Abstract
Objective
To evaluate the impact of Covid‐19 vaccination (Pfizer–BioNTech BNT162b2) during the third trimester of pregnancy on maternal and neonatal outcomes.
Design
A multicentre, retrospective computerised database.
Population
Women who gave birth at >24 weeks of gestation in Israel, between January and April 2021, with full records of Covid‐19 disease and vaccination status.
Methods
Women who received two doses of the vaccine were compared with unvaccinated women. Women who were recorded as having disease or a positive Covid‐19 polymerase chain reaction (PCR) swab during pregnancy or delivery were excluded from both study groups. Univariate analysis was followed by multivariate logistic regression.
Main outcome measures
Composite adverse maternal outcomes. Secondary outcomes were vaccination rate and composite adverse neonatal outcomes.
Results
The overall uptake of one or both vaccines was 40.2%; 712 women who received two doses of the Covid‐19 vaccine were compared with 1063 unvaccinated women. Maternal composite outcomes were comparable between the groups; however, women who received the vaccine had higher rates of elective caesarean deliveries (CDs) and lower rates of vacuum deliveries. An adjusted multivariable logistic regression analysis demonstrated that Covid‐19 vaccination was not associated with maternal composite adverse outcome (aOR 0.8, 95% CI 0.61–1.03); a significant reduction in the risk for neonatal composite adverse outcomes was observed (aOR 0.5, 95% CI 0.36–0.74).
Conclusions
In a motivated population covered by a National Health Insurance Plan, we found a 40.2% rate of vaccination for the Covid‐19 vaccine during the third trimester of pregnancy, which was not associated with adverse maternal outcomes and, moreover, decreased the risk for neonatal adverse outcomes.
Tweetable abstract
Covid‐19 vaccine during pregnancy is safe for both mother and fetus.
Keywords: Covid‐19 vaccine, healthcare coverage, outcome, pregnancy
Tweetable abstract
Covid‐19 vaccine during pregnancy is safe for both mother and fetus.
Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has posed major challenges for public health systems. 1 According to the US Centers for Disease Control and Prevention (CDC), approximately 25% of women of reproductive age hospitalised with Covid‐19 between 1 March and 22 August 2020 were pregnant. 2 The current evidence suggests that Covid‐19 infection in pregnant women is more severe compared with their non‐pregnant counterparts, with an increased risk of hospital admission, preterm birth, admission to an intensive care unit (ICU), mechanical ventilation and even death. 2 , 3 , 4
Despite the increased risk, and current deliberation by the US Food and Drug Administration (FDA) on whether to include pregnant women in clinical trials, pregnant women were not included in the initial Covid‐19 vaccine trials. 5 , 6 These randomised clinical trials reported efficacies of up to 95% of mRNA‐based vaccines in preventing Covid‐19. 6 Only recently the first vaccine trial to include pregnant women began (Pfizer–BioNTech, ClinicalTrials.gov identifier: NCT04754594). The Covid‐19 mRNA vaccines (Pfizer–BioNTech BNT162b2 and Moderna mRNA‐1273) currently used for mass vaccination do not comprise live vaccines, nor do they use an adjuvant.
Given all of the above, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal–Fetal Medicine (SMFM) recommend that Covid‐19 vaccines should not be withheld from pregnant and lactating individuals. 7 , 8 Moreover, the Israeli Ministry of Health encouraged pregnant women to get vaccinated as part of the Israeli national vaccination programme. A preliminary report on findings of mRNA Covid‐19 vaccine safety in pregnant persons was recently published and did not show obvious safety issues among pregnant women who received mRNA Covid‐19 vaccines. However, the authors of this study call for more data with a larger sample size of women vaccinated in pregnancy, to inform maternal, pregnancy and infant outcomes.
Our aim was to evaluate the Covid‐19 vaccination rate (Pfizer–BioNTech BNT162b2) during the third trimester of pregnancy and the impact on adverse maternal and neonatal outcomes.
Methods
Study design
A multicentre retrospective cohort database study was conducted in two university‐affiliated medical centres in Jerusalem, Israel. The Shaare Zedek Medical Center (SZMC) and the Bikur Holim Medical Center (BHMC) serve the population of Jerusalem (>1 200 000 patients). Together, these medical centres account for approximately 16% of all deliveries in the state of Israel, with a mean annual volume of 22 000.
The National Health Plan covers antepartum and delivery care for all women and neonates; more than 95% of deliveries are managed by the public system. Labour and delivery data were extracted from the medical record database of SZMC, which is updated in real‐time during labour and delivery by attending healthcare professionals and is audited periodically by trained technical personnel to ensure the validity of the data.
The study population included all women aged 18 years or older, with no documented previous positive SARS‐CoV‐2 polymerase chain reaction (PCR) test, who delivered between 19 January 2021 (when the first vaccinated women gave birth) and 27 April 2021. In order to evaluate the influence of the vaccine on pregnancy outcomes among unvaccinated women, women with current or previous Covid‐19 disease were excluded from both study groups.
Upon admission for labour, women were questioned regarding their Covid‐19 disease and/or vaccine status, as well as current potential symptoms of Covid‐19 and/or suspicious contacts (such as travel abroad, contact with active confirmed Covid‐19 cases in quarantine, etc.). At the time of admission and during hospitalisation, all symptomatic women, or those with a primary contact with Covid‐19, had nasopharyngeal swabs collected and tested for SARS‐CoV‐2, using a real‐time reverse‐transcription PCR test. Women excluded from the present study were those who had undocumented Covid‐19 disease or vaccine status, those who had pre‐admission Covid‐19 virus disease or positive Covid‐19 PCR test, and those who had a positive PCR test result during admission and hospitalisation.
The exposure measure of the study was uptake of the Covid‐19 vaccine, during the third trimester (>24 gestational weeks), whether it was a single dose or two doses, 21 days apart. During the study period, vaccination during the first trimester was not encouraged, and those vaccinated during the second trimester had not yet reached term. Given the urgent health significance of the report, we included the first wave of pregnant women who had received the vaccination during the third trimester. Pfizer–BioNTech BNT162b2 was studied as it is the only available and approved vaccine in Israel.
The primary outcome of the study was the composite adverse maternal outcome.
The composite adverse maternal outcome was defined by one or more of the following: chorioamnionitis, postpartum haemorrhage (PPH, estimated blood loss of >1000 ml and/or haemoglobin drop of ≥3 g/dl), endometritis, blood transfusion, a caesarean delivery (CD), ICU admission and a maternal hospital length of stay of >5 days for vaginal delivery and >7 days for CD (the usual postpartum hospital lengths of stay for vaginal delivery and CD are 48–72 hours and 96–120 hours, respectively). We also assessed each of the following variables individually: mode of delivery, chorioamnionitis, PPH, endometritis, blood transfusion and length of stay.
The secondary outcomes included were the Covid‐19 vaccination rate during the third trimester and adverse neonatal outcomes.
The composite adverse neonatal outcome was defined by one or more of the following: intrauterine fetal death (IUFD), Apgar score of ≤7 at 1 minute, Apgar score of ≤7 at 5 minutes, admission to neonatal intensive care unit (NICU), neonatal asphyxia, intracranial haemorrhage, meconium aspiration syndrome, hyperbilirubinaemia, neonatal seizures, neonatal hypoglycaemia, neonatal sepsis and use of mechanical ventilation. We also assessed each of these variables individually.
Secondary outcome analyses were only performed for women who received two doses of the vaccine.
The study was approved by the institutional ethics committee (0013‐21‐SZMC), which is responsible for both the SZMC and BHMC. The study was performed following the principles of the Declaration of Helsinki. Data were obtained from medical records and de‐identified, with no direct participation of patients; hence, informed consent was waived.
Statistical analysis
Categorical variables were presented as percentages and compared using the chi‐square or Fisher’s exact test, as appropriate. Continuous variables with a normal distribution were presented as mean and standard deviation, whereas those without a normal distribution were presented as the median and interquartile range (IQR). Comparisons were made using Student’s t‐test and Mann–Whitney U‐test for normally and non‐normally distributed data, respectively.
To assess independent associations between Covid‐19 vaccination and the primary outcome (composite adverse maternal outcome) and one of the secondary outcomes (the composite adverse neonatal outcome), two separate multivariable logistic regression models were applied. All variables found to be significantly associated with the risk of each outcome in univariate analysis (not presented) were included. The following variables were associated with the composite adverse maternal outcome (primary outcome): maternal age, parity, previous miscarriage, previous CD, fertility treatment, obesity (body mass index, BMI > 30 kg/m2), hypertensive disorder, diabetes (pre‐gestational and gestational), multifetal gestation, gestational age at delivery, epidural analgesia, non‐vertex presentation and neonatal birthweight.
The following variables were associated with the composite neonatal outcome (secondary outcome): primiparity, previous CD, fertility treatment, hypertensive disorder, diabetes (pre‐gestational and gestational), multifetal gestation, gestational age at delivery, induction of labour and neonatal birthweight.
All analyses were two‐sided, and P < 0.05 was considered to indicate statistical significance. Adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) were computed.
Analyses were carried out using the statistical package spss 25 (IBM, Armonk, NY, USA).
Results
During the study period 5745 women gave birth at SZMC (4054 deliveries) and BHMC (1691 deliveries); 2860 (49.8%) women had documented Covid‐19 disease or vaccine status, 1750 (30.5%) of whom were eligible for inclusion in the present study. Women with documented and undocumented Covid‐19 disease or vaccine status had similar demographics and obstetric characteristics, except for previous CD and fertility treatments rates (Table S1).
Comparisons between the group of women who received both doses of vaccine (n = 712) and the unvaccinated group (n = 1063) with regards to demographics and obstetric characteristics are presented in Table 1. Women who received both doses of vaccine were older (30.6 ± 5.8 versus 29.5 ± 6 years, P < 0.01), and had higher rates of previous miscarriages, previous CD and fertility treatments.
Table 1.
Demographic and obstetric characteristics of the study groups
| Unvaccinated n = 1063 | Covid‐19 vaccinated n = 712 | P | |
|---|---|---|---|
| Maternal age, years | 29.5 ± 6 | 30.6 ± 5.8 | <0.01 |
| Gravidity | 4 (2–6) | 4 (2–6) | 0.15 |
| Parity | 3 (2–5] | 3 (2–6) | 0.53 |
| Previous miscarriages | 296 (27.8%) | 240 (33.7%) | 0.01 |
| Previous caesarean delivery, any | 137 (12.9%) | 117 (16.4%) | 0.04 |
| Primipara | 211 (19.8%) | 122 (17.1%) | 0.15 |
| Fertility treatments | 24 (2.3%) | 33 (4.6%) | 0.01 |
| Hypertensive disorders of pregnancy | 19 (1.8%) | 10 (1.4%) | 0.53 |
| Diabetes (pre‐gestational + gestational) | 45 (4.2%) | 45 (6.3%) | 0.05 |
| Obesity (BMI ≥ 30 kg/m2) | 140 (13.2%) | 101 (14.2%) | 0.54 |
| Multifetal gestation | 15 (1.4%) | 16 (2.2%) | 0.19 |
Data are mean ± standard deviation, median (IQR) or number (%).
Primary outcome
Individual obstetric and maternal outcomes are presented in Table 2. The composite adverse maternal outcome was comparable between the groups; however, women who received the vaccine had higher rates of elective CD and lower rates of vacuum‐assisted vaginal delivery (VAVD). An adjusted multivariable logistic regression analysis for significant covariates and confounding factors demonstrated that Covid‐19 vaccination was not associated with the maternal composite adverse outcome (aOR 0.8, 95% CI 0.61–1.03; P = 0.08; Table 3).
Table 2.
Obstetric and maternal outcomes among the study groups
| Unvaccinated n = 1063 | Covid 19 vaccinated n = 712 | P | |
|---|---|---|---|
| Gestational age at delivery | 39.4 ± 1.6 | 39.1 ± 1.6 | <0.01 |
| Gestational age at delivery <34 weeks | 30 (2.8%) | 20 (2.8%) | 0.99 |
| Gestational age at delivery <37 weeks | 10 (0.9%) | 7 (1%) | 0.93 |
| Induction of labour | 143 (13.5%) | 107 (15%) | 0.35 |
| Oxytocin augmentation of labour | 431 (40.5%) | 333 (46.8%) | 0.01 |
| Epidural analgesia | 536 (50.4%) | 390 (54.8%) | 0.07 |
| Meconium‐stained amniotic fluid | 172 (16.2%) | 103 (14.5%) | 0.33 |
| Chorioamnionitis | 26 (2.4%) | 14 (2%) | 0.50 |
| Caesarean delivery | 115 (10.8%) | 111 (15.6%) | <0.01 |
| Elective caesarean delivery | 81 (7.6%) | 82 (11.5%) | 0.01 |
| In‐labour caesarean delivery | 20 (1.9%) | 17 (2.4%) | 0.46 |
| Home/car delivery | 19 (1.8%) | 11 (1.5%) | 0.70 |
| Vacuum‐assisted delivery | 66 (6.2%) | 23 (3.2%) | <0.01 |
| Hospitalisation length, days | 2 ± 1.9 | 2 ± 1.5 | 0.68 |
| Prolonged hospital stay | 25 (2.4%) | 11 (1.5%) | 0.24 |
| Episiotomy | 112 (10.5%) | 69 (9.7%) | 0.56 |
| Maternal ICU admission | 0 (0%) | 0 (0%) | N/A |
| Postpartum haemorrhage | 106 (10%) | 52 (7.3%) | 0.05 |
| Placental abruption | 25 (2.4%) | 8 (1.1%) | 0.06 |
| Haemoglobin drop, g/dl | 1.3 ± 1.1 | 1.2 ± 1 | 0.07 |
| Haemoglobin drop > 4 g/dl | 29 (2.7%) | 15 (2.1%) | 0.41 |
| Puerperal fever | 36 (3.4%) | 23 (3.2%) | 0.86 |
| Blood products transfusion | 7 (0.7%) | 4 (0.6%) | 0.80 |
| Composite adverse maternal outcome | 251 (23.6%) | 172 (24.2%) | 0.79 |
Data are mean ± standard deviation or number (%). Composite adverse maternal outcome was defined as one of the following: caesarean delivery, prolonged hospitalisation, postpartum haemorrhage, blood products transfusion, maternal ICU admission, chorioamnionitis or endometritis.
Table 3.
Multivariate logistic regression analysis for the association between Covid‐19 vaccination and composite maternal outcomes (adjusted odds ratio)
| P | aOR | 95% CI | ||
|---|---|---|---|---|
| Previous caesarean delivery, any | <0.01 | 5.54 | 3.96 | 7.75 |
| Parity | <0.01 | 0.77 | 0.71 | 0.84 |
| Gestational age at delivery, weeks | <0.01 | 0.75 | 0.68 | 0.83 |
| Non‐vertex presentation | <0.01 | 5.51 | 2.92 | 10.40 |
| Obesity (BMI ≥ 30 kg/m2) | <0.01 | 1.92 | 1.35 | 2.73 |
| Maternal age, years | <0.01 | 1.06 | 1.02 | 1.09 |
| Epidural analgesia | <0.01 | 0.66 | 0.51 | 0.86 |
| Fertility treatments | 0.01 | 2.60 | 1.25 | 5.42 |
| Multifetal gestation | 0.02 | 3.86 | 1.27 | 11.76 |
| Covid‐19 vaccination | 0.08 | 0.79 | 0.61 | 1.03 |
| Diabetes (pre‐gestational + gestational) | 0.12 | 1.58 | 0.89 | 2.78 |
| Hypertensive disorders of pregnancy | 0.13 | 2.13 | 0.79 | 5.73 |
| Birthweight, grams | 0.22 | 1.00 | 1.00 | 1.00 |
| Miscarriage | 0.77 | 1.05 | 0.78 | 1.40 |
Secondary outcomes
In this study, 717 women (40.2%) received either one or two doses of the Covid‐19 vaccine, whereas 1063 women did not receive any dose. Of those who received the Covid‐19 vaccine, five women (0.2%) received one dose and 712 women (40.0%) received two doses (Figure 1).
Figure 1.
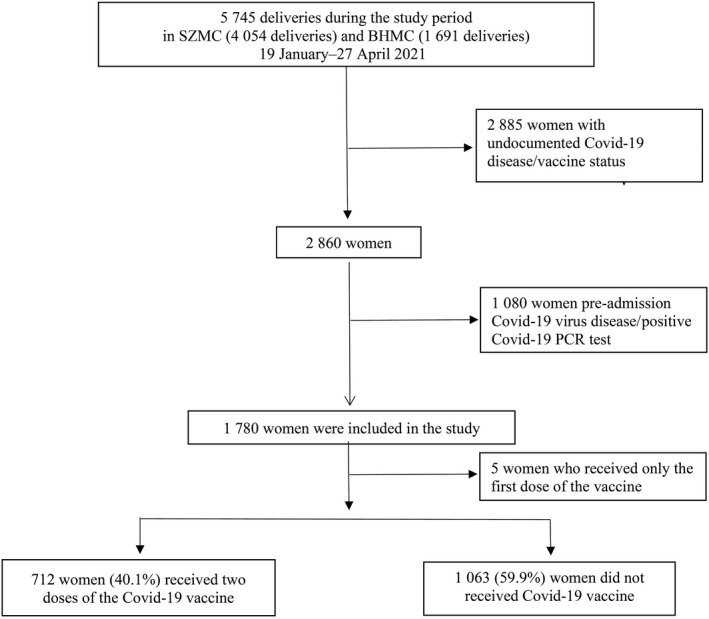
Study flow chart.
Individual neonatal outcomes are presented in Table 4. Composite adverse neonatal outcome rates were lower among women who received the vaccine, although we do not identify any individual neonatal outcome that differed between those who accepted the vaccine and those who did not.
Table 4.
Neonatal outcomes among the study groups
| Unvaccinated n = 1063 | Covid‐19 vaccinated n = 712 | P | |
|---|---|---|---|
| Birthweight > 4000 g | 3339.5 ± 466.3 | 3317.8 ± 517.8 | 0.36 |
| Birthweight, g | 27 (2.5%) | 27 (3.8%) | 0.13 |
| LGA | 85 (8%) | 68 (9.6%) | 0.26 |
| SGA | 98 (9.2%) | 81 (11.4%) | 0.14 |
| Male gender | 539 (50.7%) | 353 (49.6%) | 0.64 |
| 1‐minute Apgar score ≤ 7 | 49 (4.6%) | 30 (4.2%) | 0.69 |
| 5‐minute Apgar score ≤ 7 | 27 (2.5%) | 21 (2.9%) | 0.60 |
| Intrauterine fetal death | 5 (0.5%) | 5 (0.7%) | 0.52 |
| NICU admission | 48 (4.5%) | 29 (4.1%) | 0.65 |
| Meconium aspiration syndrome | 0 (0%) | 0 (0%) | N/A |
| Jaundice | 45 (4.6%) | 16 (3.3%) | 0.21 |
| TTN | 8 (0.8%) | 3 (0.6%) | 0.66 |
| Mechanical ventilation | 15 (1.5%) | 4 (0.8%) | 0.24 |
| Seizures | 4 (0.4%) | 1 (0.2%) | 0.52 |
| Hypoglycaemia | 23 (2.4%) | 12 (2.4%) | 0.93 |
| Sepsis | 1 (0.1%) | 1 (0.2%) | 0.62 |
| Encephalopathy | 0 (0%) | 0 (0%) | N/A |
| Intracranial haemorrhage | 0 (0%) | 0 (0%) | N/A |
| Birth asphyxia | 9 (0.9%) | 1 (0.2%) | 0.11 |
| Composite adverse neonatal outcome | 121 (11.4%) | 56 (7.9%) | 0.02 |
Data are mean ± standard deviation or number (%). LGA, large for gestational age; SGA, small for gestational age; TTN, transient tachypnoea of the newborn. Composite adverse neonatal outcome was defined as one of the following: intrauterine fetal death, 1‐minute Apgar score < 7, 5‐minute Apgar score < 7, NICU admission, neonatal asphyxia, jaundice, neonatal hypoglycaemia, meconium aspiration, TTN, mechanical ventilation, sepsis, seizures, intracranial haemorrhage and encephalopathy.
An adjusted multivariable logistic regression analysis for significant covariates and confounding factors demonstrated that Covid‐19 vaccination reduced the neonatal composite adverse outcome (aOR 0.5, 95% CI 0.36–0.74; P < 0.01; Table 5).
Table 5.
Multivariate logistic regression analysis for the association between Covid‐19 vaccination and composite neonatal outcomes (adjusted odds ratio)
| P | aOR | 95% CI | ||
|---|---|---|---|---|
| Gestational age at delivery, weeks | <0.01 | 0.70 | 0.62 | 0.79 |
| Diabetes (pre‐gestational + gestational) | <0.01 | 3.96 | 2.34 | 6.70 |
| Covid‐19 vaccination | <0.01 | 0.52 | 0.36 | 0.74 |
| Induction of labour | 0.02 | 1.66 | 1.08 | 2.55 |
| Primipara | 0.05 | 1.52 | 1.00 | 2.32 |
| Previous caesarean delivery, any | 0.18 | 1.37 | 0.87 | 2.15 |
| Birthweight, grams | 0.19 | 1.00 | 1.00 | 1.00 |
| Hypertensive disorders of pregnancy | 0.52 | 1.38 | 0.52 | 3.68 |
| Multifetal gestation | 0.58 | 1.30 | 0.51 | 3.27 |
| Fertility treatments | 0.63 | 1.21 | 0.56 | 2.62 |
Discussion
Main findings
In this study, we assessed the third‐trimester SARS‐CoV‐2 BNT162b2 mRNA vaccination impact on maternal and neonatal outcomes. Of the women without a documented previous positive Covid‐19 PCR test, 40.0% received two doses of the vaccine, and 40.2% received at least one dose, during the study period. Women who received both doses of vaccine were older, and had higher rates of previous miscarriages, previous CD and fertility treatments. Maternal outcomes were comparable and the uptake of the Covid‐19 vaccine was not associated with poorer maternal outcomes. However, we did find that the uptake of two doses of the vaccine was associated with a higher rate of elective CD and a lower rate of VAVD. In contrast, the risk for composite neonatal outcomes was twofold lower.
Interpretation
The Covid‐19 vaccine has been available in Israel since 20 December 2020. The Pfizer–BioNTech BNT162b2 vaccine is the only type of vaccine approved and available in Israel. The costs for health care and any interventions for pregnant women and children are covered by the National Health Insurance Plan. The vaccine was made available at individual branches of the health maintenance organisation (HMO) clinics as well as in regional centres under the auspices of the HMO. As of 27 April 2021, among the general population in Israel without a previously documented positive Covid‐19 PCR test, at least 54.7% had received two doses of the Covid‐19 vaccine and 58.0% have received one dose. 9 In the current study, the corresponding uptake rate of the Covid‐19 vaccine in the third trimester of pregnancy was at least 40.0% and possibly 40.2%.
The eligibility for the vaccine in Israel may be divided into two types:
1 Formal eligibility: all Israeli citizens and permanent residents of Israel are eligible to receive the vaccine under the National Health Insurance Plan. In our centre, all women meet these criteria.
2 Health condition‐derived eligibility: during the study period, women with previous Covid‐19 disease or a positive PCR test were not offered the vaccine.
In the present study, women with current or previous Covid‐19 disease were excluded in order to evaluate the influence of the vaccine on pregnancy outcomes among unvaccinated women. The health system listed no contraindications to the vaccine besides an anaphylactic reaction to previous vaccines or the first dose of the Pfizer–BioNTech BNT162b2 vaccine. As we do not have information regarding why the unvaccinated women decided not to get vaccinated, we cannot address this bias. However, the background data of the study groups is similar, and the unvaccinated group did not have pre‐existing conditions that could have led to adverse outcomes. Thus, even in the setting of any pre‐existing conditions the vaccine would still be recommended.
It is not surprising that the uptake of the Covid‐19 vaccine in pregnancy is lower than is reported in the general population. This difference could stem from several factors: (i) at the time the vaccine was offered in Israel there was a lack of evidence supporting the safety of Covid‐19 vaccination in pregnancy, and a lack of studies on its possible impact on adverse maternal and neonatal outcomes; 10 and (ii) vaccine uptake in pregnancy, for influenza vaccine or Tdap (tetanus, diphtheria and pertussis), is lower than is desirable, possibly as a result of safety concerns. 11
In terms of safety, the mRNA Covid‐19 vaccines are novel in design and, to date, are the first mRNA vaccines to have been comprehensively evaluated for the prevention of disease in people. 8 , 12 Although none of these vaccines contain a virus that replicates, nonspecific side effects from the activation of the immune system may occur. Consistently, the Advisory Committee on Immunization Practices (ACIP) branch of the CDC lists pregnancy as a precaution for the administration of these vaccines.
Safety information has been tracked by the CDC using the v‐safe smartphone application. The v‐safe data indicate no significant differences in post‐vaccination reactions in pregnant versus non‐pregnant women. 13 Furthermore, the use of this v‐safe smartphone application in 712 pregnant women who completed their pregnancies revealed no adverse pregnancy outcome, as found in our study.
Following these uncertainties, the CDC, the World Health Organization (WHO) and other agencies based their initial recommendations on a cautious approach, recommending that the vaccination of pregnant women be assessed on a case‐by‐case basis, given the risk for a severe disease course among non‐vaccinated pregnant women. 14 , 15 In Israel, as part of the national distribution, the Covid‐19 vaccine was offered to pregnant women, with preferential administration during the second or third trimester until the safety and efficacy of these novel vaccines has been validated. The Israeli population is motivated to use the healthcare services provided, especially as all are covered uniformly by the National Health Insurance Plan and are available for regional use. The administration of the vaccine was coordinated by the Ministry of Health and the HMO programmes in Isreal, 16 before the official FDA approval. 12
Indeed, the rates of uptake of the Covid‐19 vaccine in our study of roughly 40% are somewhat higher than the reported rates of uptake of other vaccines in pregnancy. Recently, there has been a reported 21.7% uptake of maternal influenza vaccine and 25.7% uptake of maternal pertussis vaccine among 323 622 pregnant women from New Zealand between 2013 and 2018. 17
Women who received the two‐dose vaccine had higher rates of elective CDs with similar rates of in‐labour CD. This could be attributed to the higher rates of previous CDs among this group, which itself is a risk factor for CD in a subsequent delivery.
Data for Covid‐19 disease or vaccine status were available for only about 50% (2860/5745) of women who gave birth at our medical centres during the study period. Although women with undocumented Covid‐19 disease or vaccine status had similar demographics and obstetric characteristics overall, the rate of documented previous or current Covid‐19 disease is relatively high (37.8%, 1080/2860), compared with the overall Covid‐19 infection rate in the general population, of approximately 10%. Nevertheless, the catchment area for our medical centre has reported one of the highest rates of Covid‐19 infection in Israel, mostly through the high impact of large families, the overcrowded population and difficulties with the educational programmes on Covid‐19 disease and vaccination reaching the various religious communities. 18 Although the infection rate in the pregnant population was still unknown, a study from Washington State demonstrated that the Covid‐19 infection rate was 70% higher in pregnant patients as compared with aged‐matched adults. The infection rate remained 30% higher even after excluding pregnant patients whose infections were detected through screening strategies of the asymptomatic population. 19 We could also postulate that, at that point in time, pregnant women tended to have numerous contacts with asymptomatic children and adolescents, and thus were a target for disease.
Although the findings of the current study are promising, there are several unanswered queries. Maternal and neonatal outcomes of maternal vaccination, during the conception period or early stages of pregnancy, remain unclear. In the current study, maternal and neonatal outcomes were found to be similar between groups, with no increase or decrease in adverse outcomes. In our opinion, the relatively high vaccination rate among pregnant women reflects the high vaccination rate in the population as a whole and is related to well‐organised and financially supported healthcare services. 16
Strengths and limitations
The strengths of this study include meticulous data collection with all records derived from a real‐time updated electronic database, thereby minimising the possibility of bias. Nonetheless, the present study has several caveats:
The retrospective design raises the possibility of biases inherent to such investigations.
We cannot exclude possible unknown factors (such as socio‐economic status), which may impact the findings; however, all of the health and vaccine costs of the population are covered by the National Healthcare System. Vaccination was free and easily accessible to all, including individuals who had difficulty reaching a vaccination centre in person. Thus, socio‐economic status is likely to have little or no influence on the rate of vaccination.
For the duration of the study, SZMC and BHMC did not screen all women who gave birth, and the vaccination availability was universal, independent of proof of no previous Covid‐19 infection. This may have led to the inclusion of unknown asymptomatic women. If this was an unintended bias then we might expect it to be similar in both groups, whereas if it was intended (i.e. women did not disclose their previous Covid‐19 symptoms or did not present for nasopharyngeal swabs), we expect this to affect the non‐vaccinated group rather the vaccinated group. As we found no difference in adverse outcomes, and Covid‐19 supposedly adversely affects the outcomes included in the present study, we may assume that this was not the case.
We have no information on the interval between the second dose of vaccination and delivery; in some cases, the interval might have been too short to reveal adverse outcomes.
The inclusion criteria for the study, women who were vaccinated during the third trimester, introduces selection bias; thus, the pregnancy outcomes of women who were vaccinated in an earlier stage of pregnancy cannot be concluded from this study.
The most significant limitation is that the current investigation may have lacked sufficient statistical power to detect small but clinically relevant differences in infrequent outcomes. Furthermore, the generalisability of our findings to other populations with different characteristics may be difficult and remains to be determined.
Documented Covid‐19 disease or vaccine status was available for only 50% of women delivered at SZMC and BHMC during the study period; however, they had overall similar demographics and obstetric characteristics.
Although we did not find differences in any individual neonatal outcome, the neonatal composite adverse outcome rate was found to be significantly lower in the vaccination group. This may be because of the relative rarity of the individual neonatal outcomes evaluated as well as the distinct biological mechanisms underlying each outcome; this finding should be further evaluated in other studies.
Conclusion
In this multicentre retrospective cohort study, we found that the uptake of Covid‐19 vaccination during the third trimester of pregnancy was not associated with an increased risk of adverse maternal outcome and lowered the risk for adverse neonatal outcome.
Disclosure of interests
None declared. Completed disclosure of interests form available to view online as supporting information.
Contribution to authorship
MR, HYS and RR: protocol development, data collection and management, data analysis, and writing and editing the article. EK, YW‐W and SG‐G: protocol development, data collection and management, and writing and editing the article.
Details of ethics approval
The study was approved by the institutional ethics committee (0013‐21‐SZMC), which is responsible for both SZMC and BHMC. The study was performed following the principles of the Declaration of Helsinki. Data were obtained from medical records and de‐identified, with no direct participation of patients; hence, informed consent was waived.
Funding
None.
Acknowledgements
None.
Supporting information
Table S1. Demographic and obstetric characteristics of women with documented and undocumented Covid‐19 disease or vaccine status.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener‐Well Y, Grisaru‐Granovsky S. Covid‐19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG 2021; 10.1111/1471-0528.16941.129:248–255.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available in accordance with privacy or ethical restrictions.
References
- 1. Ghebreyesus TA. WHO Director‐General's Opening Remarks at the Media Briefing on COVID‐19‐11 March 2020. Geneva, Switzerland: World Health Organization; 2020;11. [Google Scholar]
- 2. Delahoy MJ, Whitaker M, O'Halloran A, Chai SJ, Kirley PD, Alden N, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory‐confirmed COVID‐19—COVID‐NET, 13 states, March 1–August 22, 2020. Morb Mortal Wkly Rep 2020;69:1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status—United States, January 22–October 3, 2020. Morb Mortal Wkly Rep 2020;69:1641–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, Oduyebo T, et al. Birth and infant outcomes following laboratory‐confirmed SARS‐CoV‐2 infection in pregnancy—SET‐NET, 16 jurisdictions, March 29–October 14, 2020. Morb Mortal Wkly Rep 2020;69:1635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pregnant Women: Scientific and Ethical Considerations for Inclusion in Clinical Trials Guidance for Industry [https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm]. Accessed 25 April 2021.
- 6. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wade K. Experts in high‐risk pregnancy respond to the FDA's decision to offer the newly approved COVID‐19 vaccine to pregnant and lactating people. Soc Matern Fetal Med 2020;1:685–99. [https://s3.amazonaws.com/cdn.smfm.org/media/2632/FDA_final.pdf]. [Google Scholar]
- 8. Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid‐19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–82. 10.1056/NEJMoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaccination rate in Israel [https://datadashboard.health.gov.il/COVID‐19/general]. Accessed 29 April 2021.
- 10. Heath PT, Le Doare K, Khalil A. Inclusion of pregnant women in COVID‐19 vaccine development. Lancet Infect Dis 2020;20:1007–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Leary ST, Riley LE, Lindley MC, Allison MA, Albert AP, Fisher A, et al. Obstetrician‐Gynecologists' strategies to address vaccine refusal among pregnant women. Obstet Gynecol 2019;133:40–7. 10.1097/AOG.0000000000003005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA . FDA approves first COVID‐19 vaccine. Silver Spring, MD: FDA; [https://www.fda.gov/news‐events/press‐announcements/fda‐approves‐first‐covid‐19‐vaccine]. [Google Scholar]
- 13. CDC . Vaccine safety. V‐safe After Vaccination Health Checker. Atlanta, GA: CDC; [https://www.cdc.gov/vaccinesafety]. Accessed 8 April 2021. [Google Scholar]
- 14. Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. The Advisory Committee on Immunization Practices' updated interim recommendation for allocation of COVID‐19 vaccine ‐ United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1657–60. 10.15585/mmwr.mm695152e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Society for Maternal‐Fetal Medicine . Society for Maternal‐Fetal Medicine (SMFM) Statement: SARS‐CoV‐2 Vaccination in Pregnancy. Washington, DC: Society for Maternal‐Fetal Medicine. [Google Scholar]
- 16. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412–23. 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howe AS, Pointon L, Gauld N, Paynter J, Willing E, Turner N. Pertussis and influenza immunisation coverage of pregnant women in New Zealand. Vaccine 2020;38:6766–76. 10.1016/j.vaccine.2020.08.030 [DOI] [PubMed] [Google Scholar]
- 18. Israel Ministry of Health . Corona Data per Cities in Israel. Israel: Israel Ministry of Health; [https://data.gov.il/dataset/covid‐19]. Accessed 26 August 2021. [Google Scholar]
- 19. Lokken EM, Taylor GG, Huebner EM, Vanderhoeven J, Hendrickson S, Coler B, et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am J Obstet Gynecol 2021;225:75.e1–16. 10.1016/j.ajog.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic and obstetric characteristics of women with documented and undocumented Covid‐19 disease or vaccine status.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available in accordance with privacy or ethical restrictions.


