Abstract
Effective vaccines for prevention of severe course and lethal outcome of coronavirus disease 2019 have been developed and approved in regulatory rolling and fast‐track procedures; they are now widely distributed worldwide. Data about cutaneous side‐effects of the new mRNA‐type vaccines is scant, however. We herein report two similar cases of cutaneous adverse drug reactions (ADR) mimicking Rowell’s syndrome that occurred after the first dose of BNT162b2 and mRNA‐1273, respectively. Both patients achieved prompt clinical improvement with a short pulse of oral prednisolone and non‐steroidal inflammatory drugs. We suspect this phenomenon to occur in a timeframe of 7–14 days after vaccination due to an interferon‐γ‐driven shift towards type I immunity in susceptible individuals. As rheumatic patients were excluded from phase III clinical trials and as most countries prioritized the elderly population to receive the vaccinations first, cutaneous ADR might become more frequent once the younger part of the population is vaccinated over the course of 2021. Atypical cutaneous ADR might be misinterpreted or overlooked by non‐dermatologists. Further studies are required to determine the best suitable vaccine types for individual groups of patients.
Keywords: adverse event, coronavirus disease 2019, cutaneous lupus erythematosus, Rowell’s syndrome, vaccines
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has led to rapid invention and approval of vaccines against the causing pathogen, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Cutaneous adverse drug reactions (ADR) to mRNA‐based vaccines seem to be frequent events and include, among others, itching, erythema, swelling, pernio‐like lesions, and generalized rashes. 1 , 2 , 3 Flares of pre‐existing chronic inflammatory dermatoses might be underreported. We recently described a case of exacerbation of long‐standing subacute cutaneous lupus erythematosus (SCLE) in the course of the first dose of BNT162b2 (BioNTech/Pfizer). 4 De novo onset of Rowell’s syndrome (RS) has been observed after the first dose of the same vaccine in an elderly woman, as published previously. 5 RS was originally described as cutaneous lupus erythematosus (CLE) associated with erythema multiforme (EM)‐like lesions with immunological findings of speckled antinuclear antibodies (ANA), anti‐La/anti‐SS‐B antibodies, and detection of rheumatoid factor (RF). Due to its rarity, this entity is still a matter of debate. New diagnostic criteria were proposed 20 years ago and presupposed presence of all indispensable major criteria (lupus erythematosus including CLE, EM‐like lesions with or without involvement of mucous membranes and speckled ANA) and at least one minor criteria (chilblains, anti‐Ro/anti‐SSA or anti‐La/anti‐SSB antibodies, RF). 6 We herein report two patients who experienced clinical findings mimicking RS in the course of COVID‐19 mRNA vaccination and speculate about the pathophysiological background in light of the available data.
2. CASE REPORTS
2.1. Case 1
A 41‐year‐old male patient had been on daily medication with hydroxychloroquine 200 mg p.o. and prednisolone 5 mg p.o. for 4 years due to rheumatic joint stiffness as well as Raynaud’s syndrome and puffy fingers. As serology revealed a high titer of ANA (1:10 240) and anti‐U1‐RNP antibodies, he was diagnosed with mixed connective tissue disease. He had never experienced typical symptoms of CLE and was healthy otherwise, being a non‐smoker without any other comedication. His family history included neither skin nor rheumatic diseases. Four days after the first dose of BNT162b2 he experienced fatigue and subfebrile temperatures of 38.4°C. Within the following week, generalized annular plaques were noted (Figure 1a,b), the lesions were stinging rather than itching. A full blood count showed slight thrombocytosis; speckled ANA and anti‐U1‐RNP antibodies were still detectable in absence of RF and anti‐citrullinated cyclic protein antibodies. Negativity for anti‐SSA/SSB antibodies was identified via immunoblot. Histopathological examination of a skin biopsy from the trunk revealed a patchy lymphocytic infiltrate with discrete vacuolar alteration along the dermoepidermal junction (Figure 1c–e). To follow up on the inflammatory reaction in the skin, we performed immunohistochemical stains to detect the presence of T cells, B cells, and plasmacytoid dendritic cells, and identified a primarily T‐cellular pattern. We detected a strong antiviral response as indicated by interferon (IFN)‐induced GTP‐binding protein Mx1 (MxA) (Figure 1d). Direct immunofluorescence (DIF) was unspecific (Figure 1f). A short pulse with prednisolone 1 mg/kg bodyweight p.o. in combination with topical corticosteroids yielded prompt improvement of all symptoms. The aforementioned diagnostic criteria of RS were not fulfilled; hence, we diagnosed drug‐induced SCLE mimicking RS. Therapy with hydroxychloroquine was maintained and methotrexate 15 mg s.c. was added to prevent relapses. Interestingly, the second dose was administrated as scheduled and tolerated very well.
FIGURE 1.
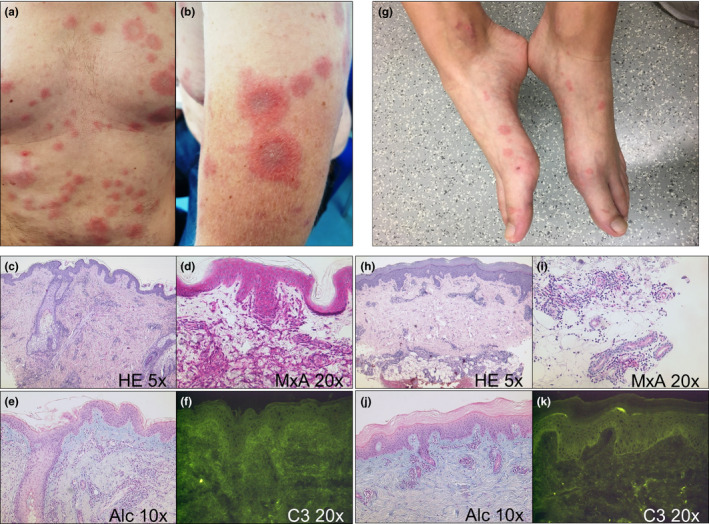
Clinical and histological findings of the patients. (a) Intense flare of generalized annular plaques ten days after the first dose of BNT162b2 on the chest and abdomen of this 41‐year‐old male patient. (b) Magnification of a lesion on the left arm. (c) Patchy perivascular and periadnexial lymphocytic infiltrate and minimal vacuolar alteration along the dermo‐epidermal junction (HE, 5x lens, original magnification 50x). (d) Both keratinocytes and inflammatory cells display a strong interferon signature as indicated by MxA (MxA, 20x lens, original magnification 200x). (e) Abundance of mucin in the papillary dermis (alcian blue, 10x lens, original magnification 100x). (f) Unspecific direct immunofluorescence (C3, 20x lens, original magnification 200x). (g) Slightly elevated annular papules and plaques without scaling on extensor sides of feet of this 22‐year‐old female patient ten days after the first dose of mRNA‐1273 vaccine. (h) Moderately dense superficial and deep perivascular lymphocytic infiltrate with slight interface dermatitis (HE, 5x lens, original magnification 50x). (i) Adnexal epithelium of eccrine sweat glands and the inflammatory infiltrate moderately express MxA (MxA, 20x lens, original magnification 200x). (j) Abundance of mucin similar to the other patient (alcian blue, 10x lens, original magnification 100x). (k) Slight granular deposits of C3 along the dermo‐epidermal junction (C3, 20x lens, original magnification 200x).
2.2. Case 2
A 22‐year‐old female patient, who was healthy except for hypothyroidism, was referred to the emergency department with abrupt onset of itchy and painful annular erythematous lesions of extensor surfaces including hands and feet (Figure 1g). Other symptoms included joint pain, fatigue, and vomiting but no fever. Ten days earlier she had received the first dose of mRNA‐1273 (Moderna) without any localized ADR except for muscle pain. Her family history included neither skin nor rheumatic diseases. Laboratory investigations were normal except for low titers of speckled ANA (1:80) and a strongly positive blot for anti‐DFS70 antibodies. Negativity for anti‐SSA/SSB antibodies was identified via immunoblot. Histopathological examination of a representative lesion from her foot showed slight interface dermatitis and marked periadnexal inflammatory infiltrates (Figure 1h–j). MxA was only partly expressed by the epithelium but strongly by the lymphocytic infiltrate around eccrine sweat glands (Figure 1i). DIF displayed slight granular deposits of C3 along the basal membrane in absence of immunoglobulins (Figure 1k). Systemic lupus erythematosus was excluded, and the presence of DFS70 antibodies argued against genuine SCLE. Not all three major criteria of RS were met and we diagnosed drug‐induced SCLE mimicking RS, accordingly. A short pulse of prednisolone 1 mg/kg bodyweight p.o. in combination with ibuprofen and topical corticosteroids led to prompt improvement of all symptoms. As the patient experienced recurrent immobilizing joint pain in the following weeks, the second dose of the vaccine was adjourned and she is currently treated with etoricoxib.
3. DISCUSSION
There is a theoretical vaccine‐derived risk for relapse or incitement of autoimmune diseases as a result of robust effects on the innate immune system. mRNA represent danger‐associated molecules which activate Toll‐like receptors resulting in type I IFN production. 7 There is preliminary evidence that mRNA vaccines may trigger cutaneous lesions of CLE, which is a somewhat expected finding considering the IFN‐driven inflammatory loop of the disease. 8 We screened the literature for cutaneous ADR to BNT162b2 and mRNA‐1273 vaccines (Table 1) and found considerable heterogeneity in the reported data, which include registry studies, summaries of pharmacovigilance reports, and case series. The most frequent findings are localized reactions including erythema and swelling which may be divided into early and late reactions (Figure 2). This is in line with the safety profile obtained from the clinical development programs of the respective agents. Generalized cutaneous eruptions including rashes of different kinds and urticarial reactions occur far less frequently, and specific dermatological diagnoses such as EM and pityriasis rosea are reported as rarities. Overall, there is a striking female predisposition which might occur for various reasons as already outlined by McMahon et al. 3 Unfortunately, data was not reported by dermatologists in many cases; hence, cutaneous ADR might not be accurately described and diagnosed and more unusual manifestations such as SCLE or RS might be overlooked. Even in the aforementioned most comprehensive registry study, only 30% of registry entries were performed by dermatologists. 3 Exacerbation of pre‐existent dermatological conditions including atopic eczema and psoriasis will open another field as only 16% of a total of 414 patients who experienced cutaneous ADR had a dermatological history in this report. Most cutaneous ADR occurred over the course of 3–10 days and were mild in nature. Management included topical corticosteroids, antihistamines, and non‐steroidal inflammatory drugs; rarely, systemic corticosteroids were necessary. Hence, the possibility of cutaneous ADR should not impose an obstacle to deliver immunizations to populations at risk of COVID‐19. As patients with skin symptoms after the first vaccine dose sometimes experience more severe reactions after the second dose, the individual approach of administration of the second dose should be determined via shared decision‐making with the patient. Comprehensive guidelines are now available to help health‐care providers in charge of vaccinations. 9 It will be important to collect further data to conclude whether reactions to mRNA vaccines deviate from other vaccine types and if they impose a larger risk to patients with pre‐existing autoimmune disease including dermatoses and connective tissue diseases. 10 As dermatologists are approached as a first instance by patients experiencing cutaneous ADR, they hold a key position in patient education and management. 11 A detailed and standardized reporting of unusual events in the course of the different deployed mRNA vaccines will enable a better understanding of underlying pathophysiological mechanisms. Until this point, it remains vague if mRNA vaccines bear a class‐specific profile of cutaneous ADR or if the specific vaccines differ in this regard.
TABLE 1.
Frequent skin manifestations following mRNA‐based coronavirus disease 2019 vaccines (mRNA‐1273, BNT162b2) in different populations as of June 2021
| Frequency and type of cutaneous ADR | Onset | Management | Type of study/population (reference) | Comments |
|---|---|---|---|---|
| 15 185 included in verum group; cutaneous ADR: mild to moderate local reactions 84–88%, delayed local reactions in ~1% | Mostly within 7 days | Local reactions resolved within 4–5 days | Phase III clinical trial of mRNA‐1273 (Baden et al.) 1 |
47.3% of patients included were female, 79.2% Caucasian, mean age was 51.4 years. Exclusion criteria included patients receiving immunosuppressive treatment and immunosuppressive condition |
| 18 860 included in verum group; cutaneous ADR: mild to moderate local reactions 66–83% | Mostly within 7 days | Local reactions mostly resolved within 1–2 days | Phase II/III clinical trial of BNT162b2 (Polack et al.) 2 |
49% of patients included were female, >80% Caucasian, median age was 52 years. Exclusion criteria included patients receiving immunosuppressive treatment and immunocompromising condition |
|
Total study population not reported; any cutaneous ADR: 414. Early local reaction (<3 days), delayed local reaction (>3 days), urticaria, morbilliform and vesicular rash, erythromelalgia, flare of existing dermatological condition, perniones, reactions to cosmetic fillers |
Median 7 days; onset after 2nd vaccine quicker | Patients responded well to topical corticosteroids and oral antihistamines; no severe sequelae | Accumulated reports to a registry of AAD and ILDS (McMahon et al. 3 ) |
Participants received mRNA‐1273 (83%) or BNT162b2 (17%), no information regarding sex and age, 98% from USA, 78% white, 90% of the patients experiencing cutaneous ADR were female, median age was 44 years. Limitations: entries limited to HCW (only 30% dermatologists), no information regarding incidence |
|
Any cutaneous ADR: 67/277 (24.2%). Local reactions: itch 17 (6.1%), redness 7 (2.5%), swelling 14 (5.1%); angioedema 12 (4.3%); tongue edema 10 (3.6%); urticaria 2 (0.7%); “skin rash” 5 (1.8%) |
No exact point of time reported; all within 3 days | No information | Prospective survey of South Korean HCW (Bae et al.) 12 | Only 4.7% of all included participants received BNT162b2 mRNA vaccine (n = 277); 66.8% were female, 68.6% were 20–39 years old; Study was conducted via self‐assessment; only onset of symptoms within 3 days was included, adverse events more common after second dose |
|
Any cutaneous ADR: 11/3170 (0.3%) Local reactions: itch 2 (0.1%), redness 2 (0.1%), swelling 2 (0.1%), urticaria 1 (0.03%); erythema and itch distant from injection site 4 (0.1%); urticarial rash + flare of AD 1 (0.03%); “skin rash” 3 (0.1%) |
1 h to 8 days | Most skin symptoms resolved spontaneously within 2–3 days without treatment | Retrospective monocentric analysis among Italian HCW (Corbeddu et al. 13 ) |
All Participants received BNT162b2 (n = 3170), no information regarding sex and age; 63.6% of the patients experiencing cutaneous ADR were female and mean age was 50 years; 63.6% of reactions occurred after the first dose. Limitations: brief report, no detailed information on the mode of reporting |
|
Any cutaneous ADR: 44/19 485 (0.2%). Local reactions 17 (0.09%); urticarial reaction 8 (0.04%); angioedema 3 (0.02%); generalized itch 6 (0.03%); erythema distant from inoculation site 2 (0.01%); other 8 (0.04%) |
60 h to 10 days | Course mostly mild and self‐limiting | Reports to the Pharmacovigilance Service in Trieste, North Italy (Farinazzo et al. 14 ) |
All participants received BNT162b2 (n = 19 485), no information regarding sex and age; 89.1% of the patients experiencing cutaneous ADR were female and mean age was 44 years, 80.4% of reactions occurred after the first dose. Limitations: potential bias of under‐ and overreporting |
|
Any ADR: 214/2000 (10.7%) Any cutaneous ADR: not exactly specified. “Rash” 8 (0.4%); analysis of EUDRAVigilance (10 December to 6 March): “rash” (2.2%) |
No information | No information | Reports to the Pharmacovigilance Service in Milan, North Italy (Gringeri et al. 15 ) |
All participants received BNT162b2 (n = 2000), no information regarding sex and age; amongst patients experiencing any ADR female/male ratio was 4.5, mean age was 47.5 years. Limitations: potential bias of under‐ and overreporting |
Abbreviations: AAD, American Academy of Dermatology; AD, atopic dermatitis; ADR, adverse drug reaction; EUDRA, European Union Drug Regulating Authorities; HCW, health‐care workers; ILDS, International League of Dermatological Societies.
FIGURE 2.
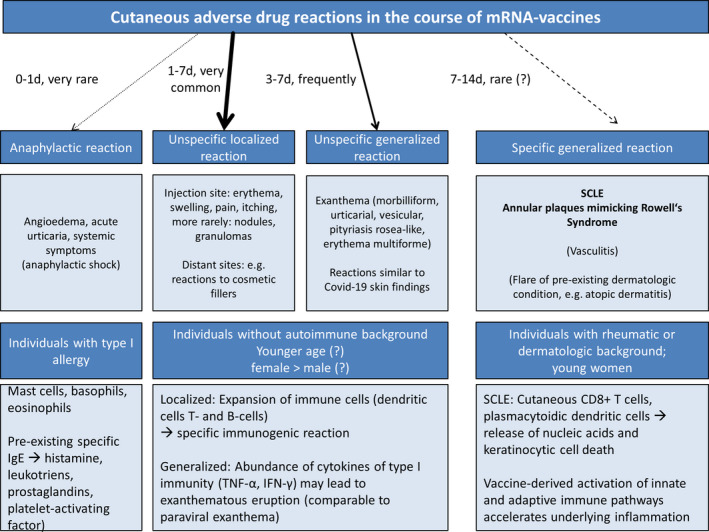
Scheme of expected cutaneous adverse drug reactions (ADR) depending on different patient groups. The vast majority of cutaneous ADR comprise unspecific localized or generalized reactions which typically occur between 1 and 7 days after the first dose; reactions after the second dose tend to occur earlier. Type I allergic reactions are very rare and occur in previously sensitized individuals (pre‐existing specific immunoglobulin E against components of the vaccine). We expect to see more specific cutaneous adverse reactions in susceptible individuals with rheumatic or dermatological background, including cutaneous lupus erythematosus, as these patients were underrepresented in clinical trials and data remains scarce. Young women are most often affected by autoimmune diseases and have not been vaccinated on a large scale with health‐care workers as an exemption.
CONFLICT OF INTEREST
The authors have been advisors and/or received speakers’ honoraria or travel expense reimbursements and/or received grants and/or participated in clinical trials of the following companies/organizations: D.N.: BMS, Novartis, GSK, Celgene, MSD, and Kiowa Kyrin; J.Z: Sigvaris Group, medi, Bayer, Juzo, and Novartis; T.B.: AbbVie, Allmiral, AnaptysBio, Arena, Asana Biosciences, Bayer Health, BioVerSys, Böhringer‐Ingelheim, BMS, Celgene, Daichi‐Sankyo, Dermavant/Roivant, DermTreat, Domain Therapeutics, DS Pharma, RAPT/FLX Bio, Galapagos/MorphoSys, Galderma, Glenmark, GSK, Incyte, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, Lilly, L’Oréal, MenloTx, Novartis, OMPharma/Vifor, Pfizer, Pierre Fabre, Sanofi/Regeneron, and UCB; J.W.: GSK, Novartis, Medac, Merck/Serono, Roche, Actelion, Pfizer, Spirig, ArrayBio, and Biogen; and C.B.: Novartis, L’Oréal, GSK. T.B. is founder of the non‐profit biotech company “Davos Biosciences” within the International Kühne‐Foundation. The remaining authors declare no conflict of interest.
ACKNOWLEDGMENTS
We thank the patients for granting permission of publishing their case details and photographs.
Niebel D, Wilhelmi J, De Vos L, Ziob J, Jaschke K, Bieber T, et al. Annular plaques mimicking Rowell’s syndrome in the course of coronavirus disease 2019 mRNA vaccines: An overlooked phenomenon? J Dermatol.2022;49:151–156. doi: 10.1111/1346-8138.16210
REFERENCES
- 1. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niebel D, Ralser‐Isselstein V, Jaschke K, Braegelmann C, Bieber T, Wenzel J. Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine. Dermatol Ther. 2021;34:e15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gambichler T, Scholl L, Dickel H, Ocker L, Stranzenbach R. Prompt onset of Rowell’s syndrome following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e415–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeitouni NC, Funaro D, Cloutier RA, Gagné E, Claveau J. Redefining Rowell’s syndrome. Br J Dermatol. 2000;142:343–6. [DOI] [PubMed] [Google Scholar]
- 7. Velikova T, Georgiev T. SARS‐CoV‐2 vaccines and autoimmune diseases amidst the COVID‐19 crisis. Rheumatol Int. 2021;41:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wenzel J. Cutaneous lupus erythematosus: new insights into pathogenesis and therapeutic strategies. Nat Rev Rheumatol. 2019;15:519–32. [DOI] [PubMed] [Google Scholar]
- 9. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology Guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases: Version 1. Arthritis Rheumatol. 2021;73:1093–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teijaro JR, Farber DL. COVID‐19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pulsipher KJ, Presley CL, Waller JD, Szeto M, Laughter M, Dellavalle R. Coronavirus vaccination adverse reactions and the role of the dermatologist. J Drugs Dermatol. 2021;20:351–2. [DOI] [PubMed] [Google Scholar]
- 12. Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV‐19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corbeddu M, Diociaiuti A, Vinci MR, Santoro A, Camisa V, Zaffina S, et al. Transient cutaneous manifestations after administration of Pfizer‐BioNTech COVID‐19 Vaccine: an Italian single‐centre case series. J Eur Acad Dermatol Venereol. 2021;35:e483–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farinazzo E, Ponis G, Zelin E, Errichetti E, Stinco G, Pinzani C, et al. Cutaneous adverse reactions after m‐RNA COVID‐19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol. 2021;35:e548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gringeri M, Mosini G, Battini V, Cammarata G, Guarnieri G, Carnovale C, et al. Preliminary evidence on the safety profile of BNT162b2 (Comirnaty): new insights from data analysis in EudraVigilance and adverse reaction reports from an Italian health facility. Hum Vaccin Immunother. 2021: 1–3. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]


