Abstract
Remdesivir, an antiviral agent for the treatment of coronavirus disease 2019 (COVID‐19), is metabolized intracellularly, with these metabolites eliminated predominantly in urine. Because of a lack of safety and pharmacokinetic (PK) data, remdesivir is not currently recommended for patients with estimated glomerular filtration rate less than 30 ml/min/1.73 m2 and those on hemodialysis. This study evaluated the PKs of remdesivir and its metabolite, GS‐441524, in patients with COVID‐19 who were and were not receiving renal replacement therapy (RRT). This study enrolled two patients with normal renal function, two with impaired renal function not receiving RRT, two receiving continuous RRT (CRRT), and three undergoing intermittent hemodialysis (IHD). Patients were administered 200 mg remdesivir on the first day, followed by 100 mg/day for 5–10 days. Serial blood samples were collected for PK analysis, and PK parameters were assessed by a noncompartmental method. Systemic exposure to remdesivir was higher in patients with impaired renal function and those receiving CRRT than in patients with normal renal function, but was similar in patients undergoing IHD and those with normal renal function. By contrast, systemic exposure to GS‐441524 was highest in patients undergoing IHD, followed by patients with impaired renal function and those receiving CRRT, and lowest in patients with normal renal function. The PK profiles of remdesivir and GS‐441524 varied according to renal function and RRT. The impact of PK changes of remdesivir and its metabolite on safety and efficacy should be considered when administering remdesivir to patients with COVID‐19 with renal impairment.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Renal impairment can alter systemic exposure to remdesivir and its metabolites. Although the pharmacokinetics (PKs) of remdesivir and its metabolite have been assessed in patients with coronarvirus disease 2019 (COVID‐19) with reduced renal function, the use of remdesivir in patients with an estimated glomerular filtration rate less than 30 ml/min/1.73 m2 and those on hemodialysis is still limited due to a lack of information.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study evaluated the effect of renal function and renal replacement therapy on the PKs of remdesivir and its metabolite, GS‐441524, in patients with COVID‐19.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Systemic exposure to remdesivir was higher in patients with impaired renal function and those receiving continuous renal replacement therapy (CRRT) than in patients with normal renal function, whereas exposure was similar in patients undergoing intermittent hemodialysis (IHD) and those with normal renal function. By contrast, systemic exposure to GS‐441524 was highest in patients undergoing IHD, followed by patients with impaired renal function and those receiving CRRT, and lowest in patients with normal renal function.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The results of this study may enable remdesivir dosage selection for patients with COVID‐19 with renal impairment who have great demand for remdesivir use. Despite this study including a small number of patients, small‐sized studies have advantages, as their results can be generated and shared rapidly, especially during the COVID‐19 pandemic.
INTRODUCTION
Remdesivir is the first and currently the only antiviral agent approved by the US Food and Drug Administration (FDA) to treat coronavirus disease 2019 (COVID‐19) in hospitalized adults and pediatric patients aged greater than or equal to 12 years and weighing at least 40 kg. The recommended regimen consists of a single intravenous loading dose of 200 mg followed by maintenance doses of 100 mg daily for 5–10 days. However, the manufacturer’s label does not recommend its use in patients with an estimated glomerular filtration rate (eGFR) less than 30 ml/min/1.73 m2. 1
Intracellular remdesivir (GS‐5734) is rapidly converted by carboxylesterase and cathepsin A to its alanine metabolite, which is monophosphorylated and ultimately converted to the pharmacologically active nucleoside triphosphate, GS‐443902. Dephosphorylation of the monophosphate form yields the nucleoside core (GS‐441524), which becomes the predominant circulating metabolite providing a potential source of the active drug through a slow intracellular re‐phosphorylation process. 2 , 3 GS‐443902 inhibits viral RNA‐dependent RNA polymerase by competing with endogenous nucleotides for incorporation into replicating viral RNA without interfering with host RNA or DNA polymerase. 4 , 5
Due to the almost complete first‐pass clearance of remdesivir, it is not suitable for oral delivery. 5 Following its intravenous infusion, the plasma concentration of remdesivir declines rapidly, accompanied by the sequential appearance of its metabolites, GS‐704277 and GS‐441524. The plasma half‐life of remdesivir is around 1 h, whereas the half‐life of GS‐441524 is ~ 24.5 h. 6 Remdesivir is mainly eliminated in the urine (74%), with the predominant form being GS‐441524 (49%) followed by remdesivir (10%) and other metabolites. Therefore, renal impairment may theoretically increase systemic exposure to remdesivir and its metabolites, especially GS‐441524. 7 , 8
Due to the lack of clinical data on remdesivir in patients with renal impairment and concerns about the accumulation of its sulfobutylether‐beta‐cyclodextrin carrier, the use of remdesivir in patients with an eGFR less than 30 ml/min/1.73 m2 and those on hemodialysis is limited. 1 Due to abnormalities in their immune systems and the prevalence of severe comorbidities, such as cardiovascular disease and diabetes mellitus, however, patients undergoing hemodialysis are at risk for a severe course of COVID‐19. 9 Moreover, about 20–40% of patients with severe COVID‐19 have been reported to develop acute kidney injury. 10 Therefore, it is urgent to generate evidence for the use of remdesivir in patients with reduced renal function.
Studies have evaluated the pharmacokinetics (PKs) of remdesivir and its metabolites in patients with COVID‐19 with reduced renal function. Higher concentrations of GS‐441524 were observed in a patient with mild renal dysfunction. 11 Remdesivir was found to have a longer half‐life (about 2 h) in patients on dialysis, accompanied by accumulation of GS‐441524, although some GS‐441524 was eliminated by hemodialysis. 12 , 13 A case report in a patient with COVID‐19 without residual renal function showed a 72% dialysis extraction rate and a 42% decrease of GS‐441524 at the end of hemodialysis. 14 These findings, however, are insufficient for determining the indications and dosages of remdesivir in patients with COVID‐19 with reduced renal function. The present study therefore evaluated the PKs of remdesivir and its primary metabolite, GS‐441524, in patients with COVID‐19 who were and were not receiving renal replacement therapy (RRT).
MATERIALS AND METHODS
Ethics
The study protocol was approved by the Institutional Review Board of the Seoul National University Hospital (IRB No. 2009‐009‐1153) and conformed with the precepts of the Declaration of Helsinki and the Korean Good Clinical Practice. All subjects provided informed written consent before any study‐related procedures were performed.
Patients
This study enrolled patients with reverse‐transcription polymerase chain reaction‐confirmed COVID‐19, who received remdesivir in the Seoul National University Hospital Biocontainment Unit from September 1 to December 31, 2020.
Study design
Remdesivir was administered intravenously over 30 min as a 200 mg loading dose on the first day, followed by a 100 mg maintenance dose once daily for 5–10 days.
Patients undergoing intermittent hemodialysis (IHD) started hemodialysis 2–2.5 h after remdesivir administration (Figure 1). IHD consisted of blood and dialysate administered at flow rates of 220–250 ml/min and 500 ml/min, respectively, for 3 h. Dialysis was performed in two patients (patients H and I) using a medium cutoff dialyzer (Theranova), and in one (patient G) using a low‐flux dialyzer (FX5). The target dose for patients undergoing continuous RRT (CRRT) was 35–45 ml/kg/h, using a ST100 dialyzer.
FIGURE 1.
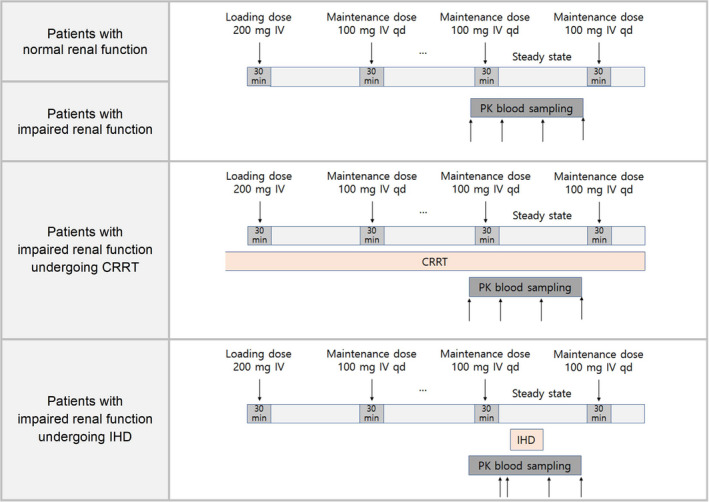
Study design. CRRT, continuous renal replacement therapy; IHD, intermittent hemodialysis; PK, pharmakinetic
Serial blood samples for PKs were collected in EDTA tubes at steady‐state after multiple doses of remdesivir. Samples were collected from patients with normal renal function and those with impaired renal function immediately before (0 h) and after (0.5 h) remdesivir infusion, at the mid‐point of a dosing interval (10–12 h after the start of dosing), and immediately before the subsequent dose of remdesivir (24 h). Blood samples were collected in the same manner from patients receiving CRRT while undergoing treatment. Blood samples were collected from patients undergoing IHD immediately before (0 h) and after (0.5 h) remdesivir infusion, immediately before (2 h) and after (5.5 h) hemodialysis, and immediately before the subsequent dose of remdesivir (24 h; Figure 1). Blood samples were immediately centrifuged at 1920 g for 15 min at 4℃. The separated plasma was aliquoted in Eppendorf tubes and stored below −70°C until analysis.
Determination of plasma concentrations of remdesivir and GS‐441524
Plasma concentrations of remdesivir and GS‐441524 were measured by liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) after liquid‐liquid extraction with ethyl acetate. Briefly, 100 μl of human plasma were mixed with 50 μl of internal standard (IS; 100 ng/ml, remdesivir‐d 5 ) and 50 μl of formic acid, and then extracted with 1 ml of ethyl acetate. Remdesivir, GS‐441524, and remdesivir‐d 5 were purchased from MedChemExpress (Monmouth Junction, NJ, USA). Plasma samples were vortex mixed and centrifuged, and the organic phase was evaporated to dryness at 40°C under a stream of nitrogen. The residue was reconstituted in 100 μl 30% aqueous acetonitrile and injected into the Agilent LC‐MS/MS system (Palo Alto, CA, USA). The analytes and IS were separated on a Gemini‐NX C18 column (100 × 3.0 mm, 3 µm; Phenomenex) under gradient conditions. The mobile phases consisted of two solutions: A, 0.1% formic acid in water, and B, 100% acetonitrile. Positive electrospray ionization in the multiple reaction monitoring (MRM) mode was used. The MRM was based on m/z transitions of 603.9 > 228.9 for remdesivir, 292.5 > 118.9 for GS‐441524, and 608.9 > 205.1 for IS. The calibration curve was linear over ranges of 10–1000 ng/ml for remdesivir and 5–500 ng/ml for GS‐441524 with all coefficients of correlation (r) greater than 0.99. The between‐run accuracy and precision were 97.39–101.4% and less than 5.823%, respectively, for remdesivir and 88.31–100.7% and less than 8.383%, respectively, for GS‐441524. Samples having remdesivir and/or GS‐441524 concentrations over the upper limit of quantification were diluted to their linear ranges with blank plasma and re‐analyzed. Dilution efficiency was confirmed using dilution quality control samples (DiQC) during the analysis of study samples. The precision and accuracy of DiQCs were 0.879% and 100.3–102.6%, respectively.
Pharmacokinetic assessment
Individual PK parameters of remdesivir and GS‐441524 were assessed using Phoenix WinNonlin version 8.2 (Certara, Princeton, NJ, USA). Maximum observed plasma concentration and time to achieve maximum observed plasma concentration were determined by evaluating individual plasma concentrations versus time profiles. Other parameters, such as the area under the plasma concentration versus the time curve within a dosing interval at steady state (AUCtau,ss), the elimination half‐life, clearance at steady state, and observed volume of distribution at steady state, were estimated by a noncompartmental method. The metabolite ratio (MR) of GS‐441524 was calculated as the AUCtau,ss of GS‐441524 divided by the AUCtau,ss of remdesivir.
RESULTS
Patients
Nine patients with confirmed COVID‐19 were enrolled and completed the study. The mean ± SD eGFRs calculated from the Chronic Kidney Disease Epidemiology Collaboration equation for patients with normal renal function, impaired renal function, impaired renal function undergoing CRRT, and IHD were 108.85 ± 11.45 ml/min/1.73 m2, 39.40 ± 8.40 ml/min/1.73 m2, 41.35 ± 1.25 ml/min/1.73 m2, and 4.30 ±1.10 ml/min/1.73 m2, respectively (Table 1). The medications administered during remdesivir treatment are listed in Table S1. None of the patients experienced any adverse events, including significant changes in renal function, that would cause remdesivir discontinuation.
TABLE 1.
Demographics and baseline characteristics of enrolled patients with COVID‐19
| Patient | Gender | Age, year | Height, cm | Body weight, kg | BSA, kg/m2 | CKD‐EPI, ml/min/1.73 m2 | Serum albumin, g/dl | Renal function and replacement therapy |
|---|---|---|---|---|---|---|---|---|
| A | Male | 70 | 168 | 57.8 | 1.64 | 97.4 | 3.3 | Normal |
| B | Female | 36 | 159 | 75.6 | 1.83 | 120.3 | 3.3 | Normal |
| C | Male | 55 | 170 | 64.1 | 1.74 | 47.8 | 2.8 | Impaired renal function |
| D | Female | 85 | 150 | 46.7 | 1.39 | 31.0 | 3.2 | Impaired renal function |
| E | Male | 58 | 170 | 106.5 | 2.24 | 42.6 | 2.7 | Impaired renal function undergoing CRRT |
| F | Male | 81 | 171 | 54.7 | 1.61 | 40.1 | 2.9 | Impaired renal function undergoing CRRT |
| G | Male | 77 | 164 | 54.3 | 1.57 | 5.4 | 3.1 | Impaired renal function undergoing IHD |
| H | Male | 63 | 173 | 67.8 | 1.81 | 2.8 | 3.7 | Impaired renal function undergoing IHD |
| I | Male | 52 | 175 | 72.2 | 1.88 | 4.7 | 3.1 | Impaired renal function undergoing IHD |
Abbreviations: BSA, body surface area; CKD‐EPI, estimated glomerular filtration rate by the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) equation; COVID‐19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; IHD, intermittent hemodialysis.
Pharmacokinetics of remdesivir and GS‐441524
The through concentrations (0 h) of remdesivir and GS‐441524 in patient A, a male patient with normal renal function, were excluded from analysis because the sample was processed inadequately. Predose blood samples (0 h) from the three patients undergoing IHD (patients G, H, and I) were lost. These concentrations were therefore set at the predose concentrations before administration of the next dose (24 h), as it was assumed that these concentrations had reached steady‐state.
The plasma‐concentration profiles of remdesivir in patients with impaired renal function and those receiving RRT differed from those in patients with normal renal function (Figure 2a). Systemic exposure (AUCtau,ss) to remdesivir was higher in patients with renal impairment not receiving RRT (27984.75 ± 655.05 μg∙h/L) and patients receiving CRRT (46468.63 ± 24181.46 μg∙h/L) than in patients with normal renal function (2844.25 ± 1290.65 μg∙h/L). However, systemic exposure to remdesivir was similar in patients undergoing IHD (2696.43 ± 1328.56 μg∙h/L) and those with normal renal function (Table 2).
FIGURE 2.
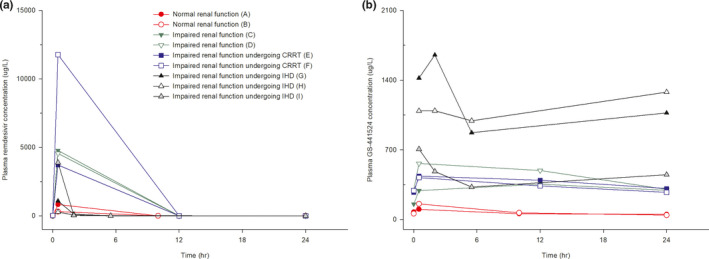
Individual plasma concentration‐time curves of (a) remdesivir and (b) GS‐441524 after intravenous infusion of 100 mg remdesivir at steady state. CRRT, continuous renal replacement therapy; IHD, intermittent hemodialysis
TABLE 2.
Pharmacokinetic parameters of remdesivir and GS‐441524 after intravenous infusion of 100 mg remdesivir at steady state
| Patients with normal renal function (N = 2) | Patients with impaired renal function (N = 2) | Patients with impaired renal function undergoing CRRT (N = 2) | Patients with impaired renal function undergoing IHD (N = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | A | B | C | D | E | F | G | H | I |
| Remdesivir | |||||||||
| Tmax,ss, h | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Cmax,ss, μg/L | 826.98 | 310.72 | 4773.30 | 4554.95 | 3713.80 | 11774.10 | 1087.30 | 276.04 | 3886.80 |
| AUCtau,ss, μg·h/L | 4134.89 | 1553.60 | 28639.80 | 27329.70 | 22287.17 | 70650.09 | 2773.39 | 1032.16 | 4283.73 |
| t 1/2, h | 0.91 | 1.08 | 1.07 | 1.09 | 1.23 | 1.00 | 0.92 | 0.86 | 0.57 |
| CLss, L/h | 24.18 | 64.37 | 3.49 | 3.66 | 4.49 | 1.42 | 36.06 | 96.88 | 23.34 |
| V ss,obs, L | 31.92 | 100.46 | 5.41 | 5.74 | 7.99 | 2.05 | 47.93 | 119.87 | 19.31 |
| GS‐441524 | |||||||||
| Tmax,ss, h | 0.5 | 0.5 | 12 | 0.5 | 0.5 | 0.5 | 2.5 | ‐ a | 0.5 |
| Cmax,ss, μg/L | 102.44 | 157.07 | 355.74 | 563.30 | 436.32 | 421.32 | 1653.45 | 1280.05 | 706.80 |
| AUCtau,ss, μg·h/L | 1582.35 | 1905.21 | 7728.20 | 11059.67 | 9203.09 | 8213.83 | 25615.76 | 26950.08 | 9785.21 |
| t 1/2, h | 25.47 | 13.27 | 43.74 | 26.20 | 48.57 | 37.80 | 70.44 | – b | 82.52 |
| MR | 0.38 | 1.23 | 0.27 | 0.40 | 0.41 | 0.12 | 9.24 | 26.11 | 2.28 |
Values are presented as the mean ± SD.
Abbreviations: AUCtau,ss, area under the plasma concentration–time curve within a dosing interval at steady state; CLss, clearance at steady state; Cmax,ss, maximum plasma concentration at steady state; CRRT, continuous renal replacement therapy; IHD, intermittent hemodialysis; MR, metabolite ratio; t 1/2, elimination half‐life; Tmax,ss, time to maximum plasma concentration at steady state; V ss,obs, observed volume of distribution at steady state.
This value was not presented because the Tmax,ss at 0 h was not clinically accordant.
This value was not presented because it was difficult to definite terminal phase.
The plasma‐concentration profiles of GS‐441524 were also affected by renal function and RRT, but their patterns differed from those of remdesivir (Figure 2b). Systemic exposure (AUCtau,ss) to GS‐441524 was higher in all groups of patients with decreased eGFR, including patients with impaired renal function not receiving RRT (9393.93 ± 1665.73 μg∙h/L), patients receiving CRRT (8708.46 ± 494.63 μg∙h/L), and patients undergoing IHD (20783.68 ± 7796.15 μg∙h/L), than in patients with normal renal function (1743.78 ± 161.43 μg∙h/L). In addition, only patients undergoing IHD had a high MR, indicating a relatively greater exposure to GS‐441524 than to remdesivir (Table 2).
Systemic exposure to remdesivir was lower and exposure to GS‐441524 was higher in a pregnant woman at 30 weeks of gestation (patient B) than in a male patient with normal renal function (patient A). Specifically, patients A and B had AUCtau,ss values of 4134.89 μg∙h/L and 1553.60 μg∙h/L, respectively, for remdesivir, and 1582.35 μg∙h/L and 1905.21 μg∙h/L, respectively, for GS‐441524 (Figure S1).
Effects of hemodialysis on the pharmacokinetics of remdesivir and GS‐441524
The plasma concentrations of both remdesivir and GS‐441524 were decreased during hemodialysis (Figure 3). The plasma concentration of remdesivir decreased from 72.83 ± 43.91 μg/L to below the limit of quantitation during hemodialysis with remdesivir having an estimated half‐life during dialysis of 1.11 ± 0.13 h. Similarly, plasma concentration of GS‐441524 decreased from 1076.40 ± 585.61 μg/L to 730.46 ± 355.07 μg/L during hemodialysis, with GS‐441524 having an estimated half‐life during dialysis of 10.52 ± 7.86 h. After the completion of hemodialysis, however, GS‐441524 concentrations were increased in all three patients, to 934.15 ± 352.27 μg/L (Figure 4).
FIGURE 3.
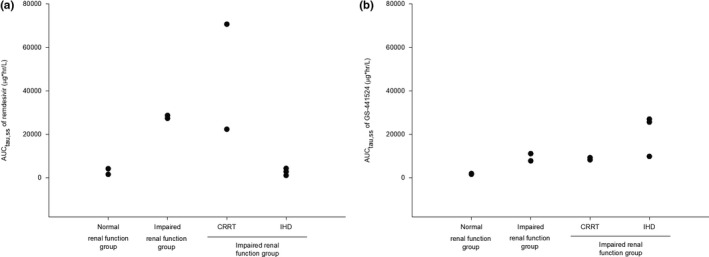
Individual systemic exposure (AUCtau,ss) of patients to (a) remdesivir and (b) GS‐441524 after intravenous infusion of 100 mg remdesivir at steady state. AUCtau,ss, area under the plasma concentration versus the time curve within a dosing interval at steady state; CRRT, continuous renal replacement therapy; IHD, intermittent hemodialysis
FIGURE 4.
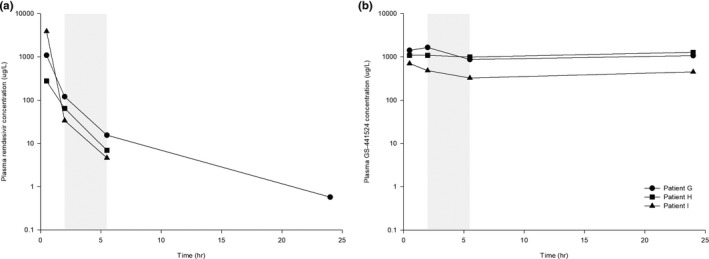
Individual plasma concentration‐time curves of (a) remdesivir and (b) GS‐441524 (right) after intravenous infusion of 100 mg remdesivir at steady state in patients with impaired renal function undergoing intermittent hemodialysis
DISCUSSION
This study evaluated changes in the PKs of remdesivir and its principal metabolite in patients varying in renal function and undergoing different types of RRT. These results can support evidence for the indications and guide dosage selection for remdesivir in renally impaired patients with COVID‐19.
Stabilizers were not added to blood samples during the sample collection process. Esterases in blood can degrade remdesivir over time. 15 , 16 This instability may be overcome by collecting blood samples in NaF tubes or by adding diluted formic acid to the plasma samples. 15 , 16 However, in this study, blood samples were centrifuged within 30 min after being drawn and each plasma sample was immediately frozen at −70℃. In addition, formic acid was added immediately to these samples after thawing on ice and before PK analysis. Therefore, the absence of a stabilizer likely did not affect the concentrations of remdesivir and GS‐441524. Moreover, the remdesivir and GS‐441524 PK profiles of patients with normal renal function were consistent with those previously reported. 1 These findings suggest that the design of this study, including the timing and processing of blood samples, was appropriate and that the bioanalysis of remdesivir and GS‐441524 was valid.
Although systemic exposure to remdesivir increased as renal function decreased, little remdesivir remained in the blood after 24 h, regardless of renal function. Thus, remdesivir did not accumulate in any of these patients. By contrast, systemic exposure to GS‐441524 was greater in patients with impaired renal function than those with normal renal function, suggesting that GS‐441524 was not sufficiently removed and showed excessive accumulation in patients with impaired renal function. In addition, GS‐441524 accumulation was greater in patients undergoing IHD than in those with normal renal function, indicating that it had not been sufficiently removed, even through dialysis.
The therapeutic range of GS‐441524 has not been determined. GS‐441524 has shown potent antiviral activity against several strains of coronaviruses. High plasma concentrations of GS‐441524 may increase its penetration through cell membranes and activate intracellular enzymatic phosphorylation pathways that supply pharmacologically active triphosphate (GS‐443902). 2 This process may enhance the effectiveness of remdesivir but might also increase its toxicity.
Remdesivir was found to be well‐tolerated after single doses of up to 225 mg and after multiple doses of 150 mg. 6 Moreover, a single dose of 225 mg of remdesivir was tolerated by a patient infected with Ebola virus. 17 In addition, a study in 157 patients with acute kidney injury or those undergoing hemodialysis found that administration of remdesivir did not result in clinically significant alanine transaminase elevations or early discontinuation due to adverse drug reactions. 18 The pharmacologically active form of remdesivir, the triphosphate (GS‐443902), has been shown to inhibit viral RNA polymerases, not host RNA or DNA polymerases. 4 , 5 Moreover, despite the increased exposure to GS‐441524 in patients with renal impairment, none of these patients had any serious adverse events or early discontinuation. These findings suggest that the accumulation of GS‐441524 in patients with renal impairment is unlikely to cause severe adverse reactions, which have limited the use of remdesivir in these patients. Nevertheless, because the evidence remains insufficient and relatively little is known about alterations in remdesivir and GS‐441524 PKs in patients with renal impairment, caution should be exercised in treating these patients with remdesivir.
This study measured only the plasma concentrations of remdesivir and GS‐441524. However, remdesivir or GS‐441524 in plasma diffuse into intracellular space and are bio‐transformed to GS‐443902, an active triphosphate form with antiviral efficacy. 19 This process may be more crucial in patients with renal impairment, because disease status may affect intracellular metabolism. A physiologically‐based pharmacokinetic model has been proposed to predict the intracellular concentration of GS‐443902 in the target tissue. 20 Use of this model in the present study, however, was limited because this study included few subjects and few sampling points, and because little is currently known about remdesivir metabolism in renally impaired patients.
The current work suffers from a number of limitations. This study was exploratory, with no intent to update the prescribing label or modifying the dosing regimen for patients with impaired renal function. The number of subjects in the present study was lower than that of recommended by the FDA’s guidance for evaluating PKs in patients with impaired renal function. The FDA has suggested including six to eight subjects in each group of patients with normal, mild, moderate, severely decreased renal function, and end‐stage renal disease. During the COVID‐19 pandemic, a small‐sized study may be advantageous, as data can be generated and shared rapidly. Although the number of patients in this study was small, two or three per each group, it is meaningful that this study found that renal function and RTT affected the PKs of remdesivir and GS‐441524. Additional clinical studies in larger numbers of patients are required to confirm these findings and to design remdesivir regimens to treat patients with COVID‐19 with impaired renal function.
CONCLUSION
The PK profiles of remdesivir and GS‐441524 were varying according to renal function and the administration of RRT. The effect of PK changes in remdesivir and its metabolite GS‐441524 on safety and efficacy should be considered when administering remdesivir to patients with COVID‐19 with renal impairment.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
P.G.C., S.I.J., S.H.L., and N.J.K. wrote the manuscript. P.G.C., S.H.L., M.D.O., and N.J.K. designed the research. P.G.C., C.K.K., S.S.H., D.K.K., S.M.L., W.B.P., M.D.O., and N.J.K. performed the research. P.G.C., S.I.J., C.K.K., S.H.L., and N.J.K. analyzed the data. L.Y. and J.‐Y.C. contributed analytical tools.
Supporting information
Figure S1
Table S1
ACKNOWLEDGEMENTS
The authors would like to thank the medical staff at Seoul National Hospital and all volunteers who participated in this trial.
Choe PG, Jeong SI, Kang CK, et al. Exploration for the effect of renal function and renal replacement therapy on pharmacokinetics of remdesivir and GS‐441524 in patients with COVID‐19: A limited case series. Clin Transl Sci. 2022;15:732–740. doi: 10.1111/cts.13194
Pyoeng Gyun Choe and Sae Im Jeong authors contributed equally to this work.
Funding information
No funding was received for this work.
REFERENCES
- 1. Fact sheet for Health Care Providers Emergency Use Authorization (EUA) of Remdesivir (GS‐5734). US Food and Drug Administration; 2020. [Google Scholar]
- 2. Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID‐19. ACS Cent Sci. 2020;6(5):672‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan VC, Muller FL. Advantages of the parent nucleoside GS‐441524 over remdesivir for covid‐19 treatment. ACS Med Chem Lett. 2020;11(7):1361‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel D, Hui HC, Doerffler E, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1‐f][triazin‐4‐amino] adenine C‐nucleoside (GS‐5734) for the treatment of Ebola and emerging viruses. J Med Chem. 2017;60(5):1648‐1661. [DOI] [PubMed] [Google Scholar]
- 5. Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID‐19: an evaluation of the evidence. Travel Med Infect Dis. 2020;35:101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Humeniuk R, Mathias A, Cao H, et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID‐19, in healthy subjects. Clin Transl Sci. 2020;13(5):896‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Summary on compassionate use: Remdesivir Gilead. European Medicines Agency; 2020. https://www.ema.europa.eu/en/documents/other/summary‐compassionate‐use‐remdesivir‐gilead_en.pdf. [Google Scholar]
- 8. Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: review of pharmacology, pre‐clinical data, and emerging clinical experience for COVID‐19. Pharmacotherapy. 2020;40(7):659‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kooman JP, van der Sande FM. COVID‐19 in ESRD and acute kidney injury. Blood Purif. 2021;50:610‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adamsick ML, Gandhi RG, Bidell MR, et al. Remdesivir in patients with acute or chronic kidney disease and COVID‐19. J Am Soc Nephrol. 2020;31(7):1384‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tempestilli M, Caputi P, Avataneo V, et al. Pharmacokinetics of remdesivir and GS‐441524 in two critically ill patients who recovered from COVID‐19. J Antimicrob Chemother. 2020;75(10):2977‐2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis MR, Pham CU, Cies JJ. Remdesivir and GS‐441524 plasma concentrations in patients with end‐stage renal disease on haemodialysis. J Antimicrob Chemother. 2021;76(3):822‐825. [DOI] [PubMed] [Google Scholar]
- 13. Lê MP, Le Hingrat Q, Jaquet P, et al. Removal of Remdesivir’s metabolite GS‐441524 by hemodialysis in a double lung transplant recipient with COVID‐19. Antimicrob Agents Chemother. 2020;64(11):e01521‐e01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sörgel F, Malin JJ, Hagmann H, et al. Pharmacokinetics of remdesivir in a COVID‐19 patient with end‐stage renal disease on intermittent haemodialysis. J Antimicrob Chemother. 2021;76(3):825‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao D, John Ling KH, Tarnowski T, et al. Validation of LC‐MS/MS methods for determination of remdesivir and its metabolites GS‐441524 and GS‐704277 in acidified human plasma and their application in COVID‐19 related clinical studies. Anal Biochem. 2021;617: 114118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alvarez J‐C, Moine P, Etting I, et al. Quantification of plasma remdesivir and its metabolite GS‐441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a Covid‐19 treated patient. Clin Chem Lab Med. 2020;58(9):1461‐1468. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs M, Rodger A, Bell DJ, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388(10043):498‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thakare S, Gandhi C, Modi T, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep. 2021;6(1):206‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gallo JM. Hybrid physiologically‐based pharmacokinetic model for remdesivir: Application to SARS‐CoV‐2. Clin Transl Sci. 2021;14(3):1082‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65(8):757‐773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1


