 | ± | 0.14 | 1.28 | ± | 0.10 | |
| Adoral shield width | 0.42 | ± | 0.06 | 0.53 | ± | 0.08 | 0.67 | ± | 0.06 | |
The length of arms varied between 10 and 79 mm and the AL/DD ratios showed a tendency to increase with DD size and ranged from 1.69 to 5.98 (Fig. 3). Also, estimations of the CV in the length of arms at each size class indicated a high dispersion of data around the mean: 33.3% in the first size class, 13.7% in the second, and 9.6% in the third.
Fig. 3.
Relationship between disk diameter (DD) and arm length/disk diameter (AL/DD) of Ophiolephis crassa individuals from the Gulf of California.
Symmetry analyses revealed that both left and right radial shields were nearly symmetric. The 95% of data had AI values between -0.051 and 0.056 for the length measurement, and between -0.073 and 0.074 for the width, indicating a high degree of symmetry.
The growth of arms related to disk diameter was positively allometric as deduced from the growth parameter (k = 1.43) and the t-student test. In turn, both the dorsal and ventral arms plates, as well as the oral, adoral, and radial shields showed a significant (t-test, p < 0.05) negative allometric growth (Fig. 4, Table 2). For these analyses, we took the largest dimension as the independent variable, that is, the width in the arm plates and the length in the shields. The growth of the length of the radial, oral, and adoral shields considering the disk diameter was isometric in the former and negatively allometric in the others (Fig. 4, Table 2). In other words, the ratio DD/RSL remains nearly constant (around 4.7) in all the size classes, whereas the equivalent ratio in the other shields increases with the disk size. Owing to the symmetry in the radial shields, the allometric analysis was only made for the right radial shield.
Fig. 4.
Adjustment to a power equation of several anatomical features (in mm) of Ophiolephis crassa from the Gulf of California. The degree of allometry is given in table 2.
Table 2.
Allometry in growth between several pairs of measurements taken for 152 individuals of Ophiolepis crassa collected off Punta Gorda, Gulf of California, Mexico
| Independent variable | Dependent variable | Growth parameter (k) | Allometry |
| DD | AL | 1.43 | + |
| DPW | DPL | 0.51 | – |
| VPW | VPL | 0.52 | – |
| RSL | RSW | 0.86 | – |
| OSL | OSW max | 0.87 | – |
| ASL | ASW | 0.91 | – |
| DD | OSL | 0.68 | – |
| DD | ASL | 0.69 | – |
| DD | RSL(right) | 1.05 | 0 |
+ positive allometry, – negative allometry, 0 isometry.
DISCUSSION
In recent years, the classification and phylogeny of ophiuroids have had important changes (Thuy and Stöhr 2016; O’Hara et al. 2018; Wilkie and Brogger 2018). In these works, authors recognized the importance of morphological characters, together with molecular data to make new proposals. However, due to changes in the shape of morphological features during growth, the taxonomic value of these structures decreases if the developmental stage of individuals is not mentioned (Stöhr 2012). In this study, we enhance the knowledge of ophiolepidid fauna by providing data on Ophiolepis crassa morphological structures regarding three size classes. These data could probably help to identify small individuals, detect clines across the distribution range of the species, and discriminate among morphologically similar species.
The values of the disk diameter of the examined sample of O. crassa ranged between 4 and 19 mm, indicating that the species is moderate in size. Some examples of brittle stars in the extreme of sizes could be Ophiodaphne formata, in which the disk diameter is 1 mm for males and 5 mm for females (Tominaga et al. 2004), and Ophiarachna incrassata, with a disc diameter of 55 mm (O’Hara 2017), probably the largest known brittle star.
The CV values showed a high percentage of variability around the mean in the length of arms, varying from 9.6% to 33.3%. This high dispersion of data (a third of the mean value in the first size class) can be attributed to arm autotomy and subsequent regeneration process. In the case of attacks of predators, brittle stars can shed a portion of an arm to distract the predator and allowing the prey to escape (O’Hara 2017). Ophiuroids exhibit an extraordinary power to regenerate their arms after a self-induced amputation; after an injury, individuals can totally regenerate the damaged arm in several weeks or months (Hendler 1996; Biressi et al. 2010). Even if predation is the most common cause of autotomy in ophiuroids, other factors, such as food availability, water quality, or physical disturbances can also affect the injury frequency and the regeneration times (Brooks et al. 2007). Potential predators of O. crassa in the Gulf of California could be the seastar Amphiaster insignis and fishes of the families Ophichthidae, Synodontidae, and Triglidae, animals seen sharing the same habitat as the brittle star (Aburto-Oropeza et al. 2010).
The AL/DD vs DD relationship (Fig. 3) confirms that the species has a surface dweller life habit. This deduction arose from the study of Twitchett et al. (2005) who classified life habits of brittle stars based on the AL/DD ratio: if AL/DD > 9, then the organisms have a burrowing life habit; if AL/DD < 5, these are epifaunal surface dwellers, with crevice dwellers as intermediate forms. Besides, the growth parameter (k = 1.43) of the DD vs AL relationship in O. crassa indicated a faster growth of arms related to disk size (Fig. 4). For all ophiuroids, arms play an important role during the feeding processes. For instance, in burrowing species, animals extend two to four arms through the sediment surface to trap food items; epifaunal detritus feeders can use their arms to accumulate sediments into small mounds from which they eat; suspension feeders uncoil their arms to expose them to major water currents, and carnivores coil the tips of arms to capture small living organisms, such as fishes (Hendler et al. 1995; Hendler 1996; Stancyk et al. 1998; O’Hara 2017). On the seabed of the Gulf of California, O. crassa feeds on detritus sinking through the water column (Aburto-Oropeza et al. 2010), probably switching from suspension to deposit feeder, depending on the sites of higher food availability.
Quantitative analyses revealed that both left and right radial shields were symmetric, but they grow in an allometric manner (Table 2). Some authors also observed considerable changes in the form of radial shields during the growth of ophiuroid individuals and emphasized the relevance of these changes to diagnose the species (Monteiro et al. 1992; Borges et al. 2015). Besides, in an exhaustive study, Borges et al. (2015) examined the morphological development of nine brittle star species and found that dorsal and ventral arm plates suffer changes of shape during growth in all the species, whereas oral and adoral shields remain almost unaltered in one of them. In the O. crassa individuals from the Gulf of California, all the microstructures here examined grow allometrically, but the degree of change they suffer is greater in the dorsal and ventral arm plates than in the radial, oral and adoral shields, as deduced from the k parameter (Fig. 4, Table 2). These k values may help to discriminate among populations or species.
CONCLUSIONS
This study, based on a large developmental growth series, provides valuable information on the morphometric traits of the ophiuroid Ophiolepis crassa. Data presented in this study offer the possibility to determine O. crassa individuals at different stages of development, can help to identify potential morphological variability of the species across their distribution range, and provides potentially relevant information for systematic, biogeographic, or phylogenetic studies.
Acknowledgments
This work was supported by the Universidad Nacional Autónoma de México. Authors thank the two anonymous reviewers for their constructive suggestions to improve the manuscript. Authors also appreciate the assistance of F. Zavala-García, A. Durán-González, L. Álvarez-Sánchez and J.L. Bortolini during the development of this work.
List of abbreviations
- AI
asymmetry index.
- AL
arm length.
- ASL
adoral shield length.
- ASW
adoral shield width.
- CV
coefficient of variation.
- DD
disk diameter.
- DPL
dorsal arm plate length.
- DPW
dorsal arm plate width.
- OSL
oral shield length.
- OSWmax
oral shield width (maximum).
- OSWmin
oral shield width (minimum).
- RSL
radial shield length.
- RSW
radial shield width.
- VPL
ventral arm plate length.
- VPW
ventral arm plate width.
Footnotes
Authors’ contributions: LSA and FSM designed the study. IRC performed the measurements. LSA analyzed the data and prepared the manuscript. All the authors discussed the results and approved the final manuscript.
Competing interests: The authors declare that they have no conflicts of interests.
Availability of data and materials: https://hdl. handle.net/20.500.12201/11327.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Aburto-Oropeza O, Caso M, Erisman B, Ezcurra E. (eds). 2010. Bitácora del mar profundo: una expedición por el golfo de California. Instituto Nacional de Ecología, UCMEXUS, Scripps Institution of Oceanography, Mexico City, Mexico.
- Alitto RAdS, Amaral ACZ, de Oliveira LD, Serrano H, Seger KR, Guilherme PDB et al. 2019. Atlantic west Ophiothrix spp. in the scope of integrative taxonomy: confirming the existence of Ophiothrix trindadensis Tommasi, 1970. PLoS ONE 14:e0210331. doi:10.1371/journal.pone.0210331. [DOI] [PMC free article] [PubMed]
- Alitto RAdS, Granadier G, Christensen AB, O’Hara T, Di Domenico M, Borges M. 2020. Unravelling the taxonomic identity of Ophiothela Verrill, 1867 (Ophiuroidea) along the Brazilian coast. J Mar Biol Assoc UK 100:413–426. doi:10.1017/S002531542000034X.
- Biressi ACM, Zou T, Dupont S, Dahlberg C, Di Benedetto C, Bonasoro F, Thorndyke M, Carnevali MDC. 2010. Wound healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): comparative morphogenesis and histogenesis. Zoomorphology 129:1‒19. doi:10.1007/s00435-009-0095-7.
- Borges M, Alitto RAdS, Amaral ACZ. 2015. From baby to adult: ontogenic series of nine species of Ophiuroidea from Atlantic Southwestern. Rev Biol Trop 63:361‒381. doi:10.15517/rbt. v63i2.23170.
- Brooks RA, Nizinski MS, Ross SW, Sulak KJ. 2007. Frequency of sublethal injury in a deepwater ophiuroid, Ophiacantha bidentata, an important component of the western Atlantic Lophelia reef communities. Mar Biol 152:307‒314. doi:10.1007/s00227-007-0690-4.
- Cepeda D, Álamo D, Sánchez N, Pardo F. 2019. Allometric growth in meiofaunal invertebrates: do all kinorhynchs show homogeneous trends? Zool J Linnean Soc 187:1041‒1060. doi:10.1093/zoolinnean/zlz083.
- Davoult D, Gounin F, Janquin MA. 1994. Experimental nutrition in the suspension-feeding ophiurid Ophiothrix fragilis (Abildgaard) as a function of chlorophyll a flux. In: David B, Guille A, Féral JP, Roux M (eds) Echinoderms through time. Balkema, Rotterdam.
- Gould SJ. 1966. Allometry and size in ontogeny and phylogeny. Biol Rev 41:587‒640. doi:10.1111/j.1469-185X.1966.tb01624.x. [DOI] [PubMed]
- Hendler G. 1996. Class Ophiuroidea. In: Blake JA, Scott PH, Lissner AL (eds) Taxonomic Atlas of the Santa Maria Basin and western Santa Barbara Channel. Vol. 14. Miscellaneous taxa. Santa Barbara Museum of Natural History, Santa Barbara, pp. 113‒179.
- Hendler G, Miller JE, Pawson DL, Kier PM. 1995. Sea stars, sea urchins and allies: echinoderms of Florida and the Caribbean. Smithsonian Institution Press, Washington DC, USA.
- Ibáñez CM, Sepúlveda RD, Sigwart JD. 2018. Comparative allometric variation in intertidal chitons (Polyplacophora: Chitonidae). Zoomorphology 137:249‒256. doi:10.1007/s00435-017-0387-2.
- Laguarda-Figueras A, Escandón-Flores N, Solís-Marín FA, Hernández-Herrejón LA, Durán-González A. 2011. Los ofiuroideos (Echinodermata: Ophiuroidea) del Golfo de California. SEMARNAT-INECC, Mexico City, Mexico.
- Maluf LY. 1988. Biogeography of the central eastern Pacific shelf echinoderms. In: Burke RD, Mladenov PV, Lambert P, Parsley R (eds) Echinoderm Biology. Proceedings of the Sixth International Echinoderm Conference. Victoria, Canada. 23‒28 August, 1988. Balkema, Rotterdam, Netherlands.
- Márquez-Borrás F, Solís-Marín FA, Mejía-Ortiz LM. 2020. Troglomorphism in the brittle star Ophionereis commutabilis Bribiesca-Contreras et al., 2019 (Echinodermata, Ophiuroidea, Ophionereididae). Subterr Biol 33:87–108. doi:10.3897/subtbiol.33.48721.
- Metaxas A, Giffin B. 2004. Dense beds of the ophiuroid Ophiacantha abyssicola on the continental slope off Nova Scotia, Canada. Deep-Sea Res 51:1307‒1317. doi:10.1016/j.dsr.2004.06.001.
- Mills VS, O’Hara T. 2013. Ophiuroids (Echinodermata: Ophiuroidea) of biogenic habitats on the continental shelf of New Zealand. Zootaxa 3613:401‒444. doi:10.11646/zootaxa.3613.5.1. [DOI] [PubMed]
- Monteiro AMG, Reis MO, Pardo EV. 1992. Morfologia comparativa e distribuição batimétrica de duas espécies de Ophiuroidea, na região costeira de Ubatuba. Bolm Inst oceanogr S Paulo 40:39‒53. doi:10.1590/S0373-55241992000100004.
- O’Hara TD. 2017. Class Ophiuroidea. In: Byrne M, O’Hara TD (eds) Australian echinoderms: biology, ecology and evolution. CSIRO Publishing, Clayton South, Australia.
- O’Hara T, Stöhr S, Hugall AF, Thuy B, Martynov A. 2018. Morphological diagnoses of higher taxa in Ophiuroidea (Echinodermata) in support of a new classification. Eur J Taxon 416:1‒35. doi:10.5852/ejt.2018.416.
- Sanvicente-Añorve L, Hermoso-Salazar M, Solís-Weiss V, Salgado-Ugarte IH. 2010. Carapace relative growth of Trapezia Latreille, 1828 (Decapoda, Brachyura), crabs that are symbionts of hard corals, from Clipperton Atoll and the Revillagigedo islands: ecological and zoogeographical implications. Crustaceana 83:1371‒1383. doi:10.1163/001121610X533520.
- Sanvicente-Añorve L, Rodríguez-Vázquez R, Lemus-Santana E, Alatorre-Mendieta M, Reguero M. 2018. Variaciones estacionales de la comunidad de quitones (Mollusca: Polyplacophora) en una zona intermareal rocosa del sur del Golfo de México. Rev Biol Mar Oceanogr 53:19‒26. doi:10.4067/S0718-19572018000100019.
- Stancyk SE, Muir C, Fujita T. 1998. Predation behavior on swimming organisms by Ophiura sarsii. In: Mooi R, Telford M (eds) Echinoderms: San Francisco. Balkema, Rotterdam.
- Stöhr S. 2012. Ophiuroid (Echinodermata) systematics –where do we come from, where do we stand and where should we go? Zoosymposia 7:147‒161. doi:10.11646/zoosymposia.7.1.14.
- Stöhr S, O’Hara T, Thuy B. 2012. Global diversity of brittle stars (Echinodermata: Ophiuroidea). PLoS ONE 7:e31940. doi:10.1371/journal.pone.0031940. [DOI] [PMC free article] [PubMed]
- Stöhr S, O’Hara T, Thuy B (eds). 2020. World Ophiuroidea database. http://www.marinespecies.org/ophiuroidea. Accessed 17 June 2020.
- Thuy B, Stöhr S. 2016. A new morphological phylogeny of the Ophiuroidea (Echinodermata) accords with molecular evidence and renders microfossils accessible for cladistics. PLoS ONE 11:e0156140. doi:10.1371/journal.pone.0156140. [DOI] [PMC free article] [PubMed]
- Tominaga H, Nakamura S, Komatsu M. 2004. Reproduction and development of the conspicuously dimorphic brittle star Ophiodaphne formata (Ophiuroidea). Biol Bull 206:25‒34. doi:10.2307/1543195. [DOI] [PubMed]
- Turon X, Codina M, Tarjuelo I, Uriz MJ, Becerro MA. 2000. Mass recruitment of Ophiothrix fragilis (Ophiuroidea) on sponges: settlement patterns and post-settlement dynamics. Mar Ecol Prog Ser 200:201‒212. doi:10.3354/meps200201.
- Twitchett RJ, Feinberg JM, O’Connor DD, Alvarez W, Mccollum LB. 2005. Early Triassic ophiuroids: their paleoecology, taphonomy, and distribution. Palaios 20:213‒223. doi:10.2110/palo.2004. p04-30.
- van Valen L. 1962. A study of fluctuating asymmetry. Evolution 16:125‒142. doi:10.1111/j.1558-5646.1962.tb03206.x.
- Warner GF. 1982. Food and feeding mechanisms: Ophiuroidea. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam.
- Wilkie IC, Brogger MI. 2018. The peristomial plates of ophiuroids (Echinodermata: Ophiuroidea) highlight an incongruence between morphology and proposed phylogenies. PLoS ONE 13:e0202046. doi:10.1371/journal.pone.0202046. [DOI] [PMC free article] [PubMed]
- Zar JH. 1999. Biostatistical analysis. Prentice Hall, Englewood Cliffs, USA.