Abstract
Background
Post‐COVID multisystem hyperinflammatory syndrome in children (MISC) has clinical and laboratory similarities with Kawasaki disease (KD). Inflammatory markers like C‐reactive protein (CRP), interleukin 6 (IL6) as well as N‐terminal probrain natriuretic peptide (NT‐proBNP) are elevated in both. This study attempts a comparative analysis of the 3 markers in an attempt at early differentiation for planning appropriate management.
Methodology
This analytical study conducted at the Institute of Child Health, Kolkata, India compared the levels of the above 3 markers at admission between 72 patients with KD, 30% of whom had coronary artery lesions (CALs) collected over a period of 18 months (Jan 2017‐June 2018), with 71 MISC patients over a period of 6 months (July 2020‐December 2020). The non‐parametric Mann‐Whitney U test was used to test for similarity in distributions of the samples of CRP, NT‐proBNP and IL6 in KD and MISC patients using correction factor for similar ranks. The 3 parameters were compared using receiver operating characteristic (ROC) curve analysis.
Results
Mean IL6 value in KD was 83.22 pg/mL and in MISC 199.91 pg/mL, which was not found to be statistically significant (P = .322 > .05).However mean NT‐proBNP (914.91 pg/mL) with CRP level (96.32 mg/L) in KD was significantly lower (P < .05 for both cases) than that in MISC (9141.16 pg/mL and 145.66 mg/L respectively). ROC analysis showed NT‐proBNP has the best sensitivity and specificity in predicting MISC.
Conclusion
NT‐proBNP and CRP are significantly higher among MISC patients; ROC analysis shows levels >935.7 pg/mL and >99.55 mg/L respectively might act as a guide to differentiate between them.
Keywords: C‐reactive protein, interleukin 6, Kawasaki disease, MISC, NT‐proBNP, PIMS
1. INTRODUCTION
The COVID‐19 pandemic originated in Wuhan in December 2019 and rapidly spread worldwide. Few months following onset of the pandemic there were global reports of a new hyperinflammatory syndrome predominantly affecting healthy children and adolescents, the majority of them requiring hospitalization and intensive care support. Named variably as pediatric hyperinflammatory multisystem syndrome (PIMS) or multisystem hyperinflammatory syndrome in children (MISC), the disease was noted to have certain clinical as well as laboratory similarities with Kawasaki disease (KD). 1 , 2 KD is an idiopathic multisystem inflammatory disease characterized by vasculitis of small and medium sized arteries, notably involving coronary arteries.
Both MISC and KD are childhood diseases presenting with fever with overlapping features like nonpurulent bilateral bulbar conjunctivitis, rashes, mucositis, lymphadenopathy and edema that sometimes make them clinically indistinguishable. Inflammatory markers like C‐reactive protein (CRP), interleukin 6 (IL‐6) are elevated in both the conditions along with serum nitrogen terminal probrain natriuretic peptide (NT‐proBNP), 3 a marker for myocardial strain. Although the 2 diseases have clinical as well as similar laboratory features, the prognosis and treatment modality vary and hence the need for early differentiation. This study attempts a comparative analysis of the above 3 common lab markers pertaining to both the diseases in an attempt to distinguish them early for planning appropriate management.
2. METHODOLOGY
The study was conducted at the Institute of Child Health, Kolkata, India. This is an analytical study comparing IL‐6, CRP 4 and NT‐proBNP (unpublished data) levels between 72 patients with KD of which 30% had coronary artery lesions (CALs), collected over a period of 18 months in the pre‐COVID era 4 (Jan 2017‐June 2018), with 71 MISC patients over a period of 6 months (July 2020‐December 2020) admitted at the same institute. The MISC patients were diagnosed on the basis of the World Health Organization criteria 5 and American Heart Association criteria 6 was used for KD diagnosis. Institutional ethics committee approval was taken (IEC/241/2021) prior to conducting the study.
All the KD and MISC patients in the study were diagnosed on the 4th‐6th days of fever. Venous blood samples for IL‐6, CRP and NT‐proBNP together with other necessary investigations were drawn at admission prior to institution of any therapy and evaluated at the biochemistry department. The serum concentrations of CRP were quantitatively determined by particle‐enhanced turbidimetry on a Roche Integra 400 Plus© biochemistry analyzer. Linearity was until 200 mg/L. Biological reference interval was <5 mg/L and serum IL‐6 was measured on the Roche Cobase411© immunoassay analyzer with a linearity until 5000 pg/mL and biological reference interval was <7 pg/mL. Estimation of NT‐proBNP was done by Roche e411 Immunoassay Autoanalyzer using electrochemiluminescence immunoassay method.
Data was collected and entered in an Excel spreadsheet.
For statistical analysis, Scipy library in Python 3 and its library functions were used. Sample values for KD and MISC were independently obtained. The non‐parametric Mann‐Whitney U test was used to test for similarity in distributions of the samples of CRP, NT‐proBNP and IL‐6 levels in KD and MISC patients using correction factor for similar ranks. The difference in distributions of values was considered significant if P value for the test was less than .05. All analyses were done in Jupyter Notebook environment. Receiver operating characteristic (ROC) curve analysis of the 3 indices at admission for both the diseases were also performed and optimum cut off values for the respective parameters was calculated by using Youden's J Index.
3. RESULTS
Seventy‐one patients with MISC were admitted during the study period from July 2020‐December 2020. The mean duration of fever at presentation (mean ± SD) was 5 ± 0.57 days for PIMS and 8.92 ± 2.82 days for the KD patients. Median age for KD was 19 months with interquartile range (IQR) of 13.75‐30.5 months and for the MISC was 11 years with IQR of 3 years.
Since the admitting institute is a non‐COVID hospital, none of the MISC patients in the study were reverse‐transcription polymerase chain reaction positive. There were 42.25% of MISC patients who had history of exposure to the virus, 91% were found to be positive for SARS‐COV2 immunoglobulin G. Intensive care admission was needed by 45% (n = 32) of the patients and 29.5% (n = 21) required inotropic support. Cardiac symptoms were present in 57.74% (n = 41), mostly as myocarditis (disproportionate tachycardia, electrocardiogram and echocardiographic changes) of which 29.5% had low ejection fraction varying from 35% to 47%, and 27% had CALs.
The majority (91.5%, n = 65) of the 71 MISC patients received intravenous immunoglobulin (IVIg), mostly at a dose of 2 g/kg. However, 7 adolescents because of the need for a large dose and consequent financial burden, were administered 1 g/kg in conjunction with corticosteroids. Methylprednisolone only was given to 4 patients and 40 children (56.3%) received steroids + IVIg. Three patients who presented only with fever and no organ involvement had a spontaneous remission without any treatment. All patients additionally received antiplatelet doses of aspirin. All of them responded to therapy, although patients with cardiac involvement and higher inflammatory markers at presentation required higher dosages of steroids (methylprednisolone 10‐30 mg/kg/d for 3‐5 days).
CRP levels were available for all MISC patients; however, data on NT‐proBNP and IL‐6 were available for 53 and 34 patients, respectively. These data were compared to similar data collected from 72 previously diagnosed KD patients (30% of them having CALs), complete data sets being available for each one of them.
Mean IL‐6 in KD of those with CALs was 143.60 pg/mL, which was about 3 times higher than in those without CALs (mean = 52.90 pg/mL); mean for the entire group being 83.22. Mean IL‐6 among the MISC patients was 199.91 pg/mL. On testing for similarity in distributions of IL‐6 levels in KD and MISC patients, the difference between the 2 groups was found to be statistically not significant (P = .322).
Testing the other 2 parameters, the mean NT‐proBNP (pg/mL) levels in KD (mean = 914.91) was significantly lower (P = .00002) than NT‐proBNP levels in MISC patients (mean = 9141.16). Again, the mean CRP levels (mg/L) in KD (mean = 96.32) was significantly lower (P = .043) than CRP in MISC patients (mean=145.66; Table 1).
TABLE 1.
Mean serum CRP, IL‐6 and NT‐proBNP levels in KD and MISC patients
| KD | MISC | P value | |
|---|---|---|---|
| CRP, mg/L | 96.32 | 145.66 | .043 (<.05) |
| IL‐6, pg/mL | 83.22 | 199.91 | .322 (>.05) |
| NT‐proBNP, pg/mL | 914.91 | 9141.16 | .00002 (<.05) |
Abbreviations: CRP, C‐reactive protein; IL‐6, interleukin 6; KD, Kawasaki disease; MISC, multisystem hyperinflammatory syndrome in children; NT‐proBNP, N‐terminal probrain natriuretic peptide.
We also compared the 3 laboratory parameters, that is CRP, IL‐6 and NT‐proBNP prior to administration of any treatment in both KD and MISC patients by performing ROC curve analysis (Figure 1) and calculating optimum cut off values for the respective parameters by using Youden's J Index. The respective areas under the curves (AUCs) with corresponding confidence intervals (CI) and P values are mentioned below the respective ROC curves. Comparative ROC curve analysis shows that among the 3 indicators, NT‐proBNP held a better diagnostic value rather than CRP and IL‐6 in differentiating KD patients from MISC.
FIGURE 1.
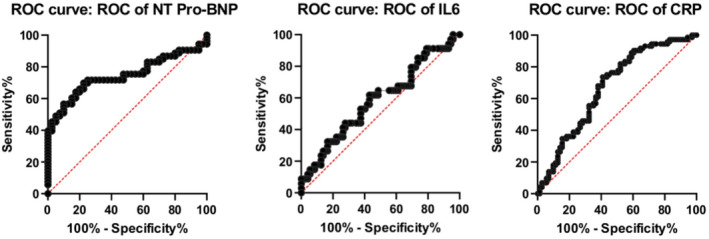
Receiver operating characteristic (ROC) curve analysis of the 3 laboratory parameters in multisystem hyperinflammatory syndrome in children (MISC) and Kawasaki disease (KD)
Table 2 shows the cut off values with respect to the 3 parameters along with their individual sensitivities and specificities; values above the cut off indicates more chances of MISC, whereas values less than the cut off points more toward KD. It is seen that NT‐proBNP values of >935.7 pg/mL have the best sensitivity (69.81%) and specificity (77.5%) in predicting MISC among all the 3 indicators, followed by CRP (>99.55 mg/L, sensitivity 59.15% and specificity 73.61%).
TABLE 2.
Sensitivity and specificity of the 3 indices for individually predicting MISC
| Lab parameters | Cut off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| CRP | 99.55 mg/L | 59.15 | 73.61 |
| IL‐6 | 72.57 pg/mL | 61.76 | 56.94 |
| NT‐proBNP | 935.7 pg/mL | 69.81 | 77.5 |
Abbreviations: CRP, C‐reactive protein; IL‐6, interleukin 6; MISC, multisystem hyperinflammatory syndrome in children; NT‐proBNP, N‐terminal probrain natriuretic peptide.
We also tried to correlate and analyze the NT‐proBNP values in MISC patients with cardiac involvement and those without (Figure 2). Of the 71 MISC patients, 39 presented with cardiac involvement, 21 had coronary artery aneurysms (z score >2.5), 24 with myocardial dysfunction (ejection fraction < 55%) and 6 had both. Correlation study was done to calculate Pearson's correlation coefficient using SPSS v.28. Analyzing NT‐proBNP values in those with myocardial dysfunction in respect of those without any cardiac involvement, the value of R is −.0291, with a P value of .900355 making the result not significant.
FIGURE 2.
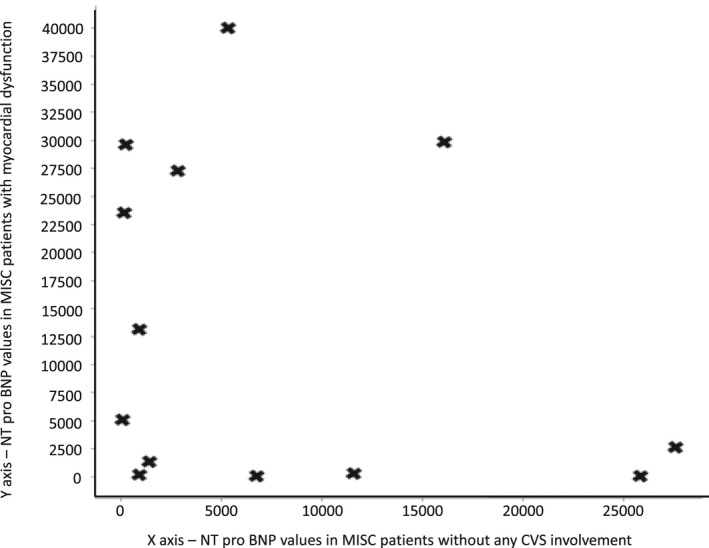
Scatter plot to show ‐terminal probrain natriuretic peptide (NT‐proBNP) values in patients with and without myocardial dysfunction
Similarly when correlation study was done between patients with MISC with coronary artery aneurysms (CAAs) and those without any cardiac involvement (Figure 3), it was found to have an R value of −.0314, with a P value of .915; again denoting no significant correlation. Hence it can be deduced that values of NT‐proBNP does not have any significant relationship with the severity of cardiac involvement.
FIGURE 3.
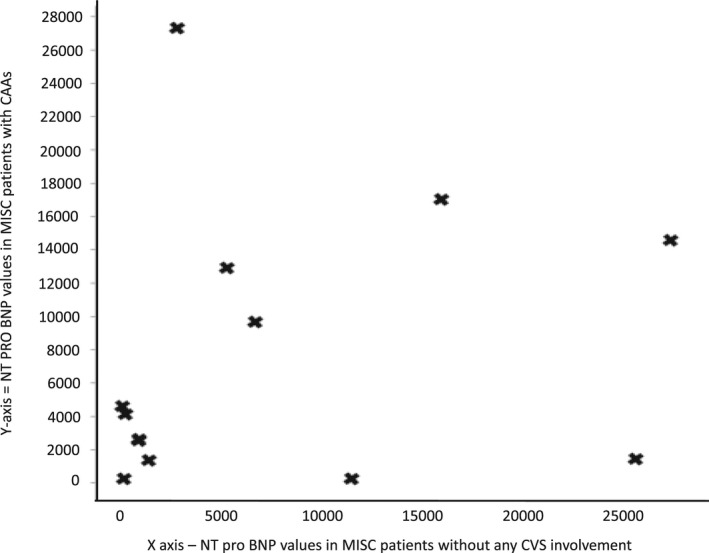
Scatter plot to show ‐terminal probrain natriuretic peptide (NT‐pro BNP) values in patients with coronary artery aneurysms (CAAs) and in those without cardiac involvement
4. DISCUSSION
With the ongoing COVID‐19 pandemic there have been increasing reports of PIMS that develop some weeks after the acute phase of a mildly symptomatic or asymptomatic SARS‐CoV‐2 infection. In the present cohort 42.25% of the patients had a history of COVID contact, exposure which occurred 4‐8 weeks prior to the onset of MISC. Although exposure occurred, none of the parents could recollect any significant symptom related to COVID.
The massive activation of pro‐inflammatory cytokines in MISC overlap that with KD and the “cytokine storm” observed in adult COVID‐19 patients, and there may be associated macrophage activation. 7 Although MISC patients have distinct clinical and immunological features, 8 , 9 young children with KD like presentation are often initially indistinguishable from KD except for serological markers of SAR COV2. Considering that the clinical course of MISC can be far more aggressive than that of KD, with development of sudden severe myocarditis along with multisystem involvement, and almost half of the admitted children (45% in our cohort) requiring intensive care and inotropic support, there is the need for an early attempt at differentiation for prompt and judicious management.
The present study attempts a comparative analysis of 3 commonly used laboratory markers, that is IL‐6, CRP and NT‐proBNP in MISC and KD and tries establishing a cutoff value at diagnosis to help in early differentiation.
IL‐6 is a pleotropic cytokine produced in response to tissue damage and infections. It helps regulate immune responses and has been found to be elevated in sepsis as well as in certain immune mediated diseases including KD and MISC. CRP is an important marker of inflammation and is used as a clinical index both in KD as well as MISC patients to reflect disease severity. 10
NT‐proBNP initially used for the management of heart disease in adults, has now become a useful adjunctive tool in the diagnosis and management of pediatric patients with KD. NT‐proBNP is an inactive fragment of BNP released when the active hormone is cleaved from the precursor molecule proBNP and has a longer half‐life in comparison to the same. Elevation of serum NT‐proBNP levels suggest myocardial stress. Over time multiple studies have shown higher NT‐proBNP levels in KD patients than their febrile controls, with a pooled sensitivity of 89% (95% CI 78‐95), and a specificity of 72% (95% CI 58‐82). The positive likelihood ratio is 3.2:1 (95% CI 2.1‐4.8). 11 , 12 Since patients with MISC present with cardiac manifestations, especially those with severe MISC and multiorgan dysfunction or those who are KD phenotype, NT‐proBNP levels clearly provide a clue to the degree of involvement and requirement of early intensive care. A meta‐analysis also showed that the key cardiac marker in patients with MISC was BNP. 13
Although previously alluded to, 14 , 15 statistical analysis in this study conclusively shows that NT‐proBNP and CRP levels are significantly higher among MISC patients in comparison to KD with elevation of these parameters; >935.7 pg/mL and >99.55 mg/L at admission respectively might act as guides as to the need for more aggressive management.
Applying the same cutoffs to the present cohort, in which 53/71 MISC patients have data available for both NT‐proBNP and CRP, 52.8% had both parameters above the cut off values at diagnosis. Further, 75% of these patients had severe cardiac involvement and required a combination of IVIg + steroids for battling the cytokine storm.
5. CONCLUSION
CRP and NT‐proBNP levels are significantly higher in MISC patients as compared to KD and can be used as laboratory markers in distinguishing these clinically overlapping entities.
ACKNOWLEDGEMENTS
The authors would like to thank Debraj Pal for the statistical analysis in the study.
Ganguly M, Nandi A, Banerjee P, et al. A comparative study of IL‐6, CRP and NT‐proBNP levels in post‐COVID multisystem inflammatory syndrome in children (MISC) and Kawasaki disease patients. Int J Rheum Dis.2022;25:27–31. 10.1111/1756-185X.14236
Funding information
None.
REFERENCES
- 1. Loke YH, Berul CI, Harahsheh AS. Multisystem inflammatory syndrome in children: is there a linkage to Kawasaki disease? Trends Cardiovasc Med. 2020;30(7):389‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐Cov‐2. JAMA. 2020;324(3):259‐269. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basu S, Bhattacharyya A, Ray K, Ghosh A. Biomarker profile in pediatric inflammatory multisystem syndrome temporarily associated with SARS‐Cov‐2 (PIMS‐TS)/multisystem inflammatory syndrome in children (MIS‐C). Indian J Biochem Biophys. 2020;57(6):687‐693. [Google Scholar]
- 4. Nandi A, Pal P, Basu S. A comparison of serum IL6 and CRP levels with respect to coronary changes and treatment response in kawasaki disease patients: a prospective study. Rheumatol Int. 2019;39(10):1797‐1801. 10.1007/s00296-019-04375-9 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Multisystem inflammatory syndrome in children and adolescents with COVID‐19. Scientific Brief, 15 May 2020. Document Number: WHO/2019‐nCoV/Sci_Brief/Multisystem_Syndrome_Children/2020.1. https://apps.who.int/iris/handle/10665/332095. Accessed 15 May, 2020. [Google Scholar]
- 6. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment and long‐term management of Kawasaki Disease: a scientific statement for health professionals from the american heart association. Circulation. 2017;135(17):927‐999. 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 7. Gupta A, Gill A, Sharma M, et al. Multi‐system inflammatory syndrome in a child mimicking Kawasaki disease. J Trop Pediatr. 2020;67(3):1–5. 10.1093/tropej/fmaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kabeerdoss J, Pilania RK, Karkhele R, Kumar TS, Danda D, Singh S. Severe COVID‐19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41(1):19‐32. 10.1007/s00296-020-04749-4. Epub 2020 Nov 21. PMID: 33219837; PMCID: PMC7680080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee PY, Day‐Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS‐CoV‐2‐induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130(11):5942‐5950. 10.1172/JCI141113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L. C‐reactive protein levels in the early stage of COVID‐19. Med Mal Infect. 2020;50(4):332‐334. 10.1016/j.medmal.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dionne A, Dahdah N. A decade of NT‐proBNP in acute Kawasaki Disease, from physiological response to clinical relevance. Children. 2018;5(10):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin K‐H, Chang S‐S, Yu C‐W. Usefulness of natriuretic peptide for the diagnosis of Kawasaki Disease: a systematic review and meta‐analysis. BMJ Open. 2015;5:e006703. 10.1136/bmjopen-2014-006703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Y, Patel J, Huang Y, Yin L, Tang L. Cardiac markers of multisystem inflammatory syndrome in children (MIS‐C) in COVID‐19 patients: a meta‐analysis. Am J Emerg Med. 2021;49:62‐70. 10.1016/j.ajem.2021.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS‐Cov‐2 mimicking Kawasaki Disease (Kawa‐COVID‐19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999‐1006. 10.1136/annrheumdis-2020-217960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383(4):347‐358. 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]


