Abstract
The COVID‐19 pandemic has driven an unprecedented level of global activity in drug discovery and clinical development for effective therapeutics targeting the coronavirus disease. There are currently 744 therapeutics being tested in 2879 clinical trials globally. Almost 90% of these clinical trials are focused on monotherapies. Combination therapies are the mainstay of antiviral therapeutics to increase the potency of the individual compounds and to combat the rapid evolution of resistance, although combination therapies have inherently complex clinical and regulatory development challenges. Increased understanding of the SARS‐CoV‐2 lifecycle and COVID‐19 pathology provides a scientific rationale for evaluating the effectiveness of different combinations. In this paper, we provide an overview of the current clinical trial landscape for combination therapeutics targeting COVID‐19 through weekly scanning of national and international clinical trial registries. Our analysis delves specifically into dual combination therapies in what can be defined as “pivotal clinical trials” (active, randomised, controlled and at least phase II), with a focus on new and repurposed therapeutic candidates that have shown positive signals and/or been granted authorisation for emergency use based on positive efficacy and safety data.
Keywords: clinical trials, combination therapy, COVID‐19, pivotal trials, SARS‐CoV‐2, treatment
1. INTRODUCTION
The coronavirus disease (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was declared a global pandemic in March 2020 and has remained a severe threat to public health and economic stability in most countries. 1 Its reproduction number (R0 > 1), and alarming hospital admissions and death rates have pushed many healthcare services to the brink. Understandably, this has also drawn the focus of considerable research towards the discovery of new chemical/molecular entities and/or repurposed interventions to prevent and treat the disease. 2 , 3 Concurrent with the rapid and highly effective development, approval and rollout of prophylactic vaccines, hundreds of mono‐ and combination therapeutics are being evaluated in thousands of trials worldwide. Interventions being trialled are predominantly monotherapies, with limited success in identifying a gold standard across the entire COVID‐19 disease pathway. However, with understanding of the natural history of the SARS‐CoV‐2 infection, the pathophysiology and clinical progression of COVID‐19, increased focus is on combination therapies in trials.
Combination therapies have been invaluable for cancer chemotherapy, and chronic viral infections such as HIV using HAART (highly active antiretroviral therapy) to reduce the potential for resistance and also restrict levels of infection due to the weak efficacy of antivirals to clear the virus. 4 , 5 Combination therapies have also been investigated in the treatment of acute viral infections such as influenza with the prediction to greatly reduce the development of rapid drug resistance, supress viral replication and achieve better efficacy and reduction in mortality than monotherapy. 4 , 6 , 7 Synergistic combinations, in which the efficacy is greater than that of the individual therapies, increases the chances for better treatment outcomes. These types of combinations frequently target a common or related pathway of the disease. Interventions may also be combined to treat two different pathologies of the disease, which may be particularly important for COVID‐19. Unfortunately, the effectiveness of combining interventions to increase antiviral efficacy is not predictive and requires testing.
The complexity of the pathology of COVID‐19 has challenged the development of appropriate therapeutics. The early stages of the disease are predominated by viral replication with minimal pathology whilst the later stages comprise decreased oxygen absorption and hyperactive immune activation (i.e., cytokine storm) (Figure 1). While certain therapies have shown benefit in a subset of the treatment population (e.g., severely ill) and/or in different clinical settings (e.g., intensive care unit), the complexity of the disease necessitates the need to look beyond monotherapies and into combining independent treatments to increase therapeutic efficacy in a much wider population and across the disease pathway. 8
FIGURE 1.
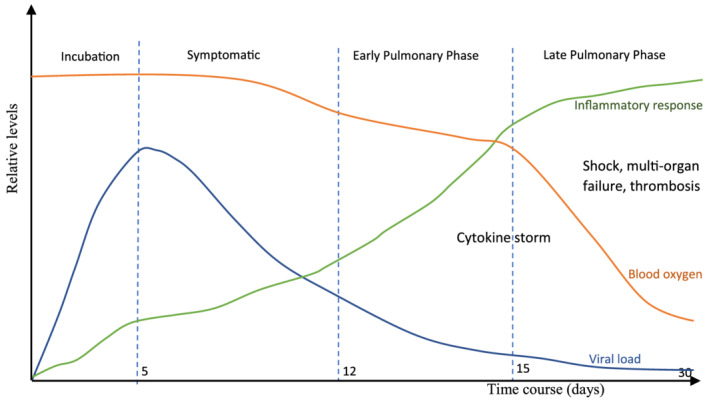
The stages of COVID‐19 showing progression of the disease. Concept of figure adapted from Ngo et al., 2021 9
There are several compelling reasons for exploring combination therapies for COVID‐19 treatment: (i) different mechanisms of action in order to treat different pathologies of the disease such as combining an antiviral (to decrease the viral load) with an immune‐modulator to suppress the hyperimmune response; (ii) increased antiviral efficacy (particularly important with the limited efficacy of current SARS‐CoV‐2 therapeutics) whilst decreasing the potential for drug resistance to the individual compounds; and (iii) synergy between compounds targeting the same or different points of the virus lifecycle or disease pathway, which may permit elevated efficacy of weak inhibitors. The use of multiple combination therapies, however, are known to be associated with increased risks in terms of safety, potential interactions and adverse effects, which may (at least partially) explain the low numbers currently being tested in trials for COVID‐19.
The aim of this paper is to provide some insight from the current data on combination therapies in this rapidly changing field and their applications in clinical practice and future trials.
2. METHODS
In March 2020, the National Institute for Health Research (NIHR) Innovation Observatory started collaborating with a multi‐agency initiative in England to identify promising therapeutic interventions with the aim to speed up access to treatments where research shows there is clinical benefit. 10 This involved weekly scanning of national and international clinical trial registries (ClinicalTrials.gov, EU Clinical Trials Register and the World Health Organisation International Clinical Trial Registry Platform [WHO ICTRP]) to identify and prioritise the most promising therapeutic interventions in use as monotherapy and/or in combination to prevent and treat COVID‐19. 11 , 12 , 13
The following search terms were used to identify relevant clinical trials: “Covid‐19”, “2019‐nCoV”, “SARS‐CoV‐2”, “2019 novel coronavirus”, “severe acute respiratory syndrome coronavirus 2”. Additionally, clinical trials were sifted and excluded at the point of data collection based on these criteria: behavioural, educational and physical activity‐related interventions; rehabilitation programmes; ventilator or mask‐based interventions; diagnostic trials; trials studying COVID‐19 epidemiology and complementary or alternative medicines. Novel, aggregated entries were created for trials testing interventions in combinations of two to six, to clearly display the different combination therapies being tested in clinical trials.
Pivotal trials (defined as active, randomised, controlled clinical trials designed to demonstrate statistically significant clinical efficacy and safety in humans for the purpose of regulatory approvals) were identified through selected trial characteristics. 14 The characteristics for selection of pivotal trials were based on: the current trial status (active); trial design (randomised); trial phase (phase II and above); estimated enrolment (trials with 100 or more participants); and trial location (UK, EU, USA, Australia or Canada).
3. RESULTS
From the analysis of data collected through NIHR Innovation Observatory scans as of 29 March 2021, 744 interventions are being tested for the prevention and treatment of COVID‐19 in 2879 clinical trials worldwide. These include antivirals, antibiotics, anti‐inflammatories/immunomodulators, cell‐based therapies, other pharmaceuticals (antimalarials, antihypertensives, antithrombotics, antifibrotics, etc.) and dietary supplements. Of the 744 interventions, approximately 37% are new (unlicensed for any indication), 44% are repurposed (established interventions licensed for other indications) and 19% do not require licensing.
Analysis of the 2879 clinical trials shows only 302 (10.5%) of these clinical trials explore combination therapies (Figure 2). 15 Further analysis of the combination therapy clinical trials shows 241 trials (79.8%) are testing dual combinations of interventions and a few are exploring other multiple combinations (Figure 3). Of the 241 dual combination therapy trials, 26 (10.8%) are pivotal trials (Table 1). These data show only five pivotal trials have reported results as of the time of data analysis, which are discussed below.
FIGURE 2.
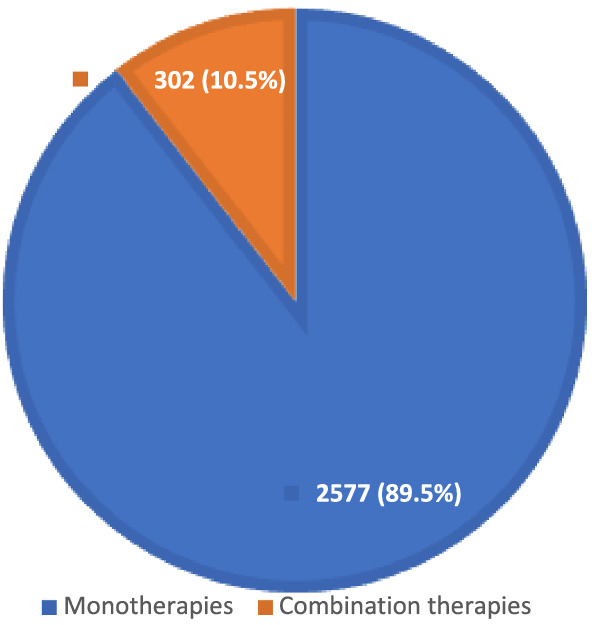
COVID‐19 monotherapy and combination therapy clinical trials as of 29 March 2021
FIGURE 3.
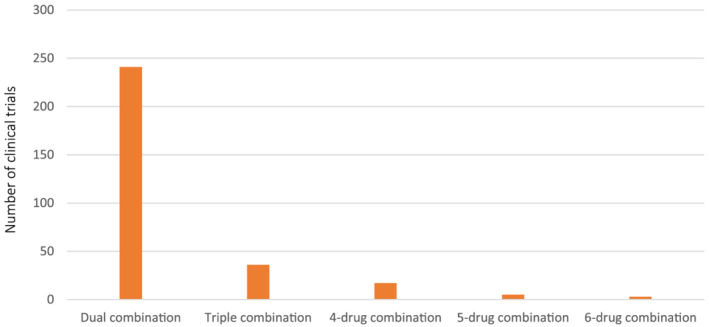
Number of COVID‐19 clinical trials testing combination therapies as of 29 March 2021
TABLE 1.
Interventions in COVID‐19 dual combination pivotal clinical trials as of 29 March 2021. 15
| Intervention 1 | Intervention 2 | Trial ID | No. of subjects | Results |
|---|---|---|---|---|
| Bamlanivimab | Etesevimab | NCT04427501 | 3160 | Yes |
| Doxycycline | Ivermectin | NCT04729140 | 150 | No |
| Hydroxychloroquine | Azithromycin | NCT04336332 | 160 | No |
| Hydroxychloroquine | Azithromycin | NCT04344444 | 600 | No |
| Hydroxychloroquine | Baricitinib | NCT04373044 | 144 | No |
| Lopinavir/ritonavir | Favipiravir | NCT04499677 | 240 | No |
| Lopinavir/ritonavir | Favipiravir | 2020‐002106‐68 | 240 | No |
| Lopinavir/ritonavir | Hydroxychloroquine | NCT04386070 | 6400 | No |
| Melatonin | Toremifene | NCT04531748 | 390 | No |
| Methylene blue | Convalescent plasma | NCT04547127 | 200 | No |
| NA‐831 (Traneurocin) | Oral polio vaccine (OPV) | NCT04540185 | 3600 | No |
| Naltrexone | Colchicine | NCT04756128 | 164 | No |
| Oseltamivir | Vidofludimus calcium | NCT04516915 | 120 | No |
| Pamapimod | Pioglitazone | 2020‐005849‐16 | 144 | No |
| Remdesivir | Bamlanivimab | NCT04501978 | 10000 | Yes |
| Remdesivir | Baricitinib | NCT04401579 | 1034 | Yes |
| Remdesivir | Hyperimmune immunoglobulin | NCT04546581 | 593 | Yes |
| Remdesivir | Interferon beta‐1a | NCT04492475 | 969 | No |
| Remdesivir | Lenzilumab | NCT04583969 | 200 | No |
| Remdesivir | Risankizumab | NCT04583956 | 200 | No |
| Remdesivir | Tocilizumab | NCT04409262 | 649 | Yes |
| Rosuvastatin | Colchicine | NCT04472611 | 466 | No |
| Vitamin B‐ complex | Nitazoxanide | NCT04343248 | 800 | No |
| Vitamin B‐ complex | Nitazoxanide | NCT04359680 | 1407 | No |
| Vitamin B‐ complex | Nitazoxanide | NCT04486313 | 1092 | No |
| Vitamin D | Aspirin | NCT04363840 | 1080 | No |
Approximately 60% (n = 26) of the dual combination pivotal trials explore combinations involving antivirals and/or immunomodulatory interventions. Others include anticoagulant, dietary supplement, antibiotic, anthelmintic, antimalarial, antioxidant, antihyperglycemic, drug withdrawal therapy, antihyperlipidemic, antirheumatic, neuroprotectant, vaccine, diagnostic agent, and natural sources of antiviral neutralising antibodies (Table 1).
4. DISCUSSION
In this analysis, 10.5% of global COVID‐19 clinical trials are testing combination therapies, with the majority exploring dual combinations. As of 29 March 2021, there are 26 dual combination pivotal trials, of which approximately 60% are exploring drug classes such as antivirals and anti‐inflammatory/immunomodulatory therapies.
Drugs, such as hydroxychloroquine, azithromycin and lopinavir/ritonavir, initially purported to have antiviral activity against SARS‐CoV‐2, have subsequently yielded little or no significant clinical benefits. 16 , 17 , 18 Studies evaluating the antiviral activity of hydroxychloroquine were mostly based on controversial evidence; but after several randomised trials, it was reported to have limited significant clinical benefit towards COVID‐19. 19 The US Food and Drug Administration (FDA) revoked the prior emergency use authorisation (EUA) for hydroxychloroquine in light of adverse effects and unlikely effectiveness to treat COVID‐19. 20
4.1. Antivirals in combination therapies
Remdesivir, a direct‐acting antiviral agent, gained global attention and was granted an EUA early in the pandemic when preliminary evidence from monotherapy trials suggested that it shortened recovery times for severely ill and hospitalised patients. 21 , 22 Analysis of the trials landscape shows that remdesivir is currently being tested in seven dual therapy pivotal trials (Table 1). Remdesivir is undergoing testing in combination with anti‐inflammatories/immunomodulators (including baricitinib, tocilizumab, bamlanivimab, risankizumab, lenzilumab) in order to target two different stages of the disease: the viral proliferation and the hyperimmune response.
The dual combination of remdesivir and baricitinib, an immunomodulatory drug, was granted an EUA by the FDA based on positive results from the ACTT‐2 trial (NCT04401579), in which there was significant improvement in clinical status and reduction in recovery time in the remdesivir‐baricitinib treatment group when compared with the remdesivir‐only group. 23 , 24 The remdesivir and tocilizumab combination, however, did not meet its endpoints in terms of improved time to hospital discharge or likelihood of death or progression to mechanical ventilation in the treatment of COVID‐19 pneumonia (NCT04409262). 25 Similarly, preliminary data from the ACTIV‐3 trial (NCT04501978) testing remdesivir and bamlanivimab dual combination did not show any benefits in hospitalised COVID‐19 patients. 26 It was reported that participants in the trial were at the late stage of the disease, hence the probable ineffectiveness of the therapy. 27 , 28 Remdesivir treatment was tested (as a standard of care) in dual combination with hyperimmune immunoglobulin (hIG), with both treatments targeting the virus: remdesivir inhibiting viral replication and the hIG neutralising SARS‐CoV‐2 (NCT04546581). This study, however, did not meet its endpoints as there was no reduction in the risk of disease progression in adults hospitalised with COVID‐19. 29 Remdesivir and interferon beta‐1a combination therapy is undergoing evaluation where the antiviral activity of remdesivir is combined with the dual antiviral and anti‐inflammatory properties of interferon beta‐1a (ACTT‐3; NCT04492475). The efficacy of the combination is compared to treatment with remdesivir alone in hospitalised patients. 30 The result of this trial and other pivotal trials testing remdesivir in dual combination are yet to be published.
With similar mechanism of action to remdesivir and shortened viral clearance time when compared with lopinavir/ritonavir, favipiravir may be another antiviral candidate for dual combination therapy trials. 31 , 32 The timing of treatment may prove to be the key element in antiviral therapies, since antivirals are mostly effective at the early stage of the infection (incubation and early symptomatic phase) (Figure 1).
4.2. Anti‐inflammatories/immunomodulators in combination therapies
Neutralising monoclonal antibodies are under evaluation in clinical trials for treatment of COVID‐19. 33 These antibodies bind and inactivate the spike glycoprotein (S protein) of SARS‐CoV‐2, hereby preventing entry of the virus particle into the host cell. 34 The efficacy of monoclonal antibodies has been tested as monotherapy and in combination, with encouraging results. Early results from trials exploring antibodies have shown significant benefit on survival and reduction in mortality for COVID‐19 patients. 33 , 35 Although the FDA revoked the EUA of bamlanivimab monotherapy due to a sustained increase in resistant variants of SARS‐CoV‐2, bamlanivimab is still undergoing evaluation in combination therapy trials. 36 In the BLAZE‐1 study (NCT04427501), ambulatory patients with mild to moderate COVID‐19 illness were randomised to receive bamlanivimab monotherapy or placebo in one group, or bamlanivimab and etesevimab (another neutralising antibody) or placebo in the second group, within 3 days of the first positive SARS‐CoV‐2 test sample collection. It was reported that there was a significant reduction in viral load at Day 11; reduction in COVID‐19‐related hospitalisations at Day 29 as well as a lesser risk for emergent resistant variants in patients who received the combination therapy compared with the monotherapy group. 37 , 38 Other combination therapy trials testing neutralising antibodies are still ongoing.
Elevations in IL‐6 levels have been reported in many COVID‐19 patients, and IL‐6 inhibitors, such as tocilizumab, have been trialled to target the excessive inflammatory response (cytokine storm) of the immune system. 39 , 40 Tocilizumab as a monotherapy has been reported to improve patient symptoms, shorten hospital stay, and reduce overall mortality from severe COVID‐19. 40 , 41 , 42 Administration of tocilizumab at the beginning of inflammation has also been suggested to improve its efficacy. 43 However, the inclusion of tocilizumab in more combination therapy pivotal trials would be required to further investigate its effectiveness.
Other anti‐inflammatory interventions being tested in ongoing dual combination pivotal trials include baricitinib and colchicine. Baricitinib has previously been described to improve the clinical efficacy of remdesivir in combination when compared with remdesivir‐only therapy. 23 It has also shown significant improvement in respiratory function in patients with COVID‐19 pneumonia when combined with corticosteroids. 44 Colchicine has been reported to decrease cytokine production and improve patient survival when administered early in the disease process. 45 Colchicine may be considered as supportive treatment for hospitalised COVID‐19 patients.
Similar to antiviral strategies, anti‐inflammatory interventions are likely to be most impactful at symptomatic and early pulmonary phases in the disease pathway (Figure 1) to prevent the progression of COVID‐19. However, the administration of combination therapies should be tailored to the individual patient based on a number of factors such as, stage of the disease, patient health status and geographic region. Administration of some anti‐inflammatory interventions, such as corticosteroids, to immunocompromised patients may alter host defence and delay the elimination of the virus, thus necessitating a risk–benefit assessment. 46
4.3. Regulatory developments in combination therapies
Pivotal trials are essential for authorisation of effective combinations based on statistically significant evidence of efficacy and safety. Regulators may authorise the emergency use of an unapproved product or unapproved use of an approved product for COVID‐19 provided the product is effective in diagnosing, treating or preventing the disease; the potential and known benefits outweigh the potential and known risks; and in the absence of adequate or approved alternatives. 47 Only three combination therapies have been granted authorisation for emergency use to treat COVID‐19 based on evidence of high scientific quality.
The first dual combination to be granted EUA by the FDA on 19 November 2020 was remdesivir and baricitinib, for the treatment of COVID‐19 in hospitalised adults and paediatric patients (2 years and older) requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). The authorisation was granted based on the totality of scientific evidence from the randomised, double‐blind, placebo‐controlled trial (NCT04401579) which suggested that potential benefits of the combination outweighed their known risks. 48
The FDA issued an EUA on 21 November 2020 for a neutralising antibody cocktail, REGN‐COV2 (casirivimab and imdevimab) for the treatment of mild to moderate COVID‐19 in adults and paediatric patients (12 years and older) at risk of progressing to severe COVID‐19 based on data from NCT04425629. 48 Recommendation for use was also granted by the European Medicines Agency (EMA) in outpatients with COVID‐19 who do not need supplemental oxygen. 49 This fixed‐dose combination was aimed at reducing mutational escape by SARS‐CoV‐2 with both antibodies binding to distinct regions of the viral target, thus eliminating the likelihood of treatment resistance. 50 There was a significant reduction in viral load, hospitalisation and emergency room visits in patients who received the antibody cocktail compared to the placebo group. 51 The use of some combinations of monoclonal antibodies has been targeted against variants with reduced susceptibility to the individual antibodies, thus yielding improved clinical outcomes. 52
Similarly, the bamlanivimab and etesevimab combination was granted EUA by the FDA on 25 February 2021 as well as recommendations for use by the EMA in March 2021 for the treatment of mild to moderate COVID‐19 in adults and paediatric patients (12 years and older) at risk of progressing to severe COVID‐19 based on data from NCT04427501. The combination is recommended to be used within 10 days of symptom onset, but not authorised in patients who require oxygen or those on chronic oxygen therapy from underlying non‐COVID‐19‐related comorbidity. 53 , 54 , 55
Different regulatory approaches apply in the assessment of pivotal clinical trials for approval; however, all are based on evidence of high scientific quality in terms of robust safety and efficacy data. Regulators can, however, be flexible in the areas of scientific data interpretation and benefit to harm balance when granting authorisations for emergency use, provided the intervention or combination therapy is used in accordance with the stipulated conditions of use. 56
4.4. Future perspectives
Based on the review of the current trial landscape, a comparatively small proportion of trials are testing combination therapies, yet these will likely prove essential for effective COVID‐19 treatment and therapeutic prophylaxis due to the complex pathophysiology and possible rates of antiviral resistance.
With no gold standard treatment and many repurposed monotherapy interventions failing to show significant benefit, there is a clear justification to move the focus to effective combination therapies. Focusing on safe and effective combinations in future randomised controlled trials may enable better overall therapeutic efficacy against COVID‐19. Large randomised combination therapy clinical trials are warranted using some interventions with significant, albeit small, benefits in monotherapy trials rather than continued duplication of ongoing trials.
Interventions have been trialled to target specific stages along the disease pathway such as viral replication, cytokine injury and thrombosis, with significant success rates. An evaluation of the timely administration of a combination of these specific interventions may effectively target multiple stages of the COVID‐19 disease pathway. Combination therapies can also be evaluated in platform trials where potential combinations can be compared simultaneously against a common control with established effectiveness. This will enable early prioritisation of effective treatment arms while saving time and resources.
Pivotal trials which test for effectiveness of therapy require large sample sizes, but with the recent administration of vaccines and a significant reduction in affected cases, conducting large trials may be quite difficult. While results from smaller trials across different trial locations could be pooled using statistical approaches such as meta‐analysis to ascertain the effectiveness of combination therapies, generalising these findings may be a challenge due to heterogeneity in trial designs and target populations. Pooling data from individual clinical trials should be initiated with trials conducted during the same phase of the pandemic, as the clinical knowledge on the management of COVID‐19 has significantly improved compared to the initial phase of the pandemic, thereby capturing valid treatment effects.
The treatment pattern for COVID‐19 is rapidly evolving and with focus on the clinical benefits of combination therapies, better treatment options will hopefully become available.
NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.57
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
S.A. wrote the manuscript with support from all authors. All authors provided critical feedback and helped shape the research, analysis and manuscript.
ACKNOWLEDGEMENTS
This project is funded by the NIHR (HSRIC‐2016‐10 009)/Innovation Observatory. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Akinbolade S, Coughlan D, Fairbairn R, et al. Combination therapies for COVID‐19: An overview of the clinical trials landscape. Br J Clin Pharmacol. 2022;88(4):1590-1597. doi: 10.1111/bcp.15089
Funding information NIHR (HSRIC‐2016‐10 009)/Innovation Observatory
REFERENCES
- 1. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Bio Medica: Atenei Parmensis. 2020;91(1):157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Willan J, King AJ, Jeffery K, Bienz N. Challenges for NHS hospitals during covid‐19 epidemic. 2020;368:m1117. [DOI] [PubMed] [Google Scholar]
- 3. Nitulescu GM, Paunescu H, Moschos SA, et al. Comprehensive analysis of drugs to treat SARS‐CoV‐2 infection: mechanistic insights into current COVID‐19 therapies. Int J Mol Med. 2020;46(2):467‐488. 10.3892/ijmm.2020.4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eggleton JS, Nagalli S. Highly Active Antiretroviral Therapy (HAART). Treasure Island, FL: StatPearls Publishing; 2020. [PubMed]
- 5. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. The Lancet. 2013;382(9903):1525‐1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melville K, Rodriguez T, Dobrovolny HM. Investigating different mechanisms of action in combination therapy for influenza. Front Pharmacol. 2018;9:1207(1207). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6206389/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Illanes‐Álvarez F, Márquez‐Ruiz D, Márquez‐Coello M, Cuesta‐Sancho S, Girón‐González JA. Similarities and differences between HIV and SARS‐CoV‐2. Int J Med Sci. 2021;18(3):846‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou Y, Wang F, Tang J, Nussinov R, Cheng F. Artificial intelligence in COVID‐19 drug repurposing. Lancet Digital Health. 2020;2(12):e667‐e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ngo BT, Marik P, Kory P, et al. The time to offer treatments for COVID‐19. Expert Opin Investig Drugs. 2021;30(5):505‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence (NICE): RAPID‐C19 . Research to access pathway for investigational drugs for COVID‐19 (RAPID‐C19). 2021. https://www.nice.org.uk/covid-19/rapid-c19. Accessed January 28, 2021.
- 11. ClinicalTrials.gov. ClinicalTrials.gov database. 2021. https://clinicaltrials.gov/. Accessed May 17, 2021.
- 12. Register ECT . European Union Clinical Trials Register. 2021. https://www.clinicaltrialsregister.eu/. Accessed May 17, 2021.
- 13. World Health Organisation (WHO) . International Clinical Trials Registry Platform (ICTRP). 2021. https://ictrptest.azurewebsites.net/Default.aspx. Accessed May 17, 2021.
- 14. Law Insider . Pivotal Clinical Trial definition. 2021. https://www.lawinsider.com/dictionary/pivotal-clinical-trial. Accessed May 14, 2021.
- 15. NIHR Innovation Observatory (NIHRIO) . COVID‐19 Therapeutics. 2021. http://www.io.nihr.ac.uk/report/covid-19-therapeutics/. Accessed April 30, 2021.
- 16. RECOVERY Collaborative Group . Effect of hydroxychloroquine in hospitalized patients with Covid‐19. N Engl J Med. 2020;383(21):2030‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO Solidarity Trial Consortium . Repurposed antiviral drugs for COVID‐19—Interim WHO SOLIDARITY trial results. N Engl J Med. 2021;384:497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naci H, Kesselheim AS, Røttingen J‐A, Salanti G, Vandvik PO, Cipriani A. Producing and using timely comparative evidence on drugs: lessons from clinical trials for covid‐19. BMJ. 2020;371:m3869. https://www.bmj.com/content/bmj/371/bmj.m3869.full.pdf [DOI] [PubMed] [Google Scholar]
- 20. US Food and Drug Administration (FDA) . Coronavirus (COVID‐19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and. Accessed May 17, 2021.
- 21. Beigel J, Tomashek K, Dodd L. Remdesivir for the treatment of Covid‐19—Final report. N Engl J Med. 2020;383(19):1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Food and Drug Administration (FDA) . FDA Approves First Treatment for COVID‐19. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19. Accessed March 3, 2021.
- 23. US Food and Drug Administration (FDA) . Letter of Authorization: EUA for baricitinib (Olumiant), in combination with remdesivir (Veklury), for the treatment of suspected or laboratory confirmed coronavirus disease 2019 (COVID‐19). 2020. https://www.fda.gov/media/143822/download. Accessed December 15, 2020.
- 24. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid‐19. N Engl J Med. 2021;384(9):795‐807. 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roche . Roche provides update on the phase III REMDACTA trial of Actemra/RoActemra plus Veklury in patients with severe COVID‐19 pneumonia. 2021. https://www.roche.com/media/releases/med-cor-2021-03-11.htm. Accessed May 17, 2021.
- 26. ACTIV‐3/TICO LY‐CoV555 Study Group . A neutralizing monoclonal antibody for hospitalized patients with Covid‐19. N Engl J Med. 2021;384(10):905‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. HIV i‐BASE . Monoclonal antibody stopped in ACTIV‐3 study: bamlanivimab shows lack of benefit in people hospitalised with COVID‐19. 2020. https://i-base.info/htb/39268 Accessed May 18, 2021.
- 28. PM Live News . Eli Lilly ends COVID‐19 antibody trial in hospitalised setting, other studies continue. 2020. http://www.pmlive.com/pharma_news/eli_lilly_ends_covid-19_antibody_trial_in_hospitalised_setting,_other_studies_continue_1354817?utm_source=pmlive&utm_medium=email&utm_campaign=pmlive_daily. Accessed March 3, 2021.
- 29. Takeda press release . CoVIg‐19 Plasma Alliance Announces Topline Results from NIH‐Sponsored Clinical Trial of Investigational COVID‐19 Hyperimmune Globulin Medicine. 2021. https://www.takeda.com/newsroom/newsreleases/2021/covig-19-plasma-alliance-announces-topline-results-from-nih-sponsored-clinical-trial-of-investigational-covid-19-hyperimmune-globulin-medicine/. Accessed August 18, 2021.
- 30. WCG CentreWatch . Adaptive COVID‐19 Treatment Trial 3 (ACTT‐3). 2021. https://www.centerwatch.com/clinical-trials/listings/249972/adaptive-covid-19-treatment-trial-3-actt-3/. Accessed May 6, 2021.
- 31. Agrawal U, Raju R, Udwadia ZF. Favipiravir: a new and emerging antiviral option in COVID‐19. Med J Armed Forces India. 2020;76(4):370‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID‐19: an open‐label control study. Engineering (Beijing). 2020;6(10):1192‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor P, Adams A, Hufford M, De La Torre I, Winthrop K, Gottlieb R. Neutralizing monoclonal antibodies for treatment of COVID‐19. Nat Rev Immunol. 2021;21(6):382‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andreano E, Nicastri E, Paciello I, et al. Extremely potent human monoclonal antibodies from COVID‐19 convalescent patients. Cell. 2021;184(7):1821‐1835.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen P, Nirula A, Heller B, et al. SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with Covid‐19. N Engl J Med. 2021;384(3):229‐237. 10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. US Food and Drug Administration (FDA) . RE: Emergency Use Authorization 090. 2021. https://www.fda.gov/media/147629/download. Accessed May 18, 2021.
- 37. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID‐19: a randomized clinical trial. JAMA. 2021;325(7):632‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. US Food and Drug Administration (FDA) . Frequently Asked Questions on the Emergency Use Authorization for Bamlanivimab and Etesevimab. 2021. https://www.fda.gov/media/145808/download. Accessed April 28, 2021.
- 39. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID‐19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7308649/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID‐19? J Transl Med. 2020;18(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee S‐S, Chakraborty C. Tocilizumab: a therapeutic option for the treatment of cytokine storm syndrome in COVID‐19. Arch Med Res. 2020;51(6)):595‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970‐10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Samaee H, Mohsenzadegan M, Ala S, Maroufi SS, Moradimajd P. Tocilizumab for treatment patients with COVID‐19: recommended medication for novel disease. Int Immunopharmacol. 2020;117:107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodriguez‐Garcia JL, Sanchez‐Nievas G, Arevalo‐Serrano J, Garcia‐Gomez C, Jimenez‐Vizuete JM, Martinez‐Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS‐CoV‐2 pneumonia: an observational cohort study. Rheumatology (Oxford). 2021;60(1):399‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reyes AZ, Hu KA, Teperman J, et al. Anti‐inflammatory therapy for COVID‐19 infection: the case for colchicine. Ann Rheum Dis. 2021;80(5):550‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. US Department of Health and Human Services . Emergency Use Authorization of Medical Products. 2017. https://www.fda.gov/media/97321/download. Accessed May 18, 2021.
- 48. US Food and Drug Administration (FDA) . FDA combating COVID‐19 with therapeutics. 2020. https://www.fda.gov/media/136832/download. Accessed December 10, 2020.
- 49. European Medicines Agency (EMA) . EMA issues advice on use of REGN‐COV2 antibody combination (casirivimab/imdevimab). 2021. https://www.ema.europa.eu/en/news/ema-issues-advice-use-regn-cov2-antibody-combination-casirivimab-imdevimab. Accessed May 25, 2021.
- 50. Baum A, Fulton BO, Wloga E, et al. Antibody cocktail to SARS‐CoV‐2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506):1014‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. US Food and Drug Administration (FDA) . FDA news release: Coronavirus (COVID‐19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID‐19. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed December 15, 2020.
- 52. National Institute of Health (NIH) . Anti‐SARS‐CoV‐2 Monoclonal Antibodies. 2021. https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/. Accessed September 2, 2021.
- 53. US Food and Drug Administration (FDA) . Emergency Use Authorization. 2021. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs. Accessed April 28, 2021.
- 54. US Food and Drug Administration (FDA) . Bamlanivimab and Etesevimab EUA Letter of Authorization. 2021. https://www.fda.gov/media/145801/download. Accessed May 18, 2021.
- 55. European Medicines Agency (EMA) . EMA issues advice on use of antibody combination (bamlanivimab/etesevimab). 2021. https://www.ema.europa.eu/en/news/ema-issues-advice-use-antibody-combination-bamlanivimab-etesevimab. Accessed April 28, 2021.
- 56. Lexchin J, Graham J, Herder M, Jefferson T, Lemmens T. Regulators, pivotal clinical trials, and drug regulation in the age of COVID‐19. Int J Health Serv. 2021;51(1):5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]


