Abstract
Introduction
In this review, cases of herpes zoster (HZ) infection following receipt of COVID‐19 vaccines will be analyzed. We also present two cases of oral HZ following the COVID‐19 vaccine and discuss this clinical anatomy.
Materials and Methods
A database search using PubMed was conducted in August 2021 and 20 articles were found to be eligible for review. Patient data and vaccine information were analyzed. In addition, two cases of oral HZ infection following the receipt of COVID‐19 vaccines are presented.
Results
A total of 399 cases were identified. The affected dermatomes mimicked the regular distribution of HZ. For the dermatomes of the face, the various reports used different ways to describe the areas involved; CNV, CNV1, CNV2, CNV3, lower jaw, forehead, and under the eyebrow (CNV, 2 cases; CNV1, 4 cases; CNV2, 3 cases; and CNV3, 3 cases). Some patients who had a history of varicella zoster virus vaccination had HZ following the COVID‐19 vaccination. Two patients with oral HZ following vaccination were found to have involvement of the greater palatine nerve.
Conclusions
Vaccine‐related HZ cases have been reported worldwide. Although many studies with a larger number of cases are ongoing, detailed information can be obtained from case reviews as reported herein.
Keywords: clinical anatomy, complications, COVID‐19, infection, oral herpes zoster, palate, pandemic, reactivation, vaccination, varicella zoster virus
1. INTRODUCTION
The Food and Drug Administration issued Emergency Use Authorizations for Pfizer/BioNTech (BNT162b2) and Moderna (mRNA‐1273) COVID‐19 vaccines in December 2020 (McMahon et al., 2021). Since then, several types of vaccines have been used worldwide. The adverse events associated with the vaccinations include anemia, skin reactions, and lymphopenia although the cause of many of these adverse effects is still unclear (Barda et al., 2021). In many countries, nationwide studies are ongoing and data from some of these are currently being analyzed (Català et al., 2021; Barda et al., 2021; Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. Updated 25 March, 2021. Available online: Website: https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting, accessed on September 7, 2021).
Recently, cases of herpes zoster (HZ) characterized by reactivation of varicella zoster virus (VZV) following COVID‐19 vaccines have been reported in the literature (Barda et al., 2021; Psichogiou et al., 2021; Rodríguez‐Jiménez et al., 2021). It is believed that VZV is latent in the spinal dorsal root ganglia (DRG) and trigeminal ganglia (TG). In cases of reactivation of the VZV in the DRG, HZ will appear in the corresponding dermatome of the sensory nerve originating from the DRG (Gagliardi et al., 2016; Psichogiou et al., 2021). The reactivation of the VZV in the TG causes HZ reactivation in the upper, middle, lower face, or oral cavity depending on involvement of V1, V2, or V3 divisions of the trigeminal nerve.
Studies have shown that vaccines such as for hepatitis A, influenza, rabies, and Japanese encephalitis can trigger the reactivation of the HZ (Eid et al., 2021; Vastarella et al., 2021; Wollina et al., 2020; Wollina et al., 2021). Although the prognosis is predictable, the etiology and association with vaccines and VZV reactivation is still unclear.
In this review, HZ cases following COVID‐19 vaccinations will be analyzed to better illustrate the involvement from different countries. We also present two cases of oral HZ following COVID‐19 vaccination.
2. MATERIALS AND METHODS
2.1. Database search
A database search using PubMed was conducted in August 2021. The following keywords were used in the search: “((herpes zoster) AND (covid‐19)) AND (vaccine)” or “((herpes zoster) AND (covid‐19)) AND (vaccination)”. Thirty‐eight articles were identified. The first author reviewed the titles and abstracts of the results and excluded studies that were duplicated or not written in English. A total of 22 studies underwent full‐text assessment. Finally, 20 studies describing HZ after COVID‐19 vaccination were included noting the publication year, first author, country, journal, and the number of cases included in the article (Table 1).
TABLE 1.
The publication year, first author, country, journal, and the number of cases included in the article
| Year | First author | Country | Journal | No. of cases | |
|---|---|---|---|---|---|
| 1 | 2021 | Rodríguez‐Jiménez | Spain | JAAD Case Rep | 5 |
| 2 | 2021 | Özdemir | Turkey | J Eur Acad Dermatol Venereol | 2 |
| 3 | 2021 | Aksu | Turkey | Clin Exp Vaccine Res | 1 |
| 4 | 2021 | Català | Spain | Br J Dermatol | 41 |
| 5 | 2021 | Lee | USA | J Cosmet Dermatol | 20 |
| 6 | 2021 | Alpalhão | Portugal | J Eur Acad Dermatol Venereol | 4 |
| 7 | 2021 | Barda | Israel | N Engl J Med | 283 |
| 8 | 2021 | Psichogiou | Greece | Vaccines (Basel) | 7 |
| 9 | 2021 | Channa | USA | JAAD Case Rep | 1 |
| 10 | 2021 | Arora | India | J Cosmet Dermatol | 1 |
| 11 | 2021 | Vastarella | Italy | J Eur Acad Dermatol Venereol | 3 |
| 12 | 2021 | Tessas | Finland | J Eur Acad Dermatol Venereol | 1 |
| 13 | 2021 | Eid | Lebanon | J Med Virol | 1 |
| 14 | 2021 | Chiu | Taiwan | QJM | 3 |
| 15 | 2021 | Bostan | Turkey | J Cosmet Dermatol | 1 |
| 16 | 2021 | Furer | Israel | Ann Rheum Dis | 6 |
| 17 | 2021 | McMahon | Multinations | J Am Acad Dermatol | 10 |
| 18 | 2021 | Furer | Israel | Rheumatology (Oxford | 6 |
| 19 | 2021 | van Dam | Netherlands | Int J Infect Dis | 2 |
| 20 | 2021 | David | USA | J Drugs Dermatol | 1 |
2.2. Data analysis
A total number of reported cases of herpes zoster after COVID‐19 vaccination was calculated based on the data found in 20 articles. The age, sex, vaccine type, dose that triggered the HZ reactivation (first or second), duration from the vaccination until the skin reaction appeared (days), and affected dermatome were noted.
In addition, two case illustrations of HZ following COVID‐19 vaccination in the oral cavity will be presented.
3. RESULTS
Twenty articles published in 2021 were analyzed. A total of 399 cases were reported from 20 articles. The affected patients' sex, mean age, and age range are shown in Table 2. The vaccine type, the dose that triggered the HZ reactivation (first or second), and the duration from the vaccination to skin reaction are shown in Table 3. BNT162b2 (Pfizer) is the most reported type of vaccine (351/399). The study by Barda et al. (2021) which had the largest number of HZ cases in this review used BNT162b2 vaccine (n = 283). HZ after inactivated COVID‐19 vaccination was reported only in reports from India and Turkey.
TABLE 2.
Patients' sex, mean age, and age range
| No. of cases for analysis | No. of articles cited for analysis and first author of the article not cited for analysis (out of 20 articles in Table 1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sex | 399 | Female | 54 | 17 | None | |||
| Male | 46 | |||||||
| Unknown | 299 | 3 | ||||||
| Mean age | 100 | 58.9 | 17 | Barda | Furer | McMahon | ||
| Age range | 59 | 21–94 | 16 | Barda | Furer | McMahon | Català | |
TABLE 3.
The vaccine type, the dose that triggered the HZ reactivation, and the duration from the vaccination to skin reaction
| No. of cases for analysis | No. of articles cited for analysis and the first author of the article not cited for analysis (out of 20 articles in Table 1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Vaccine type | 399 | BNT162b2(Pfizer) | 351 | 20 | None | |||
| mRNA‐1273 (Moderna) | 28 | |||||||
| AZD1222 (AstraZeneca) | 14 | |||||||
| mRNA COVID vaccine (not specified) | 1 | |||||||
| inactivated SARS‐CoV‐2 vaccine | 5 | |||||||
| The dose reactivating HV | 399 | First dose | 77 | 20 | None | |||
| Second dose | 32 | |||||||
| Unknown (or needed only one shot) | 290 | |||||||
| Duration (days) | 59 | Minimum | 1 | 16 | Barda | Furer | McMahon | Català |
| Maximum | 24 | |||||||
| Mean | 6.75 | |||||||
3.1. Affected dermatome
The dermatomes affected were cranial nerve V in 12 cases, the cervical region in 11 cases, the thoracic region in 24 cases, and the lumbar/sacral regions in seven cases. When multiple regions were affected in one patient, it was counted as multiple cases. For the dermatomes of the face, the various reports used different ways to describe the areas involved; CNV, CNV1, CNV2, CNV3, lower jaw, forehead, and under the eyebrow (CNV, two cases; CNV1, four cases; CNV2, three cases; CNV3, three cases). To our knowledge, no previous reports have described HZ involvement of the CN V dermatome in the oral cavity.
3.2. Prior history of herpes zoster or varicella
A total of 24 patients from seven articles mentioned a prior history of HZ or varicella and 16 patients had a prior history of these (Alpalhão & Filipe, 2021; Channa et al., 2021; Furer, Eviatar, et al., 2021; Lee et al., 2021; Psichogiou et al., 2021; Tessas & Kluger, 2021; van Dam et al., 2021).
3.3. History of the varicella zoster virus vaccination
A total of 20 patients in four articles mentioned a history of a VZV vaccination and five patients had received a VZV vaccination before the COVID‐19 vaccine (Channa et al., 2021; Furer, Zisman, et al., 2021; Lee et al., 2021; Psichogiou et al., 2021). Four out of the five patients received the VZV vaccine in 2019 or 2020 (Channa et al., 2021; Furer, Zisman, et al., 2021; Lee et al., 2021).
3.4. Other medical history of the patients
The medical history of the patients who developed HZ after COVID‐19 vaccination varied and included the following: type II diabetes mellitus, hypertension, coronary artery disease, dysrhythmia, dyslipidemia, cerebrovascular accident, chronic pulmonary obstructive disease, anxiety, and antineutrophilic cytoplasmic antibody‐related glomerulonephritis.
3.5. Treatment
Treatments described in the literature were the same as for patients in the general population with HZ.
3.6. Two cases of herpes zoster in the oral cavity following COVID‐19 vaccination
3.6.1. Case 1
A 75‐year‐old Japanese female visited the authors' (H.F. and N.F.) general dental practice for multiple unilateral small ulcers on the left hard palate. The patient had a medical history of hypertension and received a second dose of BNT162b2 vaccine 11 days before her symptoms began. Based on the clinical findings, the patient was diagnosed with oral HZ (Figure 1). There were no other symptoms in the oral cavity or other skin reaction involvement of the CN V dermatomes or other spinal nerves. The symptom disappeared in a week without treatment.
FIGURE 1.
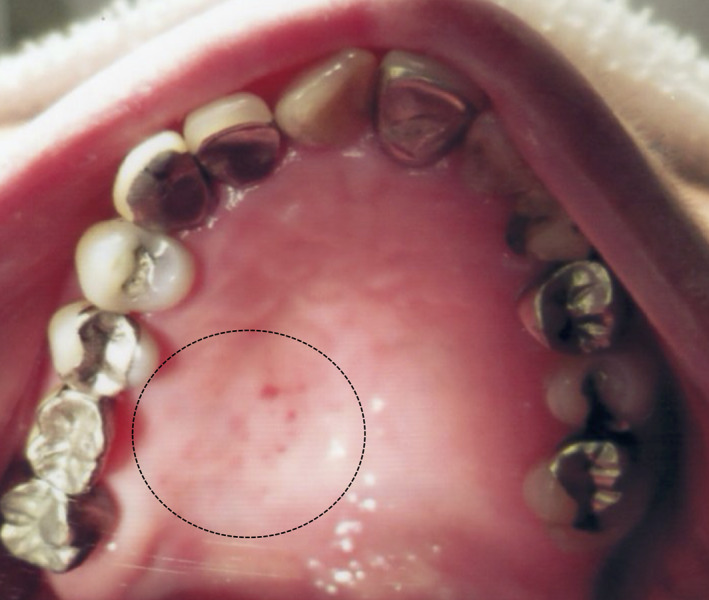
A mirror image of the multiple unilateral small ulcers on the left hard palate (dotted circle). The lesions are limited to the molar and premolar areas
3.6.2. Case 2
A 77‐year‐old Japanese female visited the authors' (H.F. and N. F.) general dental practice for multiple unilateral ulcers on the left hard palate. The patient had a history of hypertension and chronic kidney disease requiring dialysis. She received the second dose of BNT162b2 vaccine 38 days before the symptoms began. The patient was diagnosed with oral HZ based on her clinical findings (Figure 2). There were no other symptoms in the oral cavity and no other skin involvement in the CN V dermatome or other spinal nerve dermatomes. The patient was treated with amenamevir 200 mg for a week and ulcers disappeared.
FIGURE 2.
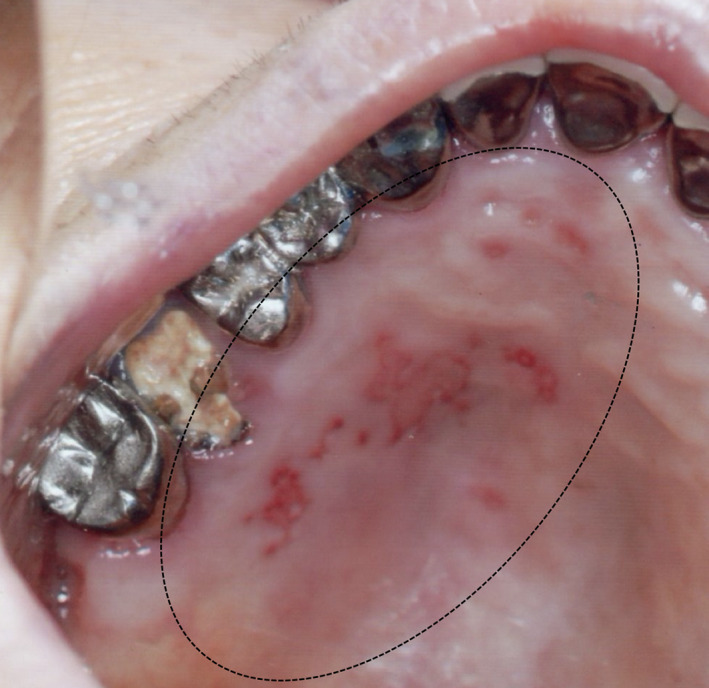
A mirror image of the multiple unilateral ulcers on the left hard palate (dotted circle). The lesions extend to the molar, premolar, canine, and incisor areas
4. DISCUSSION
4.1. Etiology
The EudraVigilance, European database of suspected adverse drug reaction reports, reported 4103 HZ cases, 590 HZ cases, and 2143 HZ cases following BNT162b2, mRNA‐1273 and AZD1222, respectively (Website: http://www.adrreports.eu/. Accessed on July 27, 2021; van Dam et al., 2021). In an Israeli study, Barda et al. (2021) reviewed 888,647 cases and found that 283 cases (0.03%) had HZ after BNT162b2 COVID‐19 vaccination. Furer, Zisman, et al. (2021) reported 6 cases of HZ (1.2%) following BNT162b2 COVID‐19 vaccination in 491 patients with autoimmune inflammatory rheumatic diseases and found none (0%) in a control group (n = 99).
In this review, we found that the reactivation of VZV following the COVID‐19 vaccines has been reported all over the world and with different types of vaccines. Reactivation of VZV following the vaccination using inactivated COVID‐19 has only been reported in India and Turkey (Aksu & Öztürk, 2021; Arora et al., 2021; Bostan & Yalici‐Armagan, 2021; Özdemir et al., 2021). The affected age had a wide range (21–94 year old) with a mean of 58.9 years. Reactivation of VZV after the first dose of vaccine seems more common than the second dose although we were unable to perform statistical analyses of this. Duration from the vaccination to skin reaction ranged from 1 to 24 days with a mean of 6.75 days. This result is similar to the case series findings of 7.6 days (three cases) reported by Vastarella et al. (2021) and 5.4 days (5 cases) reported by Rodríguez‐Jiménez et al. (2021). The median time from hospitalization after COVID‐19 infection to HZ appearing was 5.5 days (Tartari et al., 2020). Although the mechanism of vaccine‐induced HZ is still unclear some suggested vaccines might cause immunomodulation and lead to herpes virus reactivation (Özdemir et al., 2021; Walter et al., 1999).
Our review found that most of the literature involves case reports/series without control groups. It was not clear that the incidence of HZ following the COVID‐19 vaccine was higher than the incidence of HZ in the general population. One study by Barda et al. (2021) included 283 cases of HZ (70.9% of all cases in this review) after vaccination and 204 cases of HZ in a control group.
4.2. Clinical anatomy of the dermatome
HZ can be one of the causes of facial nerve palsy after vaccination (Peitersen, 2002; Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. Updated 25 March 2021. Available online: Website: https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting, accessed on September 7, 2021). The trigeminal ganglion is considered the location of the latent VZV. Ramsay‐Hunt syndrome, facial nerve palsy with other symptoms such as ear pain and vesicles in the ear, is caused by infection of the reactivated VZV of the geniculate ganglion of the facial nerve (Volpi, 2007). However, there is still no consensus of how the VZV reaches the facial and other cranial nerves.
Based on our review, any dermatome of cranial nerve V and spinal nerves can be affected by HZ following COVID‐19 vaccination, which mimics the regular HZ. Oral findings of HZ are well known (Santosh & Muddana, 2020). However, our literature review did not find any cases of VZV reactivation in the oral cavity following COVID‐19 vaccination. Therefore, the two case illustrations presented herein are unique. Although a large national database in the UK based on the spontaneous reports listed many oral lesions including oral herpes, it did not report cases of oral HZ (Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. Updated March 25, 2021. Available online: Website: https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting, accessed on September 72,021).
Interestingly, our two case illustrations had involvement of only the hard palate in the distribution of V2 (greater palatine nerve) (Figure 3). Other V2 dermatome regions of V2 for example, midface and nasal septum, were not affected.
FIGURE 3.
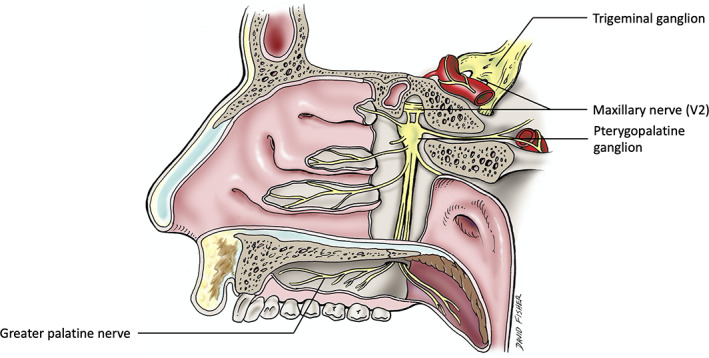
A schematic drawing of the greater palatine nerve arising from the maxillary nerve
4.3. Vaccine‐related reactivation
Brosh‐Nissimov et al. (2021) compared oropharyngeal reactivation of herpes viruses before and 1 week after BNT162b2 vaccination and concluded that they did not find any evidence of increased oropharyngeal reactivation 1 week after BNT162b2 vaccination, but not specifically VZV. There is also no definition of vaccine‐related reactivation in terms of duration. In the study by Brosh‐Nissimov et al. (2021), “1 week” was used to determine the vaccine‐related virus reactivation. Català et al. (2021) included patients with cutaneous reactions within 21 days after any dose of vaccine. However, our review revealed that the latent period of vaccine‐related HZ is from 1 to 24 days after vaccination. Additionally, our Case 2 became symptomatic 38 days after vaccination. Therefore, it is difficult to determine if this was vaccine‐related or not.
5. CONCLUSIONS
Vaccine‐related HZ cases have been reported worldwide. Although many national studies with larger number of cases are ongoing, detailed patient information can be obtained from individual case reports/series. As we identified two patients with oral HZ in a short period following COVID‐19 vaccination, other cases might be missed or misdiagnosed just a stomatitis or oral herpes. Dentists, dermatologists, and otolaryngologists should be aware that the patients who have received COVID‐19 vaccinations might present with pain in the oral cavity and oropharynx due to VZV reactivation. The dermatome in the oral cavity is often forgotten as it is not as easily seen on physical examination. Moreover, this region might be better described as an “epidermatome” or “mucotome.”
Iwanaga, J. , Fukuoka, H. , Fukuoka, N. , Yutori, H. , Ibaragi, S. , & Tubbs, R. S. (2022). A narrative review and clinical anatomy of herpes zoster infection following COVID‐19 vaccination. Clinical Anatomy, 35(1), 45–51. 10.1002/ca.23790
REFERENCES
- Aksu, S. B. , & Öztürk, G. Z. (2021). A rare case of shingles after COVID‐19 vaccine: Is it a possible adverse effect? Clinical Experimental Vaccine Research, 10(2), 198–201. 10.7774/cevr.2021.10.2.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpalhão, M. , & Filipe, P. (2021). Herpes zoster following SARS‐CoV‐2 vaccination—A series of four cases. Journal of the European Academy of Dermatology and Venereology. Online ahead of print. 10.1111/jdv.17555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora, P. , Sardana, K. , Mathachan, S. R. , & Malhotra, P. (2021). Herpes zoster after inactivated COVID‐19 vaccine: A cutaneous adverse effect of the vaccine. Journal of Cosmetic Dermatology. Online ahead of print. 10.1111/jocd.14268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda, N. , Dagan, N. , Ben‐Shlomo, Y. , Kepten, E. , Waxman, J. , Ohana, R. , Hernán, M. A. , Lipsitch, M. , Kohane, I. , Netzer, D. , Reis, B. Y. , & Balicer, R. D. (2021). Safety of the BNT162b2 mRNA Covid‐19 vaccine in a Nationwide setting. New England Journal of Medicine, 385, 1078–1090. 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan, E. , & Yalici‐Armagan, B. (2021. Jun). Herpes zoster following inactivated COVID‐19 vaccine: A coexistence or coincidence? Journal of Cosmetic Dermatology, 20(6), 1566–1567. 10.1111/jocd.14035 [DOI] [PubMed] [Google Scholar]
- Brosh‐Nissimov, T. , Sorek, N. , Yeshayahu, M. , Zherebovich, I. , Elmaliach, M. , Cahan, A. , Amit, S. , & Rotlevi, E. (2021). Oropharyngeal shedding of herpesviruses before and after BNT162b2 mRNA vaccination against COVID‐19. Vaccine, 39, 5729–5731. 10.1016/j.vaccine.2021.08.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Català, A. , Muñoz‐Santos, C. , Galván‐Casas, C. , Roncero Riesco, M. , Revilla Nebreda, D. , Solá‐Truyols, A. , Giavedoni, P. , Llamas‐Velasco, M. , González‐Cruz, C. , Cubiró, X. , Ruíz‐Villaverde, R. , Gómez‐Armayones, S. , Gil Mateo, M. P. , Pesqué, D. , Marcantonio, O. , Fernández‐Nieto, D. , Romaní, J. , Iglesias Pena, N. , Carnero Gonzalez, L. , … Guilabert, A. (2021). Cutaneous reactions after SARS‐COV‐2 vaccination: A cross‐sectional Spanish nationwide study of 405 cases. British Journal of Dermatology. Online ahead of print. 10.1111/bjd.20639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channa, L. , Torre, K. , & Rothe, M. (2021). Herpes zoster reactivation after mRNA‐1273 (Moderna) SARS‐CoV‐2 vaccination. JAAD Case Reports, 15, 60–61. 10.1016/j.jdcr.2021.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, H. H. , Wei, K. C. , Chen, A. , & Wang, W. H. (2021). Herpes zoster following COVID‐19 vaccine: Report of 3 cases. QJM. Online ahead of print. 10.1093/qjmed/hcab208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting . 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
- David, E. , & Landriscina, A. (2021). Herpes zoster following COVID‐19 vaccination. Journal of Drugs in Dermatology, 20(8), 898–900. 10.36849/JDD.6146 [DOI] [PubMed] [Google Scholar]
- Eid, E. , Abdullah, L. , Kurban, M. , & Abbas, O. (2021. Sep). Herpes zoster emergence following mRNA COVID‐19 vaccine. Journal of Medical Virology, 93(9), 5231–5232. 10.1002/jmv.27036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furer, V. , Eviatar, T. , Zisman, D. , Peleg, H. , Paran, D. , Levartovsky, D. , Zisapel, M. , Elalouf, O. , Kaufman, I. , Meidan, R. , Broyde, A. , Polachek, A. , Wollman, J. , Litinsky, I. , Meridor, K. , Nochomovitz, H. , Silberman, A. , Rosenberg, D. , Feld, J. , … Elkayam, O. (2021). Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Annals of the Rheumatic Diseases, 80, 1330–1338. 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- Furer, V. , Zisman, D. , Kibari, A. , Rimar, D. , Paran, Y. , & Elkayam, O. (2021). Herpes zoster following BNT162b2 mRNA Covid‐19 vaccination in patients with autoimmune inflammatory rheumatic diseases: A case series. Rheumatology (Oxford). Online ahead of print. 10.1093/rheumatology/keab345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi, A. M. , Gomes Silva, B. N. , Torloni, M. R. , & Soares, B. G. (2016). Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic Reviews, 3, CD008858. 10.1002/14651858.CD008858.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. , Cotter, D. , Basa, J. , & Greenberg, H. L. (2021. Jul). 20 post‐COVID‐19 vaccine‐related shingles cases seen at the Las Vegas dermatology clinic and sent to us via social media. Journal of Cosmetic Dermatology, 20(7), 1960–1964. 10.1111/jocd.14210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, D. E. , Amerson, E. , Rosenbach, M. , Lipoff, J. B. , Moustafa, D. , Tyagi, A. , Desai, S. R. , French, L. E. , Lim, H. W. , Thiers, B. H. , Hruza, G. J. , Blumenthal, K. G. , Fox, L. P. , & Freeman, E. E. (2021. Jul). Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. Journal of the American Academy of Dermatology, 85(1), 46–55. 10.1016/j.jaad.2021.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir, A. K. , Kayhan, S. , & Çakmak, S. K. (2021). Herpes zoster after inactivated SARS‐CoV‐2 vaccine in two healthy young adults. Journal of the European Academy of Dermatology and Venereology. Online ahead of print. 10.1111/jdv.17577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitersen, E. (2002). Bell's palsy: The spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Oto‐Laryngologica. Supplementum, 549, 4–30. [PubMed] [Google Scholar]
- Psichogiou, M. , Samarkos, M. , Mikos, N. , & Hatzakis, A. (2021). Reactivation of varicella zoster virus after vaccination for SARS‐CoV‐2. Vaccines (Basel)., 9(6), 572. 10.3390/vaccines9060572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Jiménez, P. , Chicharro, P. , Cabrera, L. M. , Seguí, M. , Morales‐Caballero, Á. , Llamas‐Velasco, M. , & Sánchez‐Pérez, J. (2021). Varicella‐zoster virus reactivation after SARS‐CoV‐2 BNT162b2 mRNA vaccination: Report of 5 cases. JAAD Case Rep., 12, 58–59. 10.1016/j.jdcr.2021.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosh, A. B. R. , & Muddana, K. (2020). Viral infections of oral cavity. Journal of Family Medicine and Primary Care, 9(1), 36–42. 10.4103/jfmpc.jfmpc_807_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartari, F. , Spadotto, A. , Zengarini, C. , Zanoni, R. , Guglielmo, A. , Adorno, A. , Valzania, C. , & Pileri, A. (2020). Herpes zoster in COVID‐19‐positive patients. International Journal of Dermatology, 59, 1028–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessas, I. , & Kluger, N. (2021). Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID‐19 vaccine. Journal of the European Academy of Dermatology and Venereology, 35, e620–e622. 10.1111/jdv.17422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam, C. S. , Lede, I. , Schaar, J. , Al‐Dulaimy, M. , Rösken, R. , & Smits, M. (2021). Herpes zoster after COVID‐vaccination. International Journal of Infectious Diseases, 111, 169–171. 10.1016/j.ijid.2021.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastarella, M. , Picone, V. , Martora, F. , & Fabbrocini, G. (2021). Herpes zoster after ChAdOx1 nCoV‐19 vaccine: A case series. Journal of the European Academy of Dermatology and Venereology. Online ahead of print. 10.1111/jdv.17576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi, A. (2007). Severe complications of herpes zoster. Herpes, (Suppl 2), 35–39. [PubMed] [Google Scholar]
- Walter, R. , Hartmann, K. , Fleisch, F. , Reinhart, W. H. , & Kuhn, M. (1999). Reactivation of herpesvirus infections after vaccinations? Lancet, 353, 810. [DOI] [PubMed] [Google Scholar]
- Wollina, U. , Chiriac, A. , Kocic, H. , Koch, A. , & Brzezinski, P. (2021). Cutaneous and hypersensitivity reactions associated with COVID‐19 vaccination‐a narrative review. Wiener Medizinische Wochenschrift (1946), 23, 1–7. 10.1007/s10354-021-00876-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollina, U. , Karadağ, A. S. , Rowland‐Payne, C. , et al. (2020). Cutaneous signs in COVID‐19 patients: A review. Dermatologic Therapy, 33(5), e13549. [DOI] [PMC free article] [PubMed] [Google Scholar]


