CONFLICT OF INTEREST
None declared.
Dear Editor,
Bullous pemphigoid (BP) is an autoimmune disease that tends to occur in elderly individuals. Here, we report a case that developed BP after receiving the second dose of coronavirus disease 19 (COVID‐19) vaccine.
The patient, an 83‐year‐old Japanese woman with bipolar disorder, was treated for xerotic eczema in a clinic and her eczema was relieved with topical steroids. Three days after receiving the second dose of tozinameran, the BNT162b2 mRNA COVID‐19 vaccine, erythema and blisters rapidly appeared all over the body. The patient visited the clinic 1 week later. Anti‐BP180 NC16A antibody levels were high (11 530 U/mL; reference value, <9). Skin biopsy revealed subepidermal blisters with eosinophil infiltration (Figure 1a,b), and direct immunofluorescence showed the linear deposition of immunoglobulin G at the basement membrane zone (Figure 1c). The patient was diagnosed with BP. Although oral prednisolone (PSL) was initiated at 30 mg/day (0.5 mg/kg/day), her condition was not improved. One week after the PSL initiation, she was referred to our hospital.
FIGURE 1.
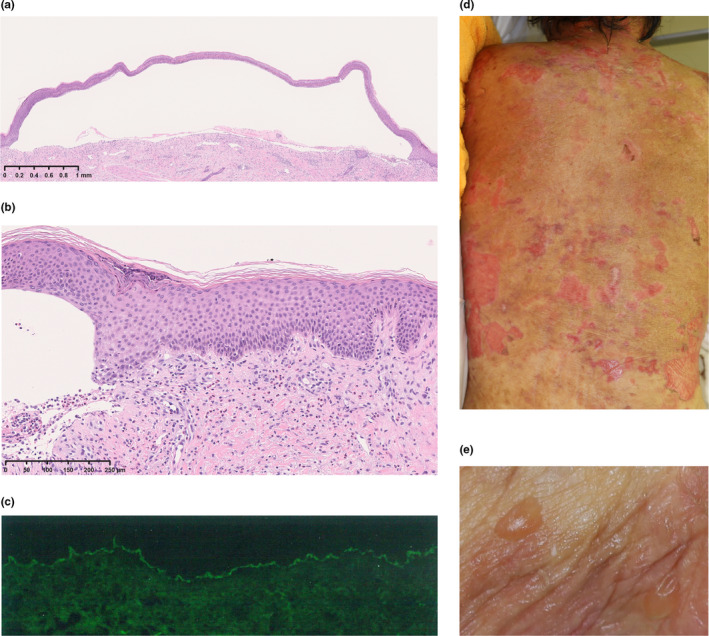
(a,b) Histopathological images (hematoxylin–eosin). (a) Subepidermal bulla (original magnification ×5) and (b) vacuolar degeneration at the epidermis−dermis junction with eosinophilic infiltration (×100). (c) Direct immunofluorescence showed the linear deposition of immunoglobulin G at the basement membrane zone. (d,e) Clinical images. (d) Diffuse erythema and erosion and (e) blisters
She had generalized erythema with erosion and blister (Figure 1d,e). The PSL dosage was increased to 60 mg/day (1 mg/kg/day). However, the blisters continued to develop. Therefore, we started steroid pulse therapy and high‐dose i.v. immunoglobulin therapy, which led to the cessation of blister formation. We readministered PSL, of which the dose was tapered gradually, and no rashes were seen at this time. In addition, she did not take a dipeptidyl peptidase‐IV inhibitor, a diabetes medicine.
In the present case, the rashes appeared immediately after the second dose of the vaccine, and we made the diagnosis with vaccine‐induced BP. BP is an autoimmune bullous disease caused by autoantibodies chiefly against BP180, a component of the hemidesmosomes in the basement membrane zone. Recently, cases of new or recurrent BP and pemphigus vulgaris after administration of COVID‐19 vaccination has been reported. 1 , 2 , 3 Interestingly, among four cases of BP180 antibody‐positive BP developing after the Comirnaty (Pfizer), all developed the symptoms after receiving the second dose of the vaccine. 2
It has been reported that BP also occurs following vaccination against influenza, tetanus, and meningococcus vaccines. 4 , 5 The mechanism of vaccine‐induced BP remains to be elucidated. The possibility of autoantibody cross‐reaction would be negative because of the lack of structural similarity between vaccines and basement membrane antigens. These vaccines may rather enhance autoimmune reaction in the patients with the predisposition to BP.
In conclusion, it would be better to know the possibility of BP development after administration of the vaccines, including COVID‐19 vaccine.
ACKNOWLEDGMENT
This research did not receive any grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
REFERENCES
- 1. Pérez‐López I, Moyano‐Bueno D, Ruiz‐Villaverde R. Bullous pemphigoid and COVID‐19 vaccine. Med Clin (Barc). 2021. [Epub ahead of print]. 10.1016/j.medcli.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tomayko MM, Damsky W, Fathy R, McMahon DE, Turner N, Valentin MN, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID‐19 vaccination. J Allergy Clin Immunol. 2021;148:750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID‐19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol. 2021. 35:e645–e647. 10.1111/jdv.17472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García‐Doval I, Mayo E, Fariña JN, Cruces MJ. Bullous pemphigoid triggered by influenza vaccination? Ecological study in Galicia, Spain. Br J Dermatol. 2006;155:820–3. [DOI] [PubMed] [Google Scholar]
- 5. Navarro‐Navarro I, Jiménez‐Gallo D, Valenzuela‐Ubiña S, Domínguez‐Gomez M, Linares‐Barrios M. Infantile bullous pemphigoid following serogroup B meningococcal vaccination. Br J Dermatol. 2021;184:e53. [DOI] [PubMed] [Google Scholar]


