Dear Editor,
We present a case of a 63‐year‐old female who developed toxic myopathy and liver damage after SARS‐CoV‐2 infection. She received her first renal transplant in 1989 for treatment of chronic glomerulonephritis. Her allograft failed in 1996 and renal function was replaced with intermittent hemodialysis until the second transplantation, which was performed in January 2001. She was treated with triple immunosuppressive therapy—tacrolimus, mycophenolate mofetil, and steroids. Also, in chronic therapy, she had atorvastatin 80 mg/day and ezetimibe 10 mg/day since April 2015, when she experienced myocardial infarction with implantation of stents in the coronary arteries. In April 2021, she was admitted to hospital due to SARS CoV‐2 infection with consequent pneumonia, which was treated with remdesivir, ceftriaxone, and dexamethasone, also with tacrolimus reduction and mycophenolate cessation. A few days after discharge from the hospital, she developed weakness of the proximal muscles of the arms and legs, which prevented her from getting up, walking, and leaning on her arms.
In laboratory tests, there were highly elevated levels of creatine kinase (CK) 9184 U/L (normal range < 153 U/L) and liver enzymes—alanine aminotransferase (ALT) 516 U/L (10–36) and aspartate aminotransferase (AST) 455 U/L (8–30). Therefore, atorvastatin and ezetimibe were immediately excluded from the therapy, which resulted in complete normalization levels of CK and liver enzymes (ALT and AST) and regression of symptoms (Figure 1).
FIGURE 1.
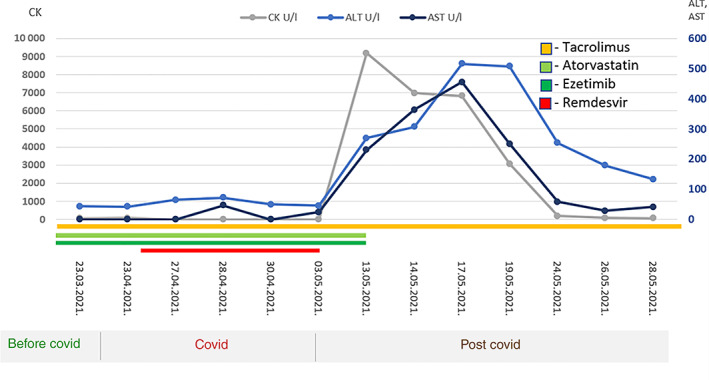
Changes in CK, ALT, and AST values over time, relative to drug administration and exclusion (remdesivir, atorvastatin, ezetimibe, and tacrolimus)
The performed immunological, virological, hepatological, and neurological diagnostic tests did not find a pathological substrate that would explain the muscular and liver lesion. Further pharmacogenetic testing verified the reduced activity of the cytochrome P450 3A4 (CYP3A4) enzyme and the patient being an intermediate metabolizer of substrate drugs—atorvastatin, tacrolimus, as well as remdesivir. Also, according to the genotyping of the transport protein organic anion transporting polypeptides 1B1 (OATP1B1), there was a significant genetic predisposition for side effects of the statin myotoxicity type because the variant SCLO1B1 521CC results in reduced statin transfer in the liver. Based on these findings, we concluded that myotoxicity and liver damage resulted from the combination of therapy with tacrolimus, remdesivir, and high doses of atorvastatin.
The reported rates of serious adverse events among all statins as a class have been deficient accounting (<1%). The most common is a slight risk for the elevation of liver enzymes and myopathy [1]. The incidence of myopathy associated with statin therapy is dose‐related. It is increased when statins are used in combination with agents that share common metabolic pathways such as other lipid‐lowering agents (fibrates and niacin), as well as immunosuppressive drugs (cyclosporine A) [2]. Increased systemic exposure to statins and consequent risk for complications has been reported in patients concomitantly treated with cyclosporin A with inhibition of drug catabolism by cytochrome CYP3A4 or drug transport by P‐glycoprotein (PGP) and organic anion transporting polypeptide OATP1B1 being associated with this effect. It is not known whether the combination of statins and tacrolimus also suffers from this drawback [3]. The common adverse event noted during compassionate use of remdesivir in patients with COVID‐19 by Grein et al. include rash, diarrhea, hypotension, abnormal liver function, and renal impairment. Serious adverse events (acute kidney injury, septic shock, and multiorgan failure) were noted in 23% patients, while 60% had at least one adverse event and 8% discontinued due to various side effect of remdesivir [4]. Until the present illness, our patient did not have any side effects associated with prescribed therapy.
To our best knowledge, this is the first case report about the combination of atorvastatin, remdesivir, ezetimibe, and tacrolimus related to myopathy and liver damage. This case report has emphasized the alert to the potential for drug–drug interactions to minimize the risk of myopathy during long‐term statin therapy in patients at high risk for coronary heart disease. Although pharmacogenomic testing is not widely available and diagnosis of drug‐induced toxicity is often set “per exclusionem,” clinicians should be aware of this differential diagnosis to minimize the risk of severe adverse events, especially in the population of immunosuppressed patients.
[Correction added on 11 November 2021, after first online publication: authors' affiliation links have been corrected.]
REFERENCES
- 1. Bottorff M, Hansten P. Long‐term safety of hepatic hydroxymethyl glutaryl coenzyme a reductase inhibitors. Arch Intern Med. 2000;160(15):2273–80. [DOI] [PubMed] [Google Scholar]
- 2. Ballantyne CM, Corsini A, Davidson MH, Holdaas H, Jacobson TA, Leitersdorf E, et al. Risk for myopathy with statin therapy in high‐risk patients. Arch Intern Med. 2003;163(5):553–64. [DOI] [PubMed] [Google Scholar]
- 3. Lemahieu WPD, Hermann M, Asberg A, Verbeke K, Holdaas H, Vanrenterghem Y, et al. Combined therapy with atorvastatin and calcineurin inhibitors: no interactions with tacrolimus. Am J Transplant. 2005;5(9):2236–43. [DOI] [PubMed] [Google Scholar]
- 4. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid‐19. N Engl J Med. 2020;382(24):2327–36. 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]


