Abstract
Objective
The primary aim of this article was to describe SARS‐CoV‐2 infection among pregnant women during the wild‐type and Alpha‐variant periods in Italy. The secondary aim was to compare the impact of the virus variants on the severity of maternal and perinatal outcomes.
Design
National population‐based prospective cohort study.
Setting
A total of 315 Italian maternity hospitals.
Sample
A cohort of 3306 women with SARS‐CoV‐2 infection confirmed within 7 days of hospital admission.
Methods
Cases were prospectively reported by trained clinicians for each participating maternity unit. Data were described by univariate and multivariate analyses.
Main outcome measures
COVID‐19 pneumonia, ventilatory support, intensive care unit (ICU) admission, mode of delivery, preterm birth, stillbirth, and maternal and neonatal mortality.
Results
We found that 64.3% of the cohort was asymptomatic, 12.8% developed COVID‐19 pneumonia and 3.3% required ventilatory support and/or ICU admission. Maternal age of 30–34 years (OR 1.43, 95% CI 1.09–1.87) and ≥35 years (OR 1.62, 95% CI 1.23–2.13), citizenship of countries with high migration pressure (OR 1.75, 95% CI 1.36–2.25), previous comorbidities (OR 1.49, 95% CI 1.13–1.98) and obesity (OR 1.72, 95% CI 1.29–2.27) were all associated with a higher occurrence of pneumonia. The preterm birth rate was 11.1%. In comparison with the pre‐pandemic period, stillbirths and maternal and neonatal deaths remained stable. The need for ventilatory support and/or ICU admission among women with pneumonia increased during the Alpha‐variant period compared with the wild‐type period (OR 3.24, 95% CI 1.99–5.28).
Conclusions
Our results are consistent with a low risk of severe COVID‐19 disease among pregnant women and with rare adverse perinatal outcomes. During the Alpha‐variant period there was a significant increase of severe COVID‐19 illness. Further research is needed to describe the impact of different SARS‐CoV‐2 viral strains on maternal and perinatal outcomes.
Keywords: Cohort studies, COVID‐19 pneumonia, Italy, pregnancy, SARS‐CoV‐2
Tweetable abstract
The rate of severe COVID‐19 disease increased during the Alpha‐variant period compared with the wild‐type period.
Linked article This article is commented on by J G Thornton, p. 232 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.16981.
Introduction
A two‐wave pattern of the COVID‐19 disease during the 2020 pandemic was observed in Italy, with the first wave during spring followed by the second wave starting in autumn and extending until the end of June 2021.
Following the unexpected onset of the pandemic at the end of February 2020, the Italian Government imposed a lockdown lasting from 9 March to 18 May 2020. During the summer, there was greater social interaction all over the country, despite the maintenance of mandatory preventive measures, such as safe interpersonal distancing and face‐mask protection. Towards the end of August 2020, the virus began to recirculate and in the autumn a second wave occurred, affecting central and southern Italy, which had previously been spared.
Since February 2021, rapid investigations sequencing and analysing the genetic code of a significant number of positive samples, at the national level, allowed us to monitor the circulation of SARS‐CoV‐2 variants. 1 In February and March 2021 the estimated national prevalence of the Alpha variant was 54.0 and 86.7%, respectively. 2 , 3
From mid‐June, thanks to the reduced circulation of the virus, the restrictions were decreased. The Delta variant began to take over in mid‐June when a prevalence of 22.7% was detected. 4 Although estimates are not available for pregnant women, it is reasonable to imagine a similar trend for this population.
Data from the UK suggest that the Alpha and Delta variants have a worse impact on maternal and perinatal outcomes. 5 , 6 Research capable of gathering sound information on the impact of different SARS‐CoV‐2 variants in pregnancy is therefore urgently needed to guide decision makers, support health professionals and inform citizens.
From the beginning of the pandemic the Italian Obstetric Surveillance System (ItOSS) launched a national population‐based prospective study, enrolling any pregnant woman with confirmed SARS‐CoV‐2 infection, admitted to hospital, until the end of June 2021. 7 , 8 , 9 ItOSS coordinates public health research in the field of obstetrics in Italy through an enhanced maternal mortality surveillance system, 10 and coordinates prospective population‐based studies on severe maternal morbidity, 11 , 12 , 13 in collaboration with the multi‐country International Network of Obstetric Survey System (INOSS). 14
The primary aim of this article was to describe SARS‐CoV‐2 infection among pregnant women during the wild‐type period and the Alpha‐variant period in Italy. The secondary aim was to compare the impact of the virus variants on the severity of maternal and perinatal outcomes.
Methods
This national population‐based prospective cohort study collects information on women with confirmed SARS‐CoV‐2 infection, admitted to any Italian hospital during pregnancy and up to 42 days after childbirth.
All Italian maternity hospitals that managed women who tested positive for SARS‐CoV‐2 were invited to participate in the project. Trained reference clinicians notified eligible women and collected comprehensive information on maternal sociodemographic characteristics, medical and obstetric history, disease management, mode of delivery, and maternal and perinatal outcomes through a dedicated online form that was revised and pre‐tested by a multidisciplinary national group of experts. Weekly email reminders and phone contacts ensured complete reporting from the participating reference clinicians.
Confirmed SARS‐CoV‐2 infection was defined as the detection of viral RNA upon reverse transcription polymerase chain reaction (RT‐PCR) testing of nasopharyngeal swab and/or blood and/or radiological diagnosis of COVID‐19 pneumonia. Until the end of March 2020, only pregnant women who were symptomatic and those defined as close contacts of someone infected with SARS‐CoV‐2 were tested. In April, the Italian regions progressively adopted universal screening policies during pregnancy. Therefore, from May 2020, all pregnant women admitted to hospital were tested for SARS‐CoV‐2, regardless of symptoms or exposure.
The present analysis refers to pregnant women with confirmed SARS‐CoV‐2 infection detected within 7 days of hospital admission during the wild‐type period and the Alpha‐variant period, defined as 25 February 2020–31 January 2021 and 1 February–30 June 2021, respectively. The cases in the first period, for which complete data were received by 30 June 2021, are consolidated, whereas reference clinicians are still adding cases to the data held for the Alpha‐variant period.
Outcomes
Outcome measures included in the study are: COVID‐19 pneumonia, confirmed by chest imaging; mechanical ventilatory support (non‐invasive mechanical ventilation, orotracheal intubation, extracorporeal membrane oxygenation (ECMO)); admission to intensive care unit (ICU); maternal mortality (maternal death during pregnancy or within 42 days from any pregnancy outcome); preterm birth (divided into 22–31 and 32–36 weeks of gestation); mode of delivery (vaginal, elective caesarean section (CS), urgent/emergency CS for COVID‐19, urgent/emergency CS for maternal/fetal indications); stillbirth (intrauterine fetal death at ≥22 weeks of gestation); admission to neonatal intensive care unit (NICU); and early neonatal mortality (death of a liveborn infant <7 days of life).
Covariates
Covariates include sociodemographic and medical characteristics that could act as potential risk factors: age (<30, 30–34 and ≥35 years); citizenship (Italian, citizenship of countries with high migration pressure (HMPCs), citizenship of other countries); 15 educational level (low, primary school or lower; medium, high school; high, bachelor’s degree or higher); previous comorbidities (at least one of the following: asthma requiring medical treatment, cardiovascular diseases, diabetes, HIV/AIDS, hypertension, lung diseases, other pathologies); and obesity (defined as a body mass index (BMI) of >30 kg/m2).
Statistical analysis
Statistical analyses were performed using the statistical package stata/mp 14.2. Frequency distributions, prevalence and odds ratios (ORs), with their 95% confidence intervals (95% CIs), were used to describe the data. Missing data were excluded when their proportion was lower than 5%, otherwise they were included in the frequency distributions.
The national SARS‐CoV‐2 incidence rate in pregnancy with 95% CI was estimated for the two periods considered. All enrolled women with continuing pregnancies or who gave birth, irrespective of the time of diagnosis, were included in the numerator. The 2019 deliveries retrieved from the national Birth Registry were used to estimate the denominators, 16 applying variations corresponding to the Italian National Statistics Institute (ISTAT) estimate for the reduction in births observed between 2019 and the corresponding months of the two study periods. 17 Finally, deliveries were weighted according to the computed time of exposure to risk of infection during pregnancy.
Frequency distributions by sociodemographic, obstetric and medical characteristics were computed for the two periods. The prevalence of COVID‐19 pneumonia, its risk factors and its trend during the study period were analysed. The birth register data were used as the background population to compare the prevalence of foreign women, CSs and preterm births reported by ItOSS with the 2019 national data.
To assess the association between presence/absence of pneumonia and its potential risk factors (woman’s age, citizenship, educational level, presence/absence of previous comorbidities, presence/absence of obesity), mutually adjusted ORs and their 95% CIs were calculated using a multiple logistic regression model. Plausible interactions were tested using the likelihood ratio test. To account for missing information, the model was applied to multiple‐imputed data (Appendix S1; Table S1). The imputation of 20 data sets was performed using chained equations; 18 Rubin’s rules were used to combine model estimates across the 20 data sets. 19 The model was also performed using only complete cases as sensitivity analysis (Table S2).
To compare the impact of the Alpha variant versus the wild type on maternal and perinatal outcomes, the prevalence of need for ventilatory support and ICU admission were described among women with pneumonia during the two periods. A logistic regression model was applied to estimate ORs and 95% CIs for ventilatory support and/or ICU admission, using the first period as the reference. ORs were adjusted for a woman’s age, citizenship, previous comorbidities and obesity. Severe outcomes (ICU admission, orotracheal intubation and maternal deaths) were compared with those recorded among the background population of hospitalised women (aged 15–49 years) testing positive for SARS‐CoV‐2. 20 Mode of delivery, gestational age at delivery and perinatal outcomes were analysed, stratified by the presence/absence of COVID‐19 pneumonia. Maternal deaths were cross‐checked with the ItOSS enhanced maternal mortality surveillance system. 21
In this observational study, no formal power calculation was performed because the sample size was governed by disease incidence.
Results
All of the 315 Italian maternity units invited (see Appendix S2) participated in the study (100% attendance rate), notifying 5734 women with confirmed SARS‐CoV‐2 infection during pregnancy and up to 42 days after childbirth from 25 February 2020 to 30 June 2021. The flow diagram in Figure 1 describes the selection of the cases included in the present analysis, represented by 3306 women with a positive SARS‐CoV‐2 test within 7 days of hospital admission, with a continuing pregnancy or who gave birth, during the wild‐type period (n = 2550) and the Alpha‐variant period (n = 756).
Figure 1.
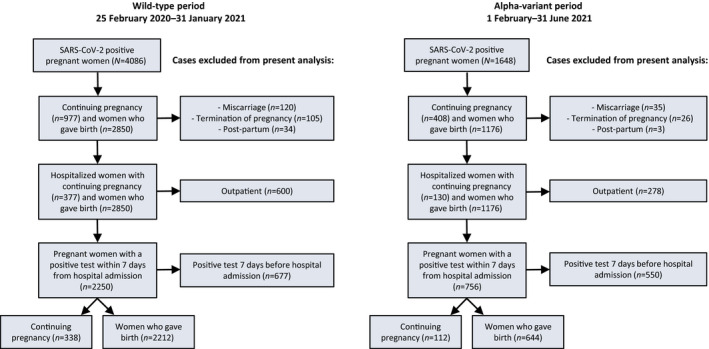
Women enrolled in the ItOSS cohort during the wild‐type period (25 February 2020–31 January 2021) and the Alpha‐variant period (1 February–30 June 2021).
The national SARS‐CoV‐2 incidence rate estimated among the ItOSS cohort of pregnant women was 23.5 per 1000 births (95% CI 22.7–24.2) during the wild‐type period and 16.6 per 1000 (95% CI 15.8–17.3) during the Alpha‐variant period, respectively.
Table 1 describes the characteristics of the enrolled women during the two periods considered. The sociodemographic characteristics do not differ between the wild‐type and Alpha‐variant periods. The percentage of women of foreign citizenship was significantly higher compared with women who gave birth in 2019 in Italy. 15 At the first positive SARS‐CoV‐2 test, the great majority (92.9%) of women were in the third trimester of pregnancy. The main reason for admission to hospital was COVID‐19 infection (57.6%) for women with a continuing pregnancy (13.6%), whereas other obstetric reasons or labour and delivery (82.7%) were the main causes for hospitalisation for those who gave birth (86.4%) (Table S3). Out of the 3306 women included in the study, 64.3% were asymptomatic at diagnosis, whereas 12.8% developed COVID‐19 pneumonia. The prevalence of pneumonia was 40.9% among women who had a continuing pregnancy and 8.4% among those who gave birth.
Table 1.
Women's characteristics during the wild‐type period (25 February 2020–31 January 2021) and the Alpha‐variant period (1 February–30 June 2021)
| Wild‐type period | Alpha‐variant period | Total | ||||
|---|---|---|---|---|---|---|
| (n = 2550) | (n = 756) | (N = 3306) | ||||
| n | % | n | % | n | % | |
| Age (56 missing): | ||||||
| <30 years | 883 | 35.2 | 242 | 32.8 | 1125 | 34.6 |
| 30–34 years | 856 | 34.1 | 273 | 37.0 | 1129 | 34.7 |
| ≥35 years | 773 | 30.8 | 223 | 30.2 | 996 | 30.6 |
| Citizenship: | ||||||
| Italian | 1792 | 70.3 | 544 | 72.0 | 2336 | 70.7 |
| HMPCs | 752 | 29.5 | 210 | 27.8 | 962 | 29.1 |
| Non‐HMPCs | 6 | 0.2 | 2 | 0.3 | 8 | 0.2 |
| Country of birth: | ||||||
| Italy, Western Europe and North America | 1640 | 64.3 | 517 | 68.4 | 2157 | 65.2 |
| East Europe | 232 | 9.1 | 97 | 12.8 | 329 | 10.0 |
| Africa | 349 | 13.7 | 76 | 10.1 | 425 | 12.9 |
| South and Central America | 138 | 5.4 | 26 | 3.4 | 164 | 5.0 |
| Asia | 191 | 7.5 | 40 | 5.3 | 231 | 7.0 |
| Level of education: | ||||||
| Low | 555 | 21.8 | 175 | 23.1 | 730 | 22.1 |
| Medium | 784 | 30.7 | 248 | 32.8 | 1032 | 31.2 |
| High | 411 | 16.1 | 133 | 17.6 | 544 | 16.5 |
| Missing | 800 | 31.4 | 200 | 26.5 | 1000 | 30.2 |
| Previous comorbidities (90 missing) | 325 | 13.0 | 89 | 12.4 | 414 | 12.9 |
| Pre‐gestational diabetes | 58 | 2.3 | 15 | 2.1 | 73 | 2.3 |
| Autoimmune disease | 46 | 1.8 | 17 | 2.4 | 63 | 2.0 |
| Chronic hypertension | 38 | 1.5 | 7 | 1.0 | 45 | 1.4 |
| BMI > 30 kg/m2 (71 missing) | 328 | 13.1 | 99 | 13.4 | 427 | 13.2 |
| Multiparous (18 missing) | 1399 | 55.2 | 436 | 57.9 | 1835 | 55.8 |
| Multiple pregnancy (1 missing) | 47 | 1.8 | 19 | 2.5 | 66 | 2.0 |
| Gestational age at diagnosis (55 missing): | ||||||
| ≤14 weeks | 35 | 1.4 | 5 | 0.7 | 40 | 1.2 |
| 15–27 weeks | 145 | 5.8 | 45 | 6.1 | 190 | 5.8 |
| ≥28 weeks | 2336 | 92.8 | 685 | 93.2 | 3021 | 92.9 |
| Presence of COVID‐19 pneumonia | 299 | 11.7 | 125 | 16.5 | 424 | 12.8 |
| Asymptomatic women (33 missing) | 1652 | 65.3 | 454 | 61.2 | 2106 | 64.3 |
| Continuing pregnancy | 338 | 13.3 | 112 | 14.8 | 450 | 13.6 |
BMI, body mass index; HMPCs, countries with high migration pressure.
Age of 30–34 years (OR 1.43, 95% CI 1.09–1.87) and ≥35 years (OR 1.62, 95% CI 1.23–2.13), citizenship from HMPCs (OR 1.75, 95% CI 1.36–2.25), previous comorbidities (OR 1.49, 95% CI 1.13–1.98) and obesity (OR 1.72, 95% CI 1.29–2.27) were significantly associated with higher occurrences of COVID‐19 pneumonia. No statistically significant association was found with educational level. Results did not change noticeably in the sensitivity analysis (Table S2).
Table 2 describes maternal and perinatal outcomes stratified by the presence/absence of COVID‐19 pneumonia during the two periods. Overall, 3.3% of the women developed severe COVID‐19 pneumonia requiring mechanical ventilatory support and/or ICU admission. A significant increase in resort to ventilatory support and/or ICU admission in the case of pneumonia was observed during the Alpha‐variant period, compared with the wild‐type period (OR 3.24, 95% CI 1.99–5.28, adjusted for age, citizenship, previous comorbidities and obesity). Except for maternal deaths, the outcomes shown in Table 2 worsened during the period characterised by the circulation of the Alpha variant. In comparison with the background population of hospitalised women aged 15–49 years testing positive for SARS‐CoV‐2, 20 the percentage of women undergoing orotracheal intubation was slightly higher (1.1 versus 0.7%), and the admission to ICU and mortality were lower among the ItOSS cohort (2.3 versus 5.5% and 0.03 versus 1.3%, respectively).
Table 2.
Women’s outcomes and perinatal outcomes
| Women’s outcomes | Wild‐type period | Alpha‐variant period | ||||||
|---|---|---|---|---|---|---|---|---|
|
No COVID‐19 pneumonia (n = 2251) |
COVID‐19 pneumonia (n = 299) |
No COVID‐19 pneumonia (n = 631) |
COVID‐19 pneumonia (n = 125) |
|||||
| n | % | n | % | n | % | n | % | |
| Oxygen therapy | 21 | 0.9 | 158 | 52.8 | 24 | 3.8 | 92 | 73.6 |
| Mechanical ventilatory support and/or ICU admission | 0 | 0.0 | 56 | 18.7 | 0 | 0.0 | 52 | 41.6 |
| Non‐invasive ventilatory support | 0 | 0.0 | 53 | 17.7 | 0 | 0.0 | 47 | 37.6 |
| Invasive ventilatory support: | 0 | 0.0 | 15 | 5.0 | 0 | 0.0 | 21 | 16.8 |
| Orotracheal intubation | 0 | 0.0 | 14 | 4.7 | 0 | 0.0 | 21 | 16.8 |
| ECMO | 0 | 0.0 | 3 | 1.0 | 0 | 0.0 | 4 | 3.2 |
| ICU admission | 0 | 0.0 | 35 | 11.7 | 0 | 0.0 | 40 | 32.0 |
| Death | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 0 | 0.0 |
| Perinatal outcomes | n = 2081 | n = 169 | n = 578 | n = 80 | ||||
| Stillbirth | 15 | 0.7 | 2 | 1.2 | 3 | 0.5 | 0 | 0.0 |
| Livebirth | 2066 | 99.3 | 167 | 98.8 | 575 | 99.5 | 80 | 100.0 |
| Neonatal death | 3 | 0.1 | 1 | 0.6 | 2 | 0.3 | 1 | 1.3 |
| NICU admission | 212 | 10.3 | 43 | 25.7 | 55 | 9.6 | 24 | 30.0 |
ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; NICU, neonatal intensive care unit.
Overall, 11.6% of 2888 livebirths were admitted to NICU, with the highest prevalence among neonates delivered by mothers with pneumonia (27.1%), compared with neonates delivered by unaffected women (10.1%). NICU admissions increased from 25.7% during the wild‐type period to 30.0% during the Alpha‐variant period (Table 2). Stillbirths (0.7%) and early neonatal deaths (0.2%) were not associated with maternal pneumonia, nor with the different viral strains. The national stillbirth rate registered in the first semester of 2020 (2.82/1000) was in line with those recorded during the previous 4 years (ranging from 2.59/1000 in 2018 to 2.86/1000 in 2015). 22
Table 3 shows mode of delivery and gestational age at birth among 2856 women who gave birth. Overall, the CS rate (34.1%) remained close to the 2019 national figure (31.8%). 16 The rate of urgent/emergency CS for COVID‐19 was consistently higher among women affected by COVID‐19 pneumonia (20.4%), compared with 0.4% in unaffected women (P < 0.001); during the Alpha‐variant period, the proportion rose to 31.2% in women with pneumonia.
Table 3.
Mode of delivery and gestational age at birth
| 25/2/2020–31/1/2021 | 1/2–30/6/2021 | |||||||
|---|---|---|---|---|---|---|---|---|
| No COVID‐19 pneumonia (n = 2049) | COVID‐19 pneumonia (n = 163) | No COVID‐19 pneumonia (n = 567) | COVID‐19 pneumonia (n = 77) | |||||
| n | % | n | % | n | % | n | % | |
| Mode of delivery (8 missing) | ||||||||
| Vaginal | 1381 | 67.6 | 74 | 46.0 | 399 | 70.4 | 23 | 29.9 |
| Elective CS | 315 | 15.4 | 19 | 11.8 | 84 | 14.8 | 8 | 10.4 |
| Urgent/emergency CS due to maternal/foetal indication | 337 | 16.5 | 43 | 26.7 | 84 | 14.8 | 22 | 28.6 |
| Urgent/emergency CS due to COVID‐19 | 10 | 0.5 | 25 | 15.5 | 0 | 0.0 | 24 | 31.2 |
| Gestational age at birth, weeks (51 missing) | ||||||||
| ≤31 | 31 | 1.5 | 16 | 10.3 | 9 | 1.6 | 8 | 10.5 |
| 32–36 | 146 | 7.2 | 38 | 24.4 | 39 | 7.1 | 25 | 32.9 |
| ≥37 | 1847 | 91.3 | 102 | 65.4 | 501 | 91.3 | 43 | 56.6 |
CS, caesarean section.
The proportion of preterm births (11.1%), mostly late preterm, was higher compared with the 2019 national figure (6.7%). 16 Among women with COVID‐19 pneumonia, the preterm birth rate was significantly higher compared with the rate in unaffected women (P < 0.001), reaching 43.4% during the Alpha‐variant period (compared with the wild‐type period: OR 1.69, 95% CI 0.94–3.04, adjusted for age, citizenship, previous comorbidities and obesity). Iatrogenic indications, defined as elective CS or induction of labour, accounted for 27.2% of the preterm births.
Discussion
Main findings
From February 2020 to June 2021, the ItOSS prospective population‐based national cohort study enrolled 3306 pregnant women with confirmed SARS‐CoV‐2 infection within 7 days of hospital admission. At the time of diagnosis, 64.3% of the cohort was asymptomatic, whereas COVID‐19 pneumonia affected 12.8% of the women. ICU admission and maternal mortality among the ItOSS cohort were lower compared with the same figures detected among the background population of infected women aged 15–49 years. 20 Overall, 3.3% needed mechanical ventilatory support and/or ICU admission. During the Alpha‐variant period, the need of ventilatory support and/or ICU admission among women affected by COVID‐19 pneumonia was two times that of the wild‐type period. Compared with the pre‐pandemic period, the rate of stillbirths and maternal and neonatal deaths remained stable. 22 Altogether, our results showed an absolute low risk of severe adverse outcomes.
Strengths and limitations
The national prospective population‐based design, the presence of trained clinicians in each hospital and the active monitoring of case reporting through monthly checks are among the main strengths of the study. Thanks to routine testing for SARS‐CoV‐2 from May 2020, the data analysis relayed a complete denominator, which significantly improved the accuracy of the outcome measures. The ItOSS data were able to distinguish hospital admissions for COVID‐related circumstances from those related to delivery or other obstetric conditions, thus favouring the interpretation of the observed different clinical patterns among women with continuing pregnancy and women who gave birth. Data analysis by pneumonia status allowed the better description of risk factors and characteristics of women at higher risk for adverse outcomes. Moreover, the inclusion in the analysis of pregnant women with a confirmed positive test within 7 days from hospital admission limited possible selection bias. The crosscheck of mortality data with the ItOSS enhanced maternal mortality surveillance is a further strength of the study. The limitations of the study include the lack of a control population and the likely missing cases of women testing positive for SARS‐CoV‐2 before May 2020, when universal testing started. The lack of a data crosscheck with the national SARS‐CoV‐2 surveillance, which does not collect information about pregnant women, is a further limitation. With the pending completion of pregnancy for all women enrolled in the cohort and with the incomplete data for the second wave of the pandemic, these findings are not conclusive.
Interpretation
Similarly to ItOSS, during the wild‐type period the prospective population‐based studies of the United Kingdom Obstetric Surveillance System (UKOSS) and the Nordic Obstetric Surveillance Study (NOSS) detected an absolute low risk for hospital admission of pregnant women as a result of severe COVID‐19 disease, and no increase in the stillbirth rate and in maternal and neonatal deaths. 23 , 24 , 25 In contrast, the passive surveillance conducted by the US Centers for Disease Control and Prevention (CDC) and the following meta‐analysis, mainly built on CDC reporting, described a higher prevalence of severe COVID‐19 disease among pregnant women and poor maternal and perinatal outcomes, compared with the ItOSS results. 26 , 27 These differences seem to be mostly related to the study design adopted. For instance, the retrospective passive CDC surveillance was affected by 64.5% missing data on pregnancy status, 26 compared with the complete reporting of the prospective population‐based studies launched in the countries participating in the INOSS. 14
The multinational INTERCOVID study reported a 22‐fold higher risk for maternal mortality among pregnant women testing positive for SARS‐CoV‐2. 28 However, all deaths were detected in low‐income countries, probably when hospital conditions became critical. In fact, the different impact of the pandemic observed between high‐ and low‐resource settings requires caution in inferring results from the unfavourable outcomes detected.
As reported by other studies, 23 , 24 , 25 , 26 , 27 , 28 we confirmed that women who were obese and women with previous comorbidities were more likely to develop severe COVID‐19 disease. Pregnant women from HMPCs were also significantly more likely to be hospitalised with severe pneumonia, probably because of their lower socio‐economic status and possible through delays in accessing health services.
Regarding the increased risk of preterm births, after excluding all cases with iatrogenic indications (27.2% of preterm births), the rate of spontaneous preterm births dropped to 8.1%, much closer to the 2019 national figure of 6.7%. 16 However, the increase in preterm births detected during the Alpha‐variant period confirms the UKOSS data and urges a careful evaluation of the impact of new variants on perinatal outcomes. 6 Luckily, as reported by UKOSS, 24 preterm babies were more often late preterm, and, even though they were more likely to be admitted to NICU, any increse in stillbirths and neonatal deaths was detected.
Unexpectedly, we did not record a significant increase in the rate of CS, as reported in many systematic reviews, 27 , 29 , 30 and in the UK and Northern Europe, 23 , 24 where usually the CS rates are lower compared with Italy. The ItOSS detected a higher rate of CS only among women affected by COVID‐19 pneumonia, whereas the increase reported by UKOSS is irrespective of symptom status. 21 The great effort to inform Italian obstetricians that COVID‐19 was not a primary indication for CS, 28 , 29 flanked by the study monitoring and periodic return of preliminary data, probably helped to limit any rise in the rate of CS. During the first weeks of the pandemic outbreak, the Istituto Superiore di Sanità (Italian National Institute of Health, ISS) published a weekly report to describe new evidence on SARS‐CoV‐2 infection among pregnant women in support of clinicians, and an average of 7500 health professionals accessed the website daily. 31 Moreover, the virus circulated more in the north of the country, which has a lower CS rate compared with southern Italy. 16 A previous ItOSS article described in detail the procedures and criticalities of peripartum care during the first wave of the pandemic in Italy. 9
The lower SARS‐CoV‐2 incidence rate detected during the Alpha‐variant period compared with the wild‐type period is likely due to the start of the vaccination campaign. In Italy the vaccine was primarily indicated for pregnant women at high risk of viral exposure (i.e. healthcare providers, caregivers) or with underlying conditions that increase the risk of severe COVID‐19. 32
Compared with the wild‐type period, we detected a significant increase in resort to ventilatory support and/or ICU admission in the case of pneumonia during the Alpha‐variant period. In contrast, maternal mortality as well as perinatal morbidity and mortality did not worsen. Similar figures have been reported by the UK during the second wave of the pandemic, assuming a possible association with the emergence of the Alpha variant described as a more pathogenic strain of SARS‐CoV‐2. 3 The ItOSS data seem to confirm this hypothesis, as roughly 85% of the viruses circulating in Italy were replaced by the Alpha variant in mid‐March 2021, 3 and the comparison of women’s sociodemographic and obstetric characteristics during the two periods did not show any significant difference to explain the poorer outcomes observed during the Alpha period. Clinicians should therefore be aware that the emergence of new viral strains could result in more severe illness, as described by the recent UKOSS paper for the Alpha and the Delta viral strains. 6
Conclusion
Further research able to correlate viral sequencing with epidemiological data is needed to confirm whether variants of the SARS‐CoV‐2 virus may be responsible for worse maternal and perinatal outcomes. The level of circulation of the virus, and the emergence of new viral strains with increased transmissibility and/or virulence, are aspects to be taken into consideration when defining public health indications, as in the case of vaccination during pregnancy. For instance, the Italian Ministry of Health and the ISS updated the interim indications on COVID‐19 vaccination during pregnancy as a result of the poorer maternal outcomes detected during the Alpha‐variant period by the ItOSS project. 32 The vaccine, originally recommended only for pregnant women at higher risk of SARS‐CoV‐2 exposure or morbidity, is now recommended for all pregnant women, starting from the second trimester. 33
During the SARS‐CoV‐2 emergency, the non‐population‐based series, at risk of duplication of the same cases and without appropriate denominators, the publication of systematic reviews affected by the low quality of the observational studies available and the premature media coverage of preprints all challenged the responsiveness of research in this field. Future research should address the need to balance speed with accuracy in producing knowledge during pandemic outbreaks.
Disclosure of interests
None declared. Completed disclosure of interests form available to view online as supporting information.
Contribution to authorship
SD conceived the study, provided overall guidance, drafted the article and reviewed the final version. EC collected data, collaborated in drafting the article, and edited and reviewed the final version. AM and MAS conducted the statistical analysis, assisted with data collection, collaborated in drafting the article and reviewed the final version. RB, MDM and MF collaborated to statistical analysis. MAA, RB, AC, PC, IC, LC, GD, EDA, SDE, GE, MPF, LL, Marco Liberati, Mariavittoria Locci, ALR, CM, GM, FM, Alessandra Meloni, ADM, LM, EP, LP, LR, AS, VS, SCAS, GS, DS, SS, MS, FT, ST, VT, CT and AV supervised, assisted with data collection and reviewed the final version. All authors have read and agreed to the published version of the article.
Details of ethics approval
The Ethics Committee of the Istituto Superiore di Sanità – Italian National Institute of Health approved the project (protocol 0010482 CE 01.00, Rome, 24 March 2020). The study protocol is available at: https://www.epicentro.iss.it/en/coronavirus/sars‐cov‐2‐pregnancy‐childbirth‐breastfeeding‐prospective‐study‐itoss (in Italian). An informed consent to participate in the study was acquired from any woman upon enrolment.
Funding
This study has not received any funding.
Acknowledgements
We thank Silvia Andreozzi, Mauro Bucciarelli and Claudia Ferraro for their technical support and assistance with the web‐based data collection system. We thank Clarissa Bostford for English editing. Our warmest thanks go to all the clinicians working in the participating maternity units (Appendix S2) for their contribution in collecting data during the study and all women who agreed to participate in the study.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Supporting information
Table S1. Differences in educational level between women with and without missing data.
Table S2. COVID‐19 pneumonia mutually adjusted odds ratios; logistic regression model applied to complete cases.
Table S3. Reasons for hospital admission.
Appendix S1. Multiple imputation.
Appendix S2. The ItOSS national network of maternity units.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Appendix A.
The ItOSS COVID‐19 working group
Maria Grazia Arena (mariagraziaarena.01@gmail.com; Department of Obstetrics and Gynaecology, University Hospital G. Rodolico – San Marco, 95123 Catania, Italy); Rosaria Boldrini (r.boldrini@sanita.it; Directorate general of digitalization, of health informative system and of statistics, Italian Ministry of Health, 00144 Rome, Italy); Roberto Brunelli (roberto.brunelli@uniroma1.it; Department of Maternal and Child Health and Urological Sciences, Sapienza University – Policlinico Umberto I, 00161 Rome, Italy); Angelo Cagnacci (angelo.cagnacci@unige.it; Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics and Maternal and Paediatric Sciences, IRCCS‐San Martino Hospital, 16132 Genova); Paola Casucci (pcasucci@regione.umbria.it; Sistema Informativo e Mobilità Sanitaria, Umbria Region, 06121 Perugia, Italy); Irene Cetin (irene.cetin@unimi.it; Unit of Obstetrics and Gynaecology, Hospital V. Buzzi, ASST Fatebenefratelli Sacco, Department of Biomedical and Clinical Sciences, University of Milan, 20154 Milan, Italy); Luigi Cobellis (luigi.cobellis@unicampania.it; Department of Women, Child and General and Specialised Surgery, University ‘Luigi Vanvitelli’, AORN Sant’Anna e San Sebastiano, 81100 Caserta, Italy); Gabriella Dardanoni (gabriella.dardanoni@regione.sicilia.it; Osservatorio Epidemiologico Assessorato Salute Regione Siciliana, Sicily Region, 90145 Palermo, Italy); Elena De Ambrosi (elena.deambrosi@gmail.com; Unit of Obstetrics and Gynaecology, Ospedale Infermi, Rimini 47923, Italy); Martina Del Manso (martina.delmanso@iss.it; Department of Infectious Diseases, Istituto Superiore di Sanità – Italian National Institute of Health, Viale Regina Elena 299, 00161 Rome, Italy); Sara D'Eusanio (sara.deusanio@ospedaliriuniti.marche.it; Department of Obstetrics and Gynaecology, University Hospital Ospedali Riuniti di Ancona, 60126 Ancona, Italy); Lorenza Driul (lorenza.driul@uniud.it; Department of Medicine, University of Udine, Gynaecology and Obstetrics Clinic, 33100 Udine, Italy); Giorgio Epicoco (giorgio.epicoco@ospedale.perugia.it; Department of Obstetrics and Gynaecology, University Hospital Santa Maria della Misericordia, 06129 Perugia, Italy); Massimo Fabiani (massimo.fabiani@iss.it; Department of Infectious Diseases, Istituto Superiore di Sanità – Italian National Institute of Health, Viale Regina Elena 299, 00161 Rome, Italy); Massimo Piergiuseppe Franchi (massimo.franchi@univr.it; Department of Obstetrics and Gynaecology, University Hospital of Verona, 37126 Verona, Italy); Livio Leo (livioleo@icloud.com; Hospital Beauregard Valle D'Aosta, 11100 Aosta, Italy); Marco Liberati (liberati10658@gmail.com; Department of Medicine and Aging Sciences, D’Annunzio University of Chieti‐Pescara, 66100 Chieti, Italy); Mariavittoria Locci (mariavittoria.locci@unina.it; Department of Neuroscience, Reproductive Sciences and Dentistry, Federico II University of Naples, 80138 Naples, Italy); Antonino Lo Re (alore@ospedalepederzoli.it; Department of Obstetrics and Gynaecology, Clinica Pederzoli, 37019 Peschiera del Garda, Italy); Claudio Martini (claudio.martini@regione.marche.it; Territorio e Integrazione Ospedale Territorio, Marche Region, 60122 Ancona, Italy); Gianpaolo Maso (gianpaolo.maso@burlo.trieste.it; Obstetrics and Gynaecology, Institute for Maternal and Child Health – IRCCS Burlo Garofolo, 34137 Trieste, Italy); Federico Mecacci (federico.mecacci@unifi.it; Department of Biomedical, Division of Obstetrics and Gynaecology, Experimental and Clinical Sciences, University of Florence, 50134 Florence, Italy); Alessandra Meloni (gineca.ameloni@gmail.com; Maternal and Neonatal Department, Azienda Ospedaliero Universitaria, 09042 Cagliari, Italy); Anna Domenica Mignuoli (anna.mignuoli@regione.calabria.it; Dipartimento Regionale Tutela della Salute, Calabria Region, 88100 Reggio Calabria, Italy); Luisa Mondo (luisa.mondo@epi.piemonte.it; Department of Epidemiology, ASL TO3 Piedmont Region, Turin 10095, Italy); Enrica Perrone (enrica.perrone@regione.emilia-romagna.it; Servizio Assistenza Territoriale, Direzione Generale Cura Della Persona, Salute e Welfare, Emilia‐Romagna Region, 40127 Bologna, Italy); Lucia Porcino (porcinolucia@gmail.com; Unit of Obstetrics and Gynaecology, Ospedali Riuniti di Reggio Calabria, 89124 Reggio Calabria, Italy); Luca Ramenghi (lucaramenghi@gaslini.org; Neonatal Intensive Care Unit, IRCCS Istituto Giannina Gaslini, 16147 Genoa, Italy); Valeria Savasi (valeria.savasi@unimi.it; Unit of Obstetrics and Gynaecology, Department of Biomedical and Clinical Sciences, ASST Fatebenefratelli Sacco, University of Milan, 20157 Milan, Italy); Sergio Crescenzo Antonio Schettini (schettini@icloud.com; San Carlo Hospital, 85100 Potenza, Italy); Gabriella Scorpio (gabriella.scorpio@ausl.pe.it; Unit of Obstetrics and Gynaecology, Presidio Ospedaliero Pescara, 65124 Pescara, Italy); Daniela Simeone (daniela.simeone@asrem.org; Ospedale Civile Antonio Cardarelli, 86100 Campobasso, Italy); Serena Simeone (serenasimeone09@gmail.com; Department of Woman and Child’s Health, Careggi University Hospital, 50141 Florence, Italy); Martin Steinkasserer (martin.steinkasserer@sabes.it; Central Teaching Hospital of Bozen, Division of Gynaecology and Obstetrics, 39100 Bozen, Italy); Fabrizio Taddei (fabrizio.taddei@apss.tn.it; Unit of Obstetrics and Gynaecology, Santa Maria del Carmine Hospital, 38068 Rovereto, Italy); Saverio Tateo (saverio.tateo@apss.tn.it; Santa Chiara Hospital, 38122 Trento, Italy); Vito Trojano (vtrojano@katamail.com; Mater Dei Hospital, 70125 Bari, Italy); Caterina Tronci (troncicgv@yahoo.it; Unit of Obstetrics and Gynaecology, SS Trinità di Cagliari Hospital, 09121 Cagliari, Italy); Antonella Vimercati (antonellavimercati@gmail.com; Department of Biomedical and Human Oncological Science (DIMO), 2nd Unit of Obstetrics and Gynaecology, University of Bari Aldo Moro, 70121 Bari, Italy).
Donati S, Corsi E, Maraschini A, Salvatore MA; the ItOSS‐COVID‐19 Working Group . SARS‐CoV‐2 infection among hospitalised pregnant women and impact of different viral strains on COVID‐19 severity in Italy: a national prospective population‐based cohort study. BJOG 2021; 10.1111/1471-0528.16980.129:221–231.
Contributor Information
E Corsi, Email: edoardo.corsi@iss.it.
the ItOSS‐COVID‐19 Working Group:
Maria Grazia Arena, Rosaria Boldrini, Roberto Brunelli, Angelo Cagnacci, Paola Casucci, Irene Cetin, Luigi Cobellis, Gabriella Dardanoni, Elena De Ambrosi, Martina Del Manso, Sara D'Eusanio, Lorenza Driul, Giorgio Epicoco, Massimo Fabiani, Massimo Piergiuseppe Franchi, Livio Leo, Marco Liberati, Mariavittoria Locci, Antonino Lo Re, Claudio Martini, Gianpaolo Maso, Federico Mecacci, Alessandra Meloni, Anna Domenica Mignuoli, Luisa Mondo, Enrica Perrone, Lucia Porcino, Luca Ramenghi, Valeria Savasi, Sergio Crescenzo Antonio Schettini, Gabriella Scorpio, Daniela Simeone, Serena Simeone, Martin Steinkasserer, Fabrizio Taddei, Saverio Tateo, Vito Trojano, Caterina Tronci, and Antonella Vimercati
References
- 1. Funk T, Pharris A, Spiteri G, Bundle N, Melidou A, Carr M, et al. Characteristics of SARS‐CoV‐2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries. Euro Surveill 2021;26:2100348. 10.2807/1560-7917.ES.2021.26.16.2100348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Istituto Superiore di Sanità, Fondazione Bruno Kessler e Ministero della Salute . Prevalenza delle varianti VOC (Variant Of Concern) in Italia: lineage B.1.1.7, P.1, P.2, lineage B.1.351, lineage B.1.525 (Indagine del 18/2/2021) [Presence of Variant Of concern in Italy: lineage B.1.1.7, P.1, P.2, lineage B.1.351, lineage B.1.525 Survey of 18/02/2021]. [Italian] [https://www.epicentro.iss.it/coronavirus/pdf/sars‐cov‐2‐monitoraggio‐varianti‐indagini‐rapide‐18‐marzo‐2021.pdf]. Accessed 29 September 2021.
- 3. Istituto Superiore di Sanità, Fondazione Bruno Kessler e Ministero della Salute . Prevalenza delle varianti VOC (Variant Of Concern) in Italia: lineage B.1.1.7, P.1, P.2, lineage B.1.351, lineage B.1.525 (Indagine del 18/3/2021) [Presence of Variant Of concern in Italy: lineage B.1.1.7, P.1, P.2, lineage B.1.351, lineage B.1.525 Survey of 18/03/2021]. [Italian] [https://www.epicentro.iss.it/coronavirus/pdf/sars‐cov‐2‐monitoraggio‐varianti‐indagini‐rapide‐18‐marzo‐2021.pdf]. Accessed 29 September 2021.
- 4. Istituto Superiore di Sanità, Fondazione Bruno Kessler e Ministero della Salute . Prevalenza delle varianti VOC (Variant Of Concern) in Italia: lineage B.1.1.7, P.1, P.2, lineage B.1.351, lineage B.1.525 (Indagine del 22/6/2021) [Presence of Variant Of concern in Italy: lineage B.1.1.7, P.1, P.2, lineage B.1.351, lineage B.1.525 Survey of 22/06/2021]. [Italian] [https://www.epicentro.iss.it/coronavirus/pdf/sars‐cov‐2‐monitoraggio‐varianti‐indagini‐rapide‐18‐marzo‐2021.pdf]. Accessed 29 September 2021.
- 5. Kadiwar S, Smith JJ, Ledot S, Johnson M, Bianchi P, Singh N, et al. Were pregnant women more affected by COVID‐19 in the second wave of the pandemic? Lancet 2021;397:1539–40. 10.1016/S0140-6736(21)00716-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vousden N, Ramakrishnan R, Bunch K, Morris E, Simpson N, Gale C, et al. Impact of SARS‐CoV‐2 variant on the severity of maternal infection and perinatal outcomes: Data from the UK Obstetric Surveillance System national cohort. Preprint. medRxiv 2021;2021.07.22.21261000. Published 2021 Jul 22. 10.1101/2021.07.22.21261000 [DOI] [Google Scholar]
- 7. Maraschini A, Corsi E, Salvatore MA, Donati S; ItOSS COVID‐19 Working Group . Coronavirus and birth in Italy: results of a national population‐based cohort study. Ann Ist Super Sanita 2020;56:378–89. 10.4415/ANN_20_03_17 [DOI] [PubMed] [Google Scholar]
- 8. Corsi E, Maraschini A, Perrone E, Salvatore MA, D'aloja P, Donati S. La preparedness dell'Italian obstetric surveillance system in occasione della pandemia da SARS‐CoV‐2: aspetti metodologici di uno studio di popolazione [The preparedness of the Italian obstetric surveillance system in the response to the emergency of the SARS‐CoV‐2 pandemic: methodological aspects of a population‐based study]. Epidemiol Prev 2020;44:81–87. 10.19191/EP20.5-6.S2.089 [DOI] [PubMed] [Google Scholar]
- 9. Donati S, Corsi E, Salvatore MA, Maraschini A, Bonassisa S, Casucci P, et al. Childbirth care among SARS‐CoV‐2 positive women in Italy. Int J Environ Res Public Health 2021;18:4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donati S, Maraschini A, Dell'Oro S, Lega I, D'Aloja P; Regional Maternal Mortality Working Group . The way to move beyond the numbers: the lesson learnt from the Italian Obstetric Surveillance System. Ann Ist Super Sanita 2019;55:363–70. 10.4415/ANN_19_04_10 [DOI] [PubMed] [Google Scholar]
- 11. Maraschini A, Lega I, D'Aloja P, Buoncristiano M, Dell'Oro S, Donati S, et al. Women undergoing peripartum hysterectomy due to obstetric hemorrhage: a prospective population‐based study. Acta Obstet Gynecol Scand 2020;99:274–82. 10.1111/aogs.13727 [DOI] [PubMed] [Google Scholar]
- 12. Ornaghi S, Maraschini A, Donati S; Regional Obstetric Surveillance System Working Group . Characteristics and outcomes of pregnant women with placenta accreta spectrum in Italy: a prospective population‐based cohort study. PLoS One 2021;16:e0252654. 10.1371/journal.pone.0252654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donati S, Fano V, Maraschini A; Regional Obstetric Surveillance System Working Group . Uterine rupture: results from a prospective population‐based study in Italy. Eur J Obstet Gynecol Reprod Biol 2021;264:70–5. 10.1016/j.ejogrb.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 14. Knight M; INOSS . The International Network of Obstetric Survey Systems (INOSS): benefits of multi‐country studies of severe and uncommon maternal morbidities. Acta Obstet Gynecol Scand 2014;93:127–31. 10.1111/aogs.12316 [DOI] [PubMed] [Google Scholar]
- 15. Istituto Nazionale di Statistica (ISTAT) . La presenza straniera in Italia: caratteristiche socio‐demografiche – Permessi di soggiorno al 1° gennaio degli anni 2001, 2002, 2003 [The foreign population living in Italy: socio‐demographic characteristics – years 2001, 2002, 2003]. [Italian] [http://www.cestim.it/sezioni/dati_statistici/italia/Istat/2004‐06%20permessi%20soggiorno%20Italia%202001%202003.pdf]. Accessed 4 August 2021.
- 16.Directorate‐general of digitalization, of health informative system and of statistics, Italian Ministry of Health. Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita – 2019 [Birth Registry – Year 2019]. [Italian] [http://www.salute.gov.it/imgs/C_17_pubblicazioni_3076_allegato.pdf]. Accessed 4 August 2021.
- 17.Istituto Nazionale di Statistica (ISTAT). La dinamica demografica durante la pandemia COVID‐19 – Anno 2020 [The demographic dynamic during the COVID‐19 pandemic – Year 2020]. [Italian] [https://www.istat.it/it/files/2021/03/REPORT‐IMPATTO‐COVIDDEMOGRAFIA_2020.pdf]. Accessed 4 August 2021.
- 18. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 19. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 20. Riccardo F, Ajelli M, Andrianou XD, Bella A, Del Manso M, Fabiani M, et al. Epidemiological characteristics of COVID‐19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Euro Surveill 2020;25:2000790. 10.2807/1560-7917.ES.2020.25.49.2000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. EpiCentro – Epidemiology for Public Health – Istituto Superiore di Sanità (ISS) – Italian National Institute of Health . The Italian Obstetric Surveillance System (ItOSS). Maternal Mortality Surveillance [https://www.epicentro.iss.it/en/itoss/maternal‐mortality‐surveillance]. Accessed 4 August 2021.
- 22.Directorate‐General of Digitalization, of Health Informative System and of Statistics, Italian Ministry of Health. Birth Registry – Year 2019. Data provided by Rosaria Boldrini.
- 23. Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: national population based cohort study. BMJ 2020;369:m2107. 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vousden N, Bunch K, Morris E, Simpson N, Gale C, O'Brien P, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS‐CoV‐2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS One 2021;16:e0251123. 10.1371/journal.pone.0251123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Engjom H, Aabakke AJM, Klungsøyr K, Svanvik T, Äyräs O, Jonasdottir E, et al. COVID‐19 in pregnancy‐characteristics and outcomes of pregnant women admitted to hospital because of SARS‐CoV‐2 infection in the Nordic countries. Acta Obstet Gynecol Scand 2021;100:1611–9. 10.1111/aogs.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: Characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status ‐ United States, January 22‐October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641–7. 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ 2020;370:m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID‐19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr 2021;175:817–26. 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khalil A, Kalafat E, Benlioglu C, O'Brien P, Morris E, Draycott T, et al. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020;25:100446. 10.1016/j.eclinm.2020.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ciapponi A, Bardach A, Comandé D, Berrueta M, Argento FJ, Rodriguez Cairoli F, et al. COVID‐19 and pregnancy: An umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS One 2021;16:e0253974. 10.1371/journal.pone.0253974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. EpiCentro – Epidemiology for Public Health – Istituto Superiore di Sanità (ISS) – Italian National Institute of Health . COVID‐19: Pregnancy, Childbirth and Breastfeeding [https://www.epicentro.iss.it/en/coronavirus/sars‐cov‐2‐pregnancy‐childbirth‐breastfeeding]. Accessed 4 August 2021.
- 32. EpiCentro – Epidemiology for Public Health – Istituto Superiore di Sanità (ISS) – Italian National Institute of Health . The Italian Obstetric Surveillance System (ItOSS). Interim Guidance “The use of COVID‐19 vaccines in pregnant and lactating patients” – Updated on January 31st 2021 [https://www.epicentro.iss.it/vaccini/pdf/ItOSS%20Vaccination%20against%20COVID‐19%20in%20pregnancy_feb.09.2021.pdf]. Accessed 4 August 2021.
- 33. EpiCentro – Epidemiology for Public Health – Istituto Superiore di Sanità (ISS) – Italian National Institute of Health . The Italian Obstetric Surveillance System (ItOSS). Interim Guidance “The use of COVID‐19 Vaccines in Pregnant and Lactating Patients” – Updated on September 22 2021 [https://www.epicentro.iss.it/vaccini/pdf/Aggiornamento%20indicazioni%20ISS%20su%20vaccino%20in%20grav_%20e%20allatt_2021.pdf]. Accessed 29 September 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Differences in educational level between women with and without missing data.
Table S2. COVID‐19 pneumonia mutually adjusted odds ratios; logistic regression model applied to complete cases.
Table S3. Reasons for hospital admission.
Appendix S1. Multiple imputation.
Appendix S2. The ItOSS national network of maternity units.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


