Abstract
Background and purpose
Population‐based studies suggest that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines may trigger immune‐mediated thrombotic thrombocytopenia (VITT) raising concerns for other autoimmune responses. The aim was to characterize neurological autoimmunity after SARS‐CoV‐2 vaccinations.
Methods
In this single‐centre prospective case study patients with neurological autoimmunity in temporal association (≤6 weeks) with SARS‐CoV‐2 vaccinations and without other triggers are reported. Clinical, laboratory and imaging data were collected with a median follow‐up of 49 days.
Results
In the study period 232,603 inhabitants from the main catchment area of our hospital (Rhein‐Neckar‐Kreis, county) received SARS‐CoV‐2 vaccinations. Twenty‐one cases (new onset n = 17, flares n = 4) diagnosed a median of 11 days (range 3–23) following SARS‐CoV‐2 vaccinations (BNT162b2 n = 12, ChAdOx1 n = 8, mRNA‐1273 n = 1) were identified. Cases included VITT with cerebral venous sinus thrombosis (n = 3), central nervous system demyelinating diseases (n = 8), inflammatory peripheral neuropathies (n = 4), myositis (n = 3), myasthenia (n = 1), limbic encephalitis (n = 1) and giant cell arteritis (n = 1). Patients were predominantly female (ratio 3.2:1) and the median age at diagnosis was 50 years (range 22–86). Therapy included administration of steroids (n = 15), intravenous immunoglobulins in patients with Guillain–Barré syndrome or VITT (n = 4), plasma exchange in cases unresponsive to steroids (n = 3) and anticoagulation in VITT. Outcomes were favourable with partial and complete remissions achieved in 71% and 24%, respectively. Two patients received their second vaccination without further aggravation of autoimmune symptoms under low‐dose immunosuppressants.
Conclusions
In this study various neurological autoimmune disorders encountered following SARS‐CoV‐2 vaccinations are characterized. Given the assumed low incidence and mostly favourable outcome of autoimmune responses, the benefits of vaccinations outweigh the comparatively small risks.
Keywords: autoimmune, cerebral venous sinus thrombosis, COVID‐19, Guillain–Barré syndrome, multiple sclerosis, myelitis, myositis
In this prospective case study, 21 consecutive cases of neurological autoimmunity, which occurred 3–23 days following SARS‐CoV‐2 vaccinations (BNT162b2 n = 12, ChAdOx1 n = 8, mRNA‐1273 n = 1), are reported. Cases included vaccine‐induced immune thrombotic thrombocytopenia (n = 3), central nervous system demyelinating diseases (n = 8), inflammatory peripheral neuropathies (n = 4), myositis (n = 3), myasthenia (n = 1), limbic encephalitis (n = 1) and giant cell arteritis (n = 1). As outcomes were favourable in most patients and the risk of autoimmune complications appears low (<1:10,000) the benefits of vaccination outweigh the comparatively small risks. Case numbers of several conditions were higher than expected compared to published incidences; however, population‐based studies are warranted for further investigation.

INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines constitute the key countermeasure of the coronavirus 2019 (COVID‐19) pandemic. Based on randomized clinical trials the European Medicines Agency has approved four COVID‐19 vaccines (Pfizer‐BioNTech BNT162b2, Moderna mRNA‐1273, AstraZeneca ChAdOx1 nCov‐19, Janssen/Johnson&Johnson Ad26.COV2.S) [1, 2, 3, 4]. Overall, severe neurological complications including autoimmune disorders affecting the nervous system are exceedingly rare (<0.1%) following SARS‐CoV‐2 vaccinations [1, 2, 3, 4]. With the exception of the Ad26.COV2.S study, patients with autoimmunity or those receiving systemic immunosuppressive treatment were excluded from these initial clinical trials [3]. Therefore, limited safety data have been generated in this particular patient cohort. However, vaccinations are particularly recommended in patients with autoimmune conditions as they may experience disease exacerbation following infection and carry a risk for severe SARS‐CoV‐2 courses [5, 6]. Recent reports suggest that several SARS‐CoV‐2 vaccines may induce platelet factor 4 (PF4) antibody‐mediated thrombotic thrombocytopenia (VITT) 5–30 days following vaccination [7, 8, 9]. Similarly, cases of facial palsy, Guillain–Barré syndrome (GBS) and transverse myelitis have been documented following SARS‐CoV‐2 vaccinations [10, 11, 12, 13, 14]. It remains unclear whether COVID‐19 vaccines trigger additional neurological autoimmune responses, particularly in patients with pre‐existing autoimmunity. In this single‐centre case study, the longitudinal characteristics of 21 patients with various neurological autoimmune reactions in the temporal context of SARS‐CoV‐2 vaccinations are defined.
METHODS
Clinical data
Adult patients with new onset or flares of autoimmune diseases within 6 weeks of vaccination against COVID‐19 who presented to the Department of Neurology or Division of Rheumatology at Heidelberg University Hospital and affiliated teaching hospitals between 1 March and 1 June 2021 were included in this case study with last follow‐up on 19 June 2021. No patients were lost to follow‐up. Patients with triggers for autoimmunity other than vaccination against SARS‐CoV‐2 such as infection, medication changes or non‐compliance were excluded from our study. At inclusion, SARS‐CoV‐2 infection was ruled out with polymerase chain reaction of endotracheal aspirates or nasopharyngeal swabs. Clinical, laboratory, radiological and outcome data were collected longitudinally. If patients were discharged from our institution, structured weekly telephone interviews were conducted with study participants or their legal guardians. Physicians involved in the outpatient care of participants were consulted for their longitudinal assessment. Descriptive analysis (frequencies, medians, ranges) of data was performed using SPSS version 27 (IBM). One VITT case was previously published [15].
Vaccination data including administered number of vaccine doses (first/second) and types from the Rhein‐Neckar‐Kreis (county), the main catchment area of our hospital, were provided by the State Ministry of Health. The number of individuals who only received their second dose during the inclusion period of this study was derived from the number of first doses administered 3 (BNT162b2), 4 (mRNA‐1273) or 10 weeks (ChAdOx1) prior to the inclusion period (1 March 2021), assuming conventional dosing intervals, that every first was followed by a second shot with the same vaccine type, and that no individual moved between counties. The total number of individuals at risk was then estimated adding up individuals who at least received their first dose and those who received their second vaccine dose only. This estimate does not include individuals who were vaccinated 4 (BNT162b2) or 5 (mRNA‐1273) to 6 weeks prior to the inclusion phase of this study and who could theoretically have fulfilled inclusion criteria by developing autoimmune diseases within 6 weeks after vaccination. However, it contains individuals vaccinated late during the inclusion phase of this study—when the number of weekly vaccinations was higher—and who may have developed autoimmunity only after the inclusion period of this study.
The study was conducted in accordance with consensus‐based clinical case reporting (CARE) guidelines [16].
Autoimmune antibody investigations
Antibody investigations were selected with respect to the suspected neurological autoimmune condition. Comprehensive autoimmune neuropathy (ANA, ANCA and cryoglobulin screen; rheumatoid factor, SS‐A, SS‐B, GM1, GM2, GM3, GD1a, GD1b, GT1b, GQ1b, asialo‐GM1, IgG and IgM; Hu, Ri, Yo PCA‐2 and Tr/DNER‐IgG; MAG‐IgM), myositis (ANA screen, Mi‐2 alpha, Mi‐2 beta, TIF1 gamma, MDA5, NXP2, SAE1, Ku, PM100, PM75, Jo‐1, SRP, PL‐7, PL‐12, EJ, OJ, Ro‐52, cN‐1A IgG), encephalitis (Hu, Ri, ANNA‐3, Yo, Tr/DNER, myelin, Ma/Ta, GAD65, amphiphysin, aquaporin‐4 [AQP4], NMDA‐R, AMOA‐R, GABAA/B‐R, LGI2, CASPR2, ZIC3, ZIC4, DPPX, glycin‐R, mGluR1, mGluR5, Rho‐GTPase activating protein 26, ITPR1, homer 3, myelin oligodendrocyte glycoprotein [MOG], recoverin, neurochondrin, GluRD2, flotillin 1/2, IgLON5, PNMA2, SOX1, titin, Zic4, GAD65, VGKC, SOX‐1, PCA‐2 antibodies) and optic neuritis/myelitis (MOG, AQP4, AGNA‐1, amphiphysin, Hu, Ri, Yo, NMDA‐R, AMPA‐R, GABAB‐R, DPPX, mGluR1, GFAP, NIF antibodies) antibody panels were performed by an external laboratory (Euroimmun) using serum and cerebrospinal fluid (CSF) samples from our patients where available. Testing for PF4 antibodies in cases of cerebral venous sinus thrombosis (CVST) was performed as previously described [9, 15].
Ethics and consents
This study (S‐373/2021) was approved by the Heidelberg University institutional review board. Written informed consent was obtained from all patients or their legal guardians.
Patient and public involvement
Patients with autoimmunity were involved in identifying research questions, defining relevant parameters, and in choosing structured telephone interviews for follow‐up prior to institutional review board application. The results of this study will be disseminated in plain language following publication.
RESULTS
During the inclusion phase of our study, 5978 patients (inpatient N = 1204, outpatient N = 4774, vaccination status not available for review) were treated at our department. In the same interval an estimated 232,603 inhabitants from the Rhein‐Neckar county (population 547,625), the main catchment area of our hospital, were vaccinated against SARS‐CoV‐2. Numbers and types of vaccine doses administered are provided in Table 1.
TABLE 1.
Local county (Rhein‐Neckar‐Kreis) SARS‐CoV‐2 vaccination data
| Type of vaccine | First dose (N, overall) | Second dose (N, overall) | Estimated second dose only (N) | Estimated vaccinated individuals (N) |
|---|---|---|---|---|
| BNT162b2 | 141,884 | 80,449 | 13,138 | 155,022 |
| ChAdOx1 | 51,730 | 6686 | 2405 | 54,135 |
| mRNA‐1273 | 19,630 | 15,590 | 1486 | 21,116 |
| Ad26.COV2.S | 2330 | N/A | 2330 | 2330 |
| Total | 215,574 | 102,725 | 17,029 | 232,603 |
Abbreviation: N, number.
Twenty‐one cases of neurological autoimmune responses (new onset n = 17, disease flares n = 4) in temporal association with SARS‐CoV‐2 vaccinations (BNT162b2 n = 12, ChAdOx1 n = 8, mRNA‐1273 n = 1) were identified. The majority of patients (n = 16) including 14 cases of newly diagnosed neurological autoimmunity resided in the Rhein‐Neckar county. Sixteen cases were noted following the first dose (BNT162b2 n = 7, ChAdOx1 n = 8, mRNA‐1273 n = 1) whereas five cases occurred after the second BNT162b2 dose.
The spectrum of autoimmunity following SARS‐CoV‐2 vaccinations is depicted in Figure 1a. Conditions—newly diagnosed according to international standards—included VITT with subsequent CVST (n = 3), central nervous system (CNS) demyelinating diseases (n = 6: optic neuritis n = 4; partial transverse myelitis n = 2), inflammatory peripheral neuropathies (n = 4: GBS n = 2, L5 radiculitis n = 1, facial palsy n = 1), myositis (n = 2), limbic encephalitis (n = 1) and giant cell arteritis (GCA) (n = 1). All cases of VITT (n = 3) and GBS (n = 2) occurred in ChAdOx1 recipients whereas all cases of newly diagnosed optic neuritis (n = 4) and myositis (n = 2) followed vaccination with BNT162b2. Flares occurred in patients with multiple sclerosis (n = 2), distal symmetric PM/Scl‐75‐positive myositis (n = 1) and myasthenia (n = 1). Two patients with known multiple sclerosis (MS) were under ongoing immunosuppressive treatment when they received their SARS‐CoV‐2 vaccination. Five patients (24%) from this series suffered from additional autoimmune conditions outside the nervous system and a positive family history for autoimmunity was noted in seven patients (33%).
FIGURE 1.
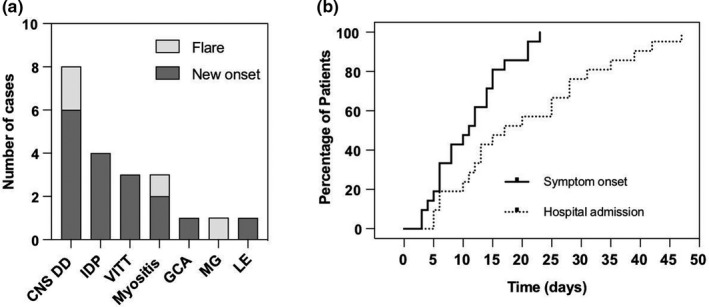
Spectrum of autoimmunity and intervals to symptom onset and hospital admission. (a) The box diagram shows neurological autoimmune diseases encountered at our institution following SARS‐CoV‐2 vaccinations. Conditions included CNS demyelinating disorders (CNS DD), inflammatory demyelinating peripheral neuropathies (IDP), vaccine‐induced immune thrombotic thrombocytopenia (VITT), myositis, giant cell arteritis (GCA), myasthenia gravis (MG) and limbic encephalitis (LE). Different grey tones indicate new onset (dark grey) or flare (light grey) of autoimmunity. (b) Inverted Kaplan–Meier curves highlight the interval from vaccination to autoimmune symptom onset (solid line, median 11 days) and hospital admission (dotted line, median 17 days)
The characteristics of our cohort are summarized in Table 2. Patients were predominantly female (ratio 3.2:1) and were vaccinated at a median age of 50 years (range 22–86). After vaccination, all participants reported mild non‐specific complaints (headache, fatigue, local pain) lasting for 1–3 days. Symptoms related to autoimmune reactions occurred after a median of 11 days (range 3–23, Figure 1b). This led to hospital admission a median of 17 days (range 5–42, Figure 1b) following vaccination.
TABLE 2.
Clinical and outcome characteristics
| Clinical data | N | (%) | Treatment/outcome | (%) | |
|---|---|---|---|---|---|
| Gender | Acute treatment | ||||
| Female | 16 | (76) | Anticoagulation | 3 | (15) |
| Male | 5 | (24) | MP pulse | 15 | (75) |
| Age (range, years) | 50 (22–86) | IVIG | 4 | (20) | |
| Autoimmunity | PLEX | 3 | (15) | ||
| New onset | 17 | (81) | Long‐term IS treatment | 3 | (15) |
| Exacerbation | 4 | (19) | |||
| Additional prior AD | 5 | (24) | Clinical outcome | ||
| Familial AD predisposition | 7 | (33) | Median f/u, (range, days) | 49 (20–105) | |
| Prior IS | 4 | (19) | PD | 0 | (0) |
| Median intervals | SD | 1 | (5) | ||
| To symptoms (range, days) | 11 (3–23) | PR | 15 | (71) | |
| To admission (range, days) | 17 (5–42) | CR | 5 | (24) |
Abbreviations: AD, autoimmune diseases; CR, complete remission; f/u, follow‐up; IS, immunosuppression; IVIG, intravenous immunoglobulins; MP, methylprednisolone; N, number; PD, progressive disease; PLEX, plasma exchange; PR, partial remission; SD, stable disease.
Laboratory findings at diagnosis revealed thrombocytopenia (median 53/nl, range 21–93) in patients with VITT only. C‐reactive protein elevation (median 11.6 mg/l; range 7.1–89 mg/l) without clinical, other laboratory or radiological signs of infection was noted in nine patients.
Diagnostic modalities including autoimmune antibody panels were selected with respect to the suspected autoimmune disease. Examples of radiological findings in patients with autoimmunity following SARS‐CoV‐2 vaccinations are shown in Figure 2. CVST in VITT was diagnosed using contrast‐enhanced magnetic resonance venography in all three patients (Figure 2a–d). Congestive bleeding was observed in two cases. One patient had additional systemic venous thromboses in the inferior cava, both iliacal and the right femoral veins. PF4 antibodies were positive in both patients where they were assessed. Two patients with VITT had known heterozygous factor V Leiden mutations. No additional risk factors for CVST were found (e.g., oral contraception, smoking status, overweight etc.) and in all three patients whole‐body computed tomography revealed no evidence of malignancy.
FIGURE 2.
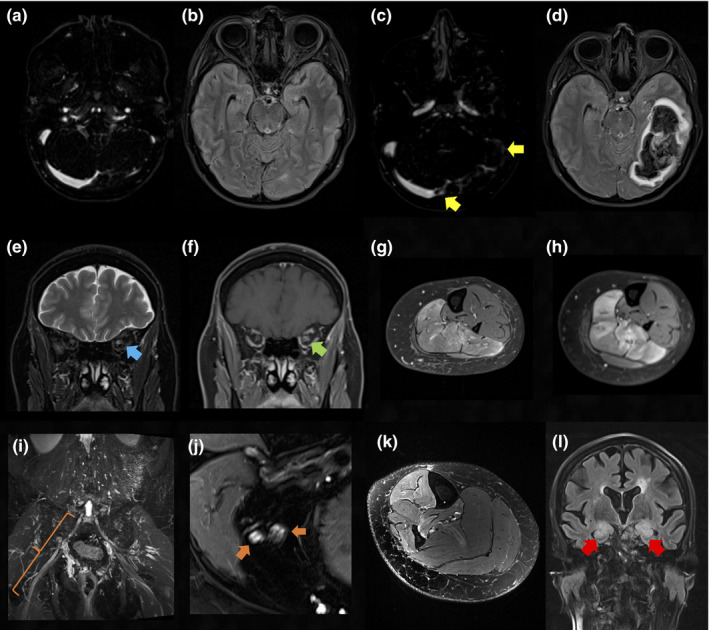
Radiological findings in patients with autoimmunity following SARS‐CoV‐2 vaccinations. (a), (b) Four days prior to the onset of supraventricular tachycardia symptoms: aside from a hypoplastic left sigmoid sinus findings are unremarkable in contrast‐enhanced magnetic resonance venography (CE‐MRV) (a) and axial fluid attenuated inversion recovery (FLAIR) sequence (b). (c) Follow‐up CE‐MRV reveals an occlusive thrombus of the left sigmoid sinus and a non‐occlusive thrombus of the right transverse sinus (yellow arrows). (d) The corresponding axial FLAIR sequence shows resulting congestive bleeding in the left temporal and occipital lobe. (e) Coronal T2‐weighted (T2w) and (f) gadolinium contrast‐enhanced (Gd CE) T1w orbital sequences reveal T2w hyperintensity (e) (blue arrow) and associated mild contrast enhancement of the left optic nerve (f) (green arrow) consistent with optic neuritis. (g), (h) Axial, fat saturated, Gd CE T1w saturated images at the level of the left lower leg 6 weeks before (g) and 7 days after (h) vaccination demonstrate progressive contrast uptake of the left soleus muscle indicating reactivation of focal myositis. (i) Coronal T2w MRI reveals hyperintense enlargement of the right L5 nerve root (orange bracket) consistent with L5 radiculitis. (j), (k) Axial T2w sequence with spectral fat saturation at the level of the distal right thigh (j) and right lower leg (k) are shown. Somatotopic L5 lesion pattern with hyperintense, fascicular enlargement of the common peroneal nerve and the ventral section of the tibial nerve (j) (orange arrows) and subsequent denervation oedema of the fibularis longus, extensor digitorum longus, tibialis anterior and posterior muscles (k) are evident. (l) Coronal FLAIR sequence demonstrates bilateral hippocampal hyperintensities (red arrows) and mild swelling in line with limbic encephalitis [Color figure can be viewed at wileyonlinelibrary.com]
Patients with suspected CNS demyelinating conditions underwent brain and spine magnetic resonance imaging (MRI) in all cases. Consistent with acute inflammation, MRI revealed contrast‐enhancing lesions of the optic nerves (optic neuritis n = 4, Figure 2e,f), the thoracic cord (partial transverse myelitis n = 2) and juxtacortical regions (MS n = 2). Lumbar puncture was performed in all patients with new onset of neuroinflammatory conditions (n = 6). It revealed mild lymphocytic pleocytosis (median 7/μl, range 3–13/μl) and CSF‐restricted oligoclonal bands (type 2 n = 5, type 3 n = 1) in all and disruption of the blood–brain barrier as indicated by elevated CSF/serum albumin ratios in half (n = 3) of the cases. MOG and AQP4 antibodies were assessed in patients with optic neuritis and myelitis, yielding negative results in all cases.
Patients with myositis had elevated creatine kinase levels at diagnosis (median 9086 U/l, range 868–11,105 U/l). MRI of the lower extremities revealed T2‐weighted hyperintense muscle signals and contrast uptake in all cases (Figure 2g,h). Autoimmune myositis antibody panels were evaluated in newly diagnosed patients with PM/Scl‐75 and SAE1 antibody titres detected in two patients, respectively. Muscle biopsy confirmed the diagnosis in one of two cases with new onset myositis and it was deferred in the second case due to favourable clinical response to steroids. Dermatological examination of all three patients was unremarkable. Echocardiogram revealed no evidence for cardiac involvement; tumour search with whole‐body computed tomography and gynaecological evaluation were negative in all patients.
Participants with peripheral inflammatory neuropathies underwent lumbar puncture in all except one case. Consistent with GBS, CSF revealed albuminocytological dissociation. Electroneurography showed demyelinating polyneuropathy with loss of F‐waves (n = 2). In the cases of facial palsy and L5 radiculitis, MRI demonstrated T2‐weighted hyperintensities of the facial nerve and the L5 nerve root, respectively. The latter was accompanied by subsequent affection of the common peroneal and the tibial nerve (Figure 2i–k). CSF analysis showed mild lymphocytic pleocytosis (6/μl and 7/μl) and oligoclonal bands were positive in the CSF but not the serum (type 2). Autoimmune neuropathy panel was negative in all four patients.
In the case of limbic encephalitis MRI demonstrated bilateral hippocampal fluid attenuated inversion recovery (FLAIR) hyperintensities (Figure 2l) and lumbar puncture revealed mild lymphocytic pleocytosis (13/μl), oligoclonal bands type 2 and mild disruption of the blood–brain barrier. The encephalitis antibody panel and tumour search were negative. A flare of previously stable mild ocular myasthenia gravis was diagnosed by rapidly progressive bulbar crisis supported by corresponding elevation of acetylcholine‐receptor antibody titre (8.29 nmol/l, normal <0.4). Diagnosis of GCA was established based on patient age (>50 years), new onset of localized headache, temporal artery tenderness and halo sign on temporal artery duplex ultrasound.
Most patients presented with mild neurological symptoms and were treated on a normal neurological ward (n = 15), four patients were referred to the stroke/intermediate care unit (VITT n = 2, GBS n = 1, GCA n = 1) and two patients required mechanical ventilation and treatment in the neurological intensive care unit (VITT n = 1; myasthenia n = 1).
Patients with VITT were therapeutically anticoagulated (unfractionated heparin perfusor and low molecular weight heparin n = 1, argatroban perfusor n = 2) and received high‐dose intravenous immune globulins (IVIGs, 1 g/kg) for 2 days (n = 2). Heparin, which is contraindicated in VITT, had been used before the pathomechanism of VITT was understood, which later resulted in guidelines recommending the use of anticoagulants administered to treat heparin‐induced thrombocytopenia (direct thrombin inhibitors, e.g., argatroban; direct and indirect Xa inhibitors, e.g., apixaban or fondaparinux). Patients with other neurological autoimmune disorders were treated with immunosuppressants (n = 15) including intravenous methylprednisolone pulse therapy (n = 9: CNS demyelinating disorders n = 7; limbic encephalitis n = 1; L5 radiculitis n = 1), oral prednisolone therapy (overall n = 8: myositis n = 3; MS flares n = 2; GCA/facial palsy/myasthenia n = 1), IVIGs (overall n = 4: GBS n = 2) and plasma exchange (overall n = 3: myasthenia n = 1; optic neuritis n = 1; limbic encephalitis n = 1). Mechanical ventilation was required in two patients (VITT n = 1; myasthenia n = 1). In the follow‐up period, long‐term immunosuppressive treatment was modified in two patients with pre‐existing MS and initiated in one patient with myasthenia.
Median follow‐up after diagnosis or flare of autoimmune disease was 49 days (range 20–105). Complete and partial clinical remission were achieved in five (24%) and 15 (71%) patients, respectively. Disease was stable in one patient (5%) and no patient experienced progressive symptoms after treatment was initiated. Two patients with CNS demyelinating disorders diagnosed following their first vaccination dose received their second BNT162b2 dose without aggravation of their symptoms within the first 24 and 32 days under low‐dose oral prednisolone therapy.
DISCUSSION
Recent population‐based studies found a significantly increased risk for PF4‐antibody‐mediated VITT with subsequent CVST following ChAdOx1 vaccinations [17, 18]. Similarly, analysing the vaccine adverse events reporting database the US Food and Drug Administration noted a potential association between Ad26.COV2.S vaccinations and GBS and revised the vaccine fact sheet accordingly [19]. Although the subject of ongoing research, these findings suggest that SARS‐CoV‐2 vaccinations may trigger neurological autoimmune responses in rare cases with pathomechanisms and risk factors warranting further investigation.
In this case study, a large series of neurological autoimmunity in temporal association with various SARS‐CoV‐2 vaccines (BNT162b2, ChAdOx1 and mRNA‐1273) is reported. No concurrent triggers such as infection, medication changes or non‐compliance were identified. The spectrum of autoimmune diseases was broad and included many conditions, which had not been described in temporal association with COVID‐19 vaccinations before.
Case numbers of several newly diagnosed autoimmune conditions in local county inhabitants were higher than expected compared to previously published incidences. In the 3 months’ inclusion period, these included VITT with subsequent CVST (2/54,135 ChAdOx1 recipients), GBS (2/54,135 ChAdOx1 recipients), optic neuritis (3/155,022 BNT162b2 recipients), polymyositis (2/155,022 BNT162b2 recipients), myelitis (1/54,135 ChAdOx1; 1/155,022 BNT162b2 recipients). In comparison, previously published annual incidences of CVST, GBS, optic neuritis, polymyositis and transverse myelitis ranged between 0.2 and 1.3/100,000, 0.8 and 2.4/100,000, 3.7 and 5.4/100,000, 0.1 and 0.2/100,000 and 0.1 and 2.4/100,000 respectively [20, 21, 22, 23, 24, 25, 26]. However, this study was not powered to investigate incidences of neurological autoimmune responses following SARS‐CoV‐2 vaccinations due to the single‐centre design and a rather small main catchment area in comparison to recent population‐based studies [17, 18]. This observation might therefore also be attributed to variations of normal background incidences. Except for VITT, larger population‐based studies—ideally with control groups—are therefore warranted to further investigate these findings.
Many conditions found in temporal association with vaccinations in this study were previously reported as potential autoimmune sequels of SARS‐CoV‐2 infection with similar clinical and laboratory characteristics [27, 28, 29, 30, 31, 32, 33, 34]. Vaccines containing SARS‐CoV‐2 antigens may enhance autoimmunity by similar mechanisms including polyclonal or bystander activation, epitope spreading or molecular mimicry [35]. Alternatively, the inflammatory stimulus posed by vaccination may enhance autoimmunity in predisposed patients, particularly by driving pre‐existing autoimmune pathways similar to pathogenesis of immune related adverse events following administration of immune checkpoint inhibitors [35, 36, 37]. In patients with new onset of autoimmune diseases, vaccination could have unmasked patients with previously asymptomatic autoimmunity. Lastly, although recent population‐based studies linked SARS‐CoV‐2 vaccinations to VITT and GBS incidence was found to be increased following Ad26.COV2.S administration, the possibility that new onset or flares of other neurological autoimmune conditions merely coincided with vaccinations against SARS‐CoV‐2 cannot be fully excluded.
The majority of cases in this study occurred following BNT162b2 or ChAdOx1 vaccinations, which probably reflects their frequency of administration in Germany. Of note, cases of VITT were found following ChAdOx1 vaccinations only in agreement with previous cases reported in the temporal context of ChAdOx1 and Ad26.COV2.S vaccinations [7, 8, 9]. In line with this clinical observation, a recent Scottish population‐based study found an increased risk for VITT following administration of the ChAdOx1 but not the BNT162b2 vaccine [17]. Cases of GBS, which were previously described irrespective of the vaccine type, also only occurred after ChAdOx1 vaccinations whereas cases of optic neuritis and myositis were found following BNT162b2 vaccinations only. The interval from vaccination to onset of autoimmune responses resembled previously reported cases of PF4‐antibody‐mediated VITT, GBS, facial palsy and transverse myelitis which occurred 1–4 weeks after vaccination [7, 8, 9, 13, 14].
Laboratory findings upon diagnosis were remarkable for elevated C‐reactive protein without other findings suggestive of infection in nine patients. This may correspond with excessive immune reaction following vaccination but must not necessarily characterize vaccinated persons with higher risk for autoimmune reactions. Thrombocyte counts were reduced in patients with VITT only in line with previous reports [7, 8, 9].
Relevant antibodies were detected in four of 15 patients with new onset of autoimmunity following vaccinations. These included detection of PF4 antibodies in cases of VITT as well as detection of myositis‐associated antibodies in two cases. CSF restricted oligoclonal bands (type 2 n = 8, type 3 n = 1), however, were detected in most of the remaining patients with new onset of neurological autoimmunity indicating an intrathecal immune reaction following vaccination. Low diagnostic yield of antibody panels parallels cases of immune related adverse events [36, 37]. In particular, in patients with CNS demyelinating disorders, which constituted the largest subgroup in our cohort, antibodies were rarely identified following immune checkpoint inhibitors [36, 37].
Treatment included administration of immunosuppressants in most cases. IVIGs were given to patients with GBS, and plasma exchange was chosen in the setting of severe autoimmunity or cases unresponsive to prior immunosuppressive therapy. As per recently published recommendations, two of three patients with VITT were therapeutically anticoagulated with argatroban and additionally received a 2‐day treatment with high‐dose IVIGs [9]. The latter had beneficial effects on thrombocyte counts and the clinical course of our patients as recently suggested by Bourguignon et al. [38].
Overall symptoms responded well to treatment including cases of VITT, which had a poor outcome in most previous case series [7, 8, 9]. Levels of acute‐phase proteins corresponded well with clinical responses and may assist to monitor the disease course particularly in patients who require mechanical ventilation. According to our experience, vigilant inpatient or outpatient monitoring under continuous immunosuppressive treatment may allow administration of the second vaccine dose in selected cases of less severe autoimmune reactions. However, a recent prospective cohort study in solid organ transplant patients under immunosuppressive therapy found low antibody titres following vaccination in more than half of the patients [39]. Whilst correlation of antibody titres with vaccine efficacy is pending and thresholds are yet to be determined, lower titres could correspond to a shorter or less sufficient antiviral protection. Strategies such as longer dosing intervals or a third booster shot may improve antibody titres in immunocompromised individuals and warrant further investigation [40].
A limitation of our study is the cohort size although previous reports of VITT and GBS included an even smaller number of patients [7, 8, 9]. The single university centre design of our study may result in a selection bias. Individuals with more severe symptoms may have consulted our institution whereas others may have simply presented to their local healthcare providers. Whilst a temporal association is described, a causative relationship between vaccination and autoimmunity also cannot be established. The strengths of our study include the clinically important research questions, particularly given the increasing global rollout of SARS‐CoV‐2 vaccines, the characterization of various autoimmune phenomena after vaccination, and the longitudinal observation of patients. Awareness and clinical suspicion of autoimmunity following SARS‐CoV‐2 vaccination are critical as early initiation of immunosuppressive treatment appears to result in favourable outcomes including cases of VITT.
It is important to note that autoimmune events constitute rare adverse events after SARS‐CoV‐2 vaccination. Within the local county, neurological autoimmunity was newly diagnosed in 14/232,603 vaccinated individuals (<1:10,000) and most conditions responded well to immunosuppressive therapy. Of 12,021 participants in the ChAdOx1 vaccine trial seven patients (0.1%) experienced severe neurological adverse events including cases of ischaemic stroke (n = 1, <0.1%), multiple sclerosis (n = 1, <0.1%) and transverse myelitis (n = 1, <0.1%), all of which responded well to subsequent treatment [10]. As COVID‐19 represents a life‐threatening infection in some patients with a case fatality rate of 2% in Germany—although the actual number is probably lower as many infections will have remained undetected—the benefits of SARS‐CoV‐2 vaccinations outweigh the rather small risk of autoimmune complications [41].
CONCLUSIONS
In this study, the characteristics of a broad spectrum of neurological autoimmunity encountered following SARS‐CoV2 vaccinations are defined. Like previously reported cases of VITT, autoimmunity typically occurred within 1–4 weeks following vaccination. Compared to published incidences, higher case numbers than expected were noted in Rhein‐Neckar‐Kreis residents for VITT and GBS in ChAdOx1 recipients and for optic neuritis and myositis in BNT162b2 recipients. As this study was not powered to calculate incidences due to the single‐centre design and rather small main catchment area, large population‐based studies with control groups are warranted to further investigate this observation. If autoimmune responses after vaccination are suspected early initiation of immunosuppressive treatment is warranted, which appears to result in favourable responses but might limit the generation of SARS‐CoV‐2‐antibodies. A second vaccination dose may be considered in selected patients with autoimmune reactions following their first vaccine dose under continuous immunosuppressive treatment and vigilant clinical monitoring. Based on the small number of cases of autoimmunity following SARS‐CoV‐2 vaccinations in comparison to the overall number of vaccines administered autoimmune complications appear rare. As COVID‐19 constitutes a life‐threatening infection in some patients, the benefits of vaccination outweigh the comparatively small risk of treatable autoimmune complications.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Leon D Kaulen: Conceptualization (lead); data curation (lead); formal analysis (equal); investigation (lead); methodology (lead); project administration (lead); writing—original draft (lead). Sofia Doubrovinskaia: Data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); writing—review and editing (supporting). Christoph Mooshage: Data curation (supporting); formal analysis (supporting); investigation (supporting); writing—review and editing (supporting). Berit Jordan: Data curation (supporting); formal analysis (supporting); writing—review and editing (supporting). Jan Purrucker: Data curation (supporting); investigation (supporting); writing—review and editing (supporting). Carmen Haubner: Data curation (supporting); investigation (supporting); writing—review and editing (supporting). Corinna Seliger: Data curation (supporting); investigation (supporting); writing—review and editing (supporting). Hanns‐Martin Lorenz: Data curation (supporting); investigation (supporting); writing—review and editing (supporting). Simon Nagel: Data curation (supporting); investigation (supporting); writing—review and editing (supporting). Brigitte Wildemann: Data curation (supporting); investigation (supporting); writing—review and editing (supporting). Martin Bendszus: Data curation (supporting); formal analysis (supporting); investigation (supporting); writing—review and editing (supporting). W. Wick: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); investigation (supporting); supervision (supporting); writing—review and editing (supporting). Silvia Schönenberger: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); writing—original draft (equal); writing—review and editing (lead).
ACKNOWLEDGEMENTS
The State Ministry of Health (Baden‐Württemberg) is thanked for kindly providing SARS‐CoV‐2 vaccination data for the Rhein‐Neckar‐Kreis (county). Dr Wagner‐Wiening, Dr Gaile and Mr Falk who were involved in data curation are specifically thanked. Open access funding enabled and organized by ProjektDEAL.
Kaulen LD, Doubrovinskaia S, Mooshage C, et al. Neurological autoimmune diseases following vaccinations against SARS‐CoV‐2: a case series. Eur J Neurol.2022;29:555–563. 10.1111/ene.15147
Funding information
Dr Kaulen reports no disclosures. Dr Doubrovinskaia reports no disclosures. Dr Mooshage reports no disclosures. Dr Jordan reports no disclosures. Dr Purrucker reports consultation fees and travel expenses from Abbott, Akcea, Bayer, Boehringer Ingelheim, Daiichi Sanyo and Pfizer (all unrelated to the present work). Dr Haubner reports no disclosures. Dr Seliger reports no disclosures. Dr Lorenz reports no disclosures. Dr Nagel reports consultation fees from Brainomix and payment for lectures including service on speakers’ bureaus from Boehringer Ingelheim and Pfizer (all unrelated to the present work). Dr Wildemann reports no disclosures. Dr Bendszus reports no disclosures. Dr Wick reports no disclosures. Dr Schönenberger reports no disclosures
DATA AVAILABILITY STATEMENT
Anonymized data will be shared on reasonable request from qualified researchers.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. New Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S COVID‐19 vaccine. New Engl J Med. 2021;384(19):1824‐1835. 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. New Engl J Med. 2020;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boekel L, Hooijberg F, van Kempen ZLE, et al. Perspective of patients with autoimmune diseases on COVID‐19 vaccination. Lancet Rheumatol. 2021;3(4):e241‐e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS‐CoV‐2 infection in a North American Registry of Patients with Multiple Sclerosis. JAMA Neurol. 2021;78(6):699‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. New Engl J Med. 2021;384(22):2124‐2130. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muir K‐L, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. New Engl J Med. 2021;384(20):1964‐1965. 10.1056/NEJMc2105869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. New Engl J Med. 2021;384(22):2092‐2101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS‐CoV‐2 vaccines. Lancet Infect Dis. 2021;21(4):450‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cirillo N, Doan R. Bell's palsy and SARS‐CoV‐2 vaccines—an unfolding story. Lancet Infect Dis. 2021;21(9):1210‐1211. 10.1016/S1473-3099(21)00273-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen CM, Ramsamy S, Tarr AW, et al. Guillain–Barré syndrome variant occurring after SARS‐CoV‐2 vaccination. Ann Neurol. 2021;90:315‐318. 10.1002/ana.26144 [DOI] [PubMed] [Google Scholar]
- 14. Maramattom BV, Krishnan P, Paul R, et al. Guillain–Barré syndrome following ChAdOx1‐S/nCoV‐19 vaccine. Ann Neurol. 2021;90:3012‐3014. 10.1002/ana.26143 [DOI] [PubMed] [Google Scholar]
- 15. Althaus K, Möller P, Uzun G, et al. Antibody‐mediated procoagulant platelets in SARS‐CoV‐2‐vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106(8):2170. 10.3324/haematol.2021.279000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus‐based clinical case reporting guideline development. J Med Case Rep. 2013;7(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simpson CR, Shi T, Vasileiou E, et al. First‐dose ChAdOx1 and BNT162b2 COVID‐19 vaccines and thrombocytopenic, thromboembolic and haemorrhagic events in Scotland. Nat Med. 2021;27(7):1290‐1297. 10.1038/s41591-021-01408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schulz JB, Berlit P, Diener HC, et al. COVID‐19 vaccine‐associated cerebral venous thrombosis in Germany. Ann Neurol. 2021;90:627‐639. 10.1002/ana.26172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Food and Drug Administration . Coronavirus (COVID‐19) Update: July 13, 2021. 2021. https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐july‐13‐2021. Accessed July 14, 2021.
- 20. Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis. Stroke. 2012;43(12):3375‐3377. [DOI] [PubMed] [Google Scholar]
- 21. Hense S, Schink T, Kreisel SH, et al. Estimation of background incidence rates of Guillain–Barré syndrome in Germany—a retrospective cohort study with electronic healthcare data. Neuroepidemiology. 2014;43(3‐4):244‐252. [DOI] [PubMed] [Google Scholar]
- 22. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain–Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32(2):150‐163. [DOI] [PubMed] [Google Scholar]
- 23. Braithwaite T, Subramanian A, Petzold A, et al. Trends in optic neuritis incidence and prevalence in the UK and association with systemic and neurologic disease. JAMA Neurol. 2020;77(12):1514‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martínez‐Lapiscina EH, Fraga‐Pumar E, Pastor X, et al. Is the incidence of optic neuritis rising? Evidence from an epidemiological study in Barcelona (Spain), 2008–2012. J Neurol. 2014;261(4):759‐767. [DOI] [PubMed] [Google Scholar]
- 25. Meyer A, Meyer N, Schaeffer M, Gottenberg J‐E, Geny B, Sibilia J. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology. 2015;54(1):50‐63. [DOI] [PubMed] [Google Scholar]
- 26. Young J, Quinn S, Hurrell M, Taylor B. Clinically isolated acute transverse myelitis: prognostic features and incidence. Mult Scler. 2009;15(11):1295‐1302. [DOI] [PubMed] [Google Scholar]
- 27. Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS‐CoV‐2. Ann Rheum Dis. 2021;80(3):e42. [DOI] [PubMed] [Google Scholar]
- 28. Zambreanu L, Lightbody S, Bhandari M, et al. A case of limbic encephalitis associated with asymptomatic COVID‐19 infection. J Neurol Neurosurg Psychiatry. 2020;91(11):1229. [DOI] [PubMed] [Google Scholar]
- 29. Restivo DA, Centonze D, Alesina A, Marchese‐Ragona R. Myasthenia gravis associated with SARS‐CoV‐2 infection. Ann Int Med. 2020;173(12):1027‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou S, Jones‐Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody‐associated optic neuritis and myelitis in COVID‐19. J Neuroophthalmol. 2020;40(3):398‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lima MA, Silva MTT, Soares CN, et al. Peripheral facial nerve palsy associated with COVID‐19. J Neurovirol. 2020;26(6):941‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aschman T, Schneider J, Greuel S, et al. Association between SARS‐CoV‐2 infection and immune‐mediated myopathy in patients who have died. JAMA Neurol. 2021;78(8):948. 10.1001/jamaneurol.2021.2004 [DOI] [PubMed] [Google Scholar]
- 33. Munz M, Wessendorf S, Koretsis G, et al. Acute transverse myelitis after COVID‐19 pneumonia. J Neurol. 2020;267(8):2196‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zuo YA‐O, Estes SK, Ali RA, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID‐19. Sci Transl Med. 2020;12(570):eabd3876. 10.1126/scitranslmed.abd3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agmon‐Levin N, Paz Z, Israeli E, Shoenfeld Y. Vaccines and autoimmunity. Nat Rev Rheumatol. 2009;5(11):648‐652. [DOI] [PubMed] [Google Scholar]
- 36. Marini A, Bernardini A, Gigli GL, et al. Neurologic adverse events of immune checkpoint inhibitors. Neurology. 2021;96(16):754. [DOI] [PubMed] [Google Scholar]
- 37. Braaten TJ, Brahmer JR, Forde PM, et al. Immune checkpoint inhibitor‐induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis. 2020;79(3):332‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine‐induced thrombotic thrombocytopenia. New Engl J Med. 2021;385:720‐728. 10.1056/NEJMoa2107051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA COVID‐19 vaccine in solid‐organ transplant recipients. New Engl J Med. 2021;385(7):661‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on reasonable request from qualified researchers.


